Decrease of Pirimiphos-Methyl and Deltamethrin Residues in Stored Rice with Post-Harvest Treatment
Abstract
:1. Introduction
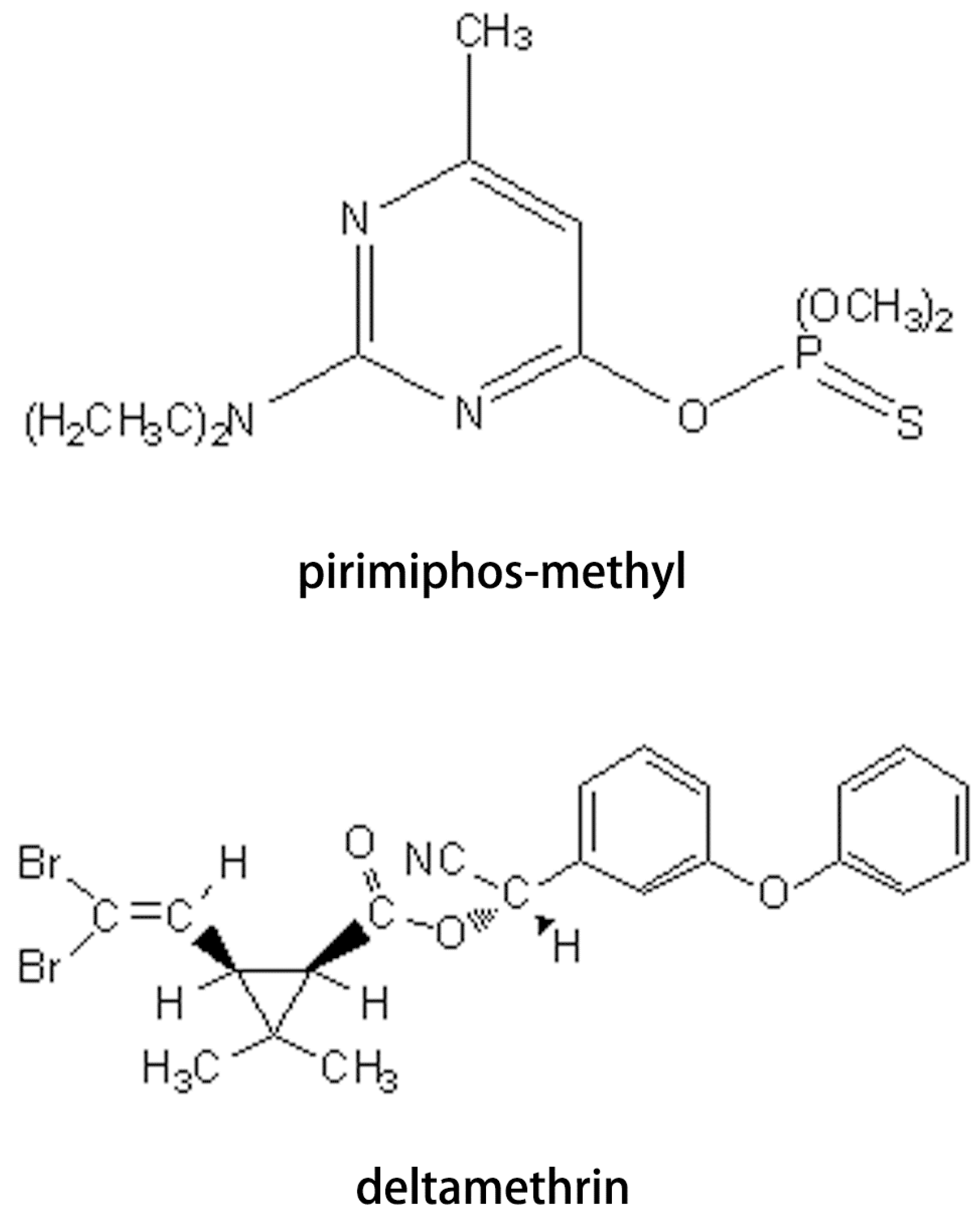
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Trials
2.3. Analytical Procedures
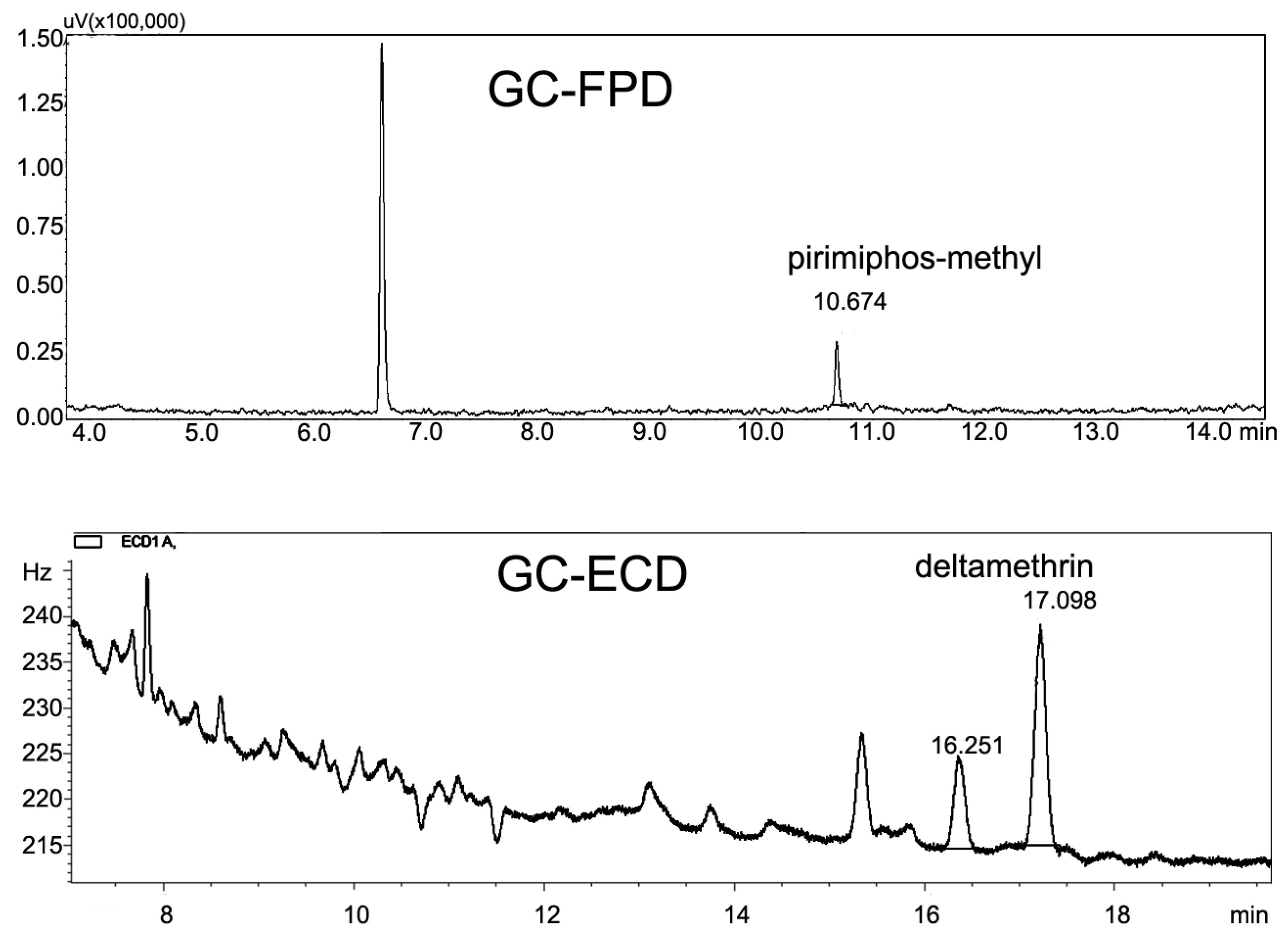
2.4. Instruments and Chromatographic Conditions
2.5. Statistical Method
3. Results and Discussion
3.1. Method Validation
| Pesticide | Spiked Level (mg/kg) | Average Recovery (n = 5%) | RSD (%) | LOQ (mg/kg) | Regression Equation | R2 |
|---|---|---|---|---|---|---|
| Pirimiphos-methyl | 0.01 | 96.3 ± 7.6 | 7.9 | 0.01 | y = 6936300x + 26524 | 1 |
| 0.1 | 107 ± 3.8 | 3.5 | ||||
| 0.5 | 95.5 ± 1.9 | 2.0 | ||||
| Deltamethrin | 0.06 | 78.3 ± 3.3 | 3.8 | 0.06 | y = 3207.4x + 66.442 | 0.9991 |
| 0.1 | 106 ± 3.7 | 3.1 | ||||
| 0.5 | 98.2 ± 3.2 | 2.9 |
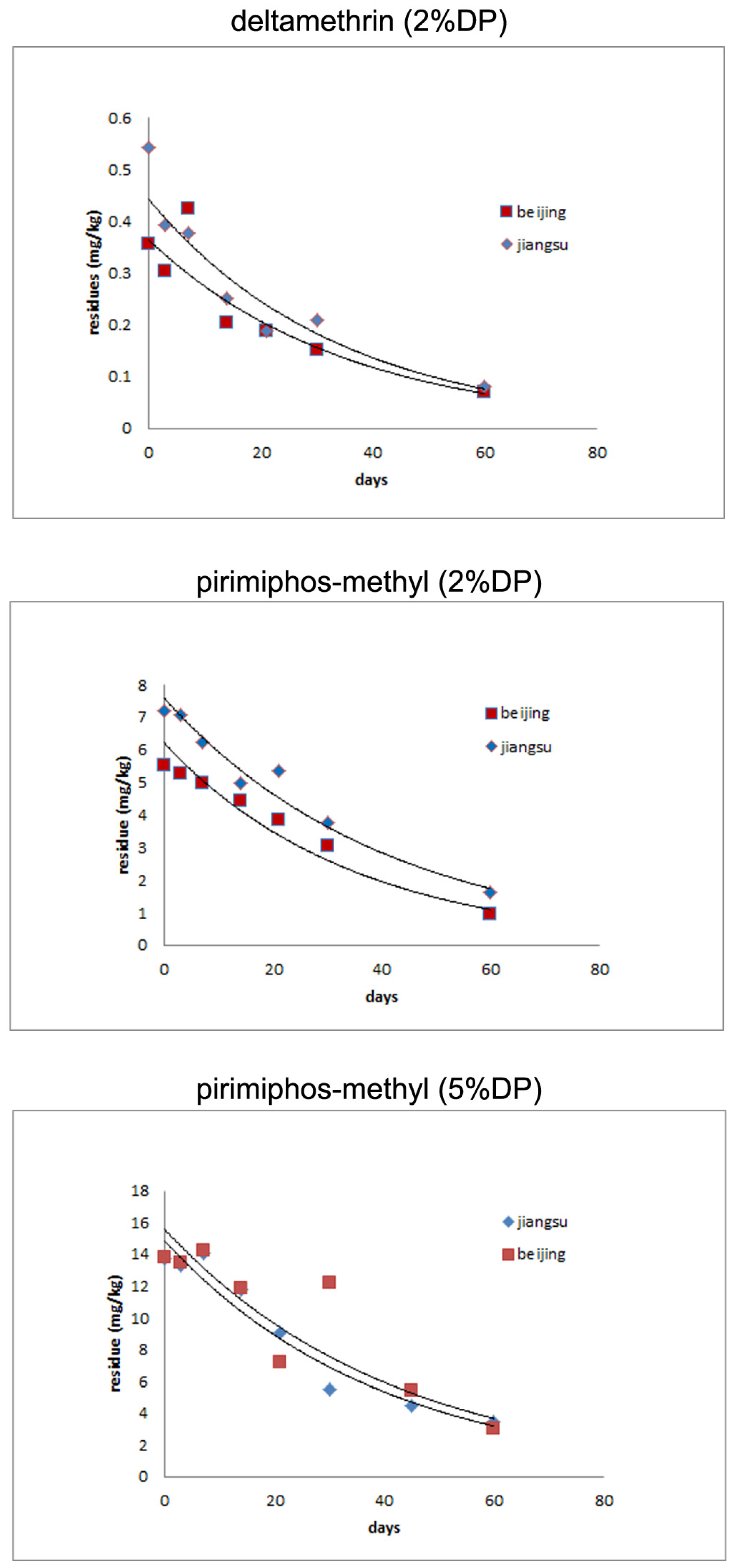
3.2. Residue Dynamics of Pirimiphos-methyl and Deltamethrin
3.3. Residues of Pirimiphos-methyl and Deltamethrin in Stored Rice
| Site | PHI (Days) | Pirimiphos-methyl | Deltamethrin | ||
|---|---|---|---|---|---|
| Application Rate a.i. mg/kg | Residue (mg/kg) | Application Rate a.i. mg/kg | Residue (mg/kg) | ||
| Beijing | 30 | 4.5 | 3.1 | 0.5 | 0.17 |
| 60 | 1.6 | 0.13 | |||
| 30 | 6.75 | 4.0 | 0.75 | 0.13 | |
| 60 | 3.0 | 0.13 | |||
| Jiangsu | 30 | 4.5 | 3.6 | 0.5 | 0.15 |
| 60 | 1.7 | 0.14 | |||
| 30 | 6.75 | 4.5 | 0.75 | 0.18 | |
| 60 | 3.8 | 0.14 | |||
| Site | PHI (Days) | Pirimiphos-methyl | |
|---|---|---|---|
| Application Rate (a.i. mg/kg) | Residue (mg/kg) | ||
| Beijing | 45 | 10 | 4.5 |
| 60 | 3.0 | ||
| 45 | 15 | 5.4 | |
| 60 | 4.5 | ||
| Jiangsu | 45 | 10 | 4.5 |
| 60 | 3.4 | ||
| 45 | 15 | 6.4 | |
| 60 | 4.5 | ||
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Uygun, U.; Senoz, B.; Koksel, H. Dissipation of organophosphorus pesticides in wheat during pasta processing. Food Chem. 2008, 109, 355–360. [Google Scholar] [CrossRef]
- Afridi, I.A.K.; Parveen, Z.; Masud, S.Z. Stability of organophosphate and pyrethroid pesticides on wheat in storage. J. Stored Prod. Res. 2001, 37, 199–204. [Google Scholar] [CrossRef]
- Hou, X.; Han, M.; Dai, X.; Yang, X.; Yi, S. A multi-residue method for the determination of 124 pesticides in rice by modified QuEChERS extraction and gas chromatography—Tandem mass spectrometry. Food Chem. 2013, 138, 1198–1205. [Google Scholar] [CrossRef]
- Fleurat-Lessard, F.; Chaurand, M.; Marchegay, G.; Abecassis, J. Effects of processing on the distribution of pirimiphos-methyl residues in milling fractions of durum wheat. J. Stored Prod. Res. 2007, 43, 384–395. [Google Scholar] [CrossRef]
- Barlow, S.M.; Sullivan, F.M.; Lines, J. Risk assessment of the use of deltamethrin on bednets for the prevention of malaria. Food Chem. Toxicol. 2001, 39, 407–422. [Google Scholar] [CrossRef]
- Kljajić, P.; Perić, I. Residual effects of deltamethrin and malathion on different populations of Sitophilus granarius (L.) on treated wheat grains. J. Stored Prod. Res. 2009, 45, 45–48. [Google Scholar] [CrossRef]
- López-Fernández, O.; Rial-Otero, R.; González-Barreiro, C.; Simal-Gándara, J. Surveillance of fungicidal dithiocarbamate residues in fruits and vegetables. Food Chem. 2012, 134, 366–374. [Google Scholar] [CrossRef]
- Pose-Juan, E.; Rial-Otero, R.; Martinez-Carballo, E.; Lopez-Periago, E.; Simal-Gandara, J. Determination of metalaxyl and identification of adjuvants in wettable powder pesticide technical formulas. Anal. Bioanal. Chem. 2009, 394, 1535–1544. [Google Scholar] [CrossRef]
- Gonzalezrodriguez, R.; Rialotero, R.; Canchogrande, B.; Simalgandara, J. Occurrence of fungicide and insecticide residues in trade samples of leafy vegetables. Food Chem. 2008, 107, 1342–1347. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, X.Y.; Li, Y.S.; Yang, S.P.; Shen, J.Z.; Zhang, S.X. Simultaneous determination of type-A and type-B trichothecenes in rice by UPLC-MS/MS. Anal. Method. 2012, 4, 4077–4082. [Google Scholar] [CrossRef]
- Rial-Otero, R.; Cancho-Grande, B.; Perez-Lamela, C.; Simal-Gandara, J.; Arias-Estevez, M. Simultaneous determination of the herbicides diquat and paraquat in water. J. Chromatogr. Sci. 2006, 44, 539–542. [Google Scholar] [CrossRef]
- Zhao, L.W.; Chen, X.C.; Liu, F.M.; Ge, J.; You, X.W. Determination of monosulfuron-ester residues in grains, straw, green plants and soil of wheat by modified QuEChERS and LC-MS/MS. Anal. Method. 2013, 5, 2267–2272. [Google Scholar] [CrossRef]
- Anastassiades, M.; Maštovská, K.; Lehotay, S.J. Evaluation of analyte protectants to improve gas chromatographic analysis of pesticides. J. Chromatogr. A 2003, 1015, 163–184. [Google Scholar] [CrossRef]
- Zhang, W.W.; Xu, J.; Dong, F.S.; Liu, X.G.; Zhang, Y.; Tao, Y.; Wu, X.H.; Zheng, Y.Q. Simultaneous determination of three strobilurin fungicide residues in fruits, vegetables and soil by a modified quick, easy, cheap, effective, rugged (QuEChERS) method coupled with gas chromatography-tandem mass spectrometry. Anal. Method. 2013, 5, 7102–7109. [Google Scholar]
- Nguyen, T.D.; Han, E.M.; Seo, M.S.; Kim, S.R.; Yun, M.Y.; Lee, D.M.; Lee, G.-H. A multi-residue method for the determination of 203 pesticides in rice paddies using gas chromatography/mass spectrometry. Anal. Chim. Acta 2008, 619, 67–74. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, L.; Zhou, L.; Zhang, F.; Kang, S.; Pan, C. Multi-walled carbon nanotubes as alternative reversed-dispersive solid phase extraction materials in pesticide multi-residue analysis with QuEChERS method. J. Chromatogr. A 2012, 1225, 17–25. [Google Scholar]
- Pareja, L.; Fernández-Alba, A.R.; Cesio, V.; Heinzen, H. Analytical methods for pesticide residues in rice. Trac-Trend. Anal. Chem. 2011, 30, 270–291. [Google Scholar] [CrossRef]
- Liao, Q.G.; Zhou, Y.M.; Luo, L.G.; Wang, L.B.; Feng, X.H. Determination of twelve herbicides in tobacco by a combination of solid-liquid-solid dispersive extraction using multi-walled carbon nanotubes, dispersive liquid-liquid micro-extraction, and detection by GC with triple quadrupole mass spectrometry. Microchim. Acta 2013, 181, 163–169. [Google Scholar]
- Hou, X.; Lei, S.; Qiu, S.; Guo, L.; Yi, S.; Liu, W. A multi-residue method for the determination of pesticides in tea using multi-walled carbon nanotubes as a dispersive solid phase extraction absorbent. Food Chem. 2014, 153, 121–129. [Google Scholar] [CrossRef]
- Deng, X.; Guo, Q.; Chen, X.; Xue, T.; Wang, H.; Yao, P. Rapid and effective sample clean-up based on magnetic multiwalled carbon nanotubes for the determination of pesticide residues in tea by gas chromatography–mass spectrometry. Food Chem. 2014, 145, 853–858. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yu, C.; Li, Y.; Zhang, Q.; Zou, N.; Gu, K.; Li, X.; Pan, C. Decrease of Pirimiphos-Methyl and Deltamethrin Residues in Stored Rice with Post-Harvest Treatment. Int. J. Environ. Res. Public Health 2014, 11, 5372-5381. https://doi.org/10.3390/ijerph110505372
Yu C, Li Y, Zhang Q, Zou N, Gu K, Li X, Pan C. Decrease of Pirimiphos-Methyl and Deltamethrin Residues in Stored Rice with Post-Harvest Treatment. International Journal of Environmental Research and Public Health. 2014; 11(5):5372-5381. https://doi.org/10.3390/ijerph110505372
Chicago/Turabian StyleYu, Chuanshan, Yanjie Li, Qian Zhang, Nan Zou, Kejia Gu, Xuesheng Li, and Canping Pan. 2014. "Decrease of Pirimiphos-Methyl and Deltamethrin Residues in Stored Rice with Post-Harvest Treatment" International Journal of Environmental Research and Public Health 11, no. 5: 5372-5381. https://doi.org/10.3390/ijerph110505372




