Identifying Determinants of Oncomelania hupensis Habitats and Assessing the Effects of Environmental Control Strategies in the Plain Regions with the Waterway Network of China at the Microscale
Abstract
:1. Introduction
2. Materials and Methods
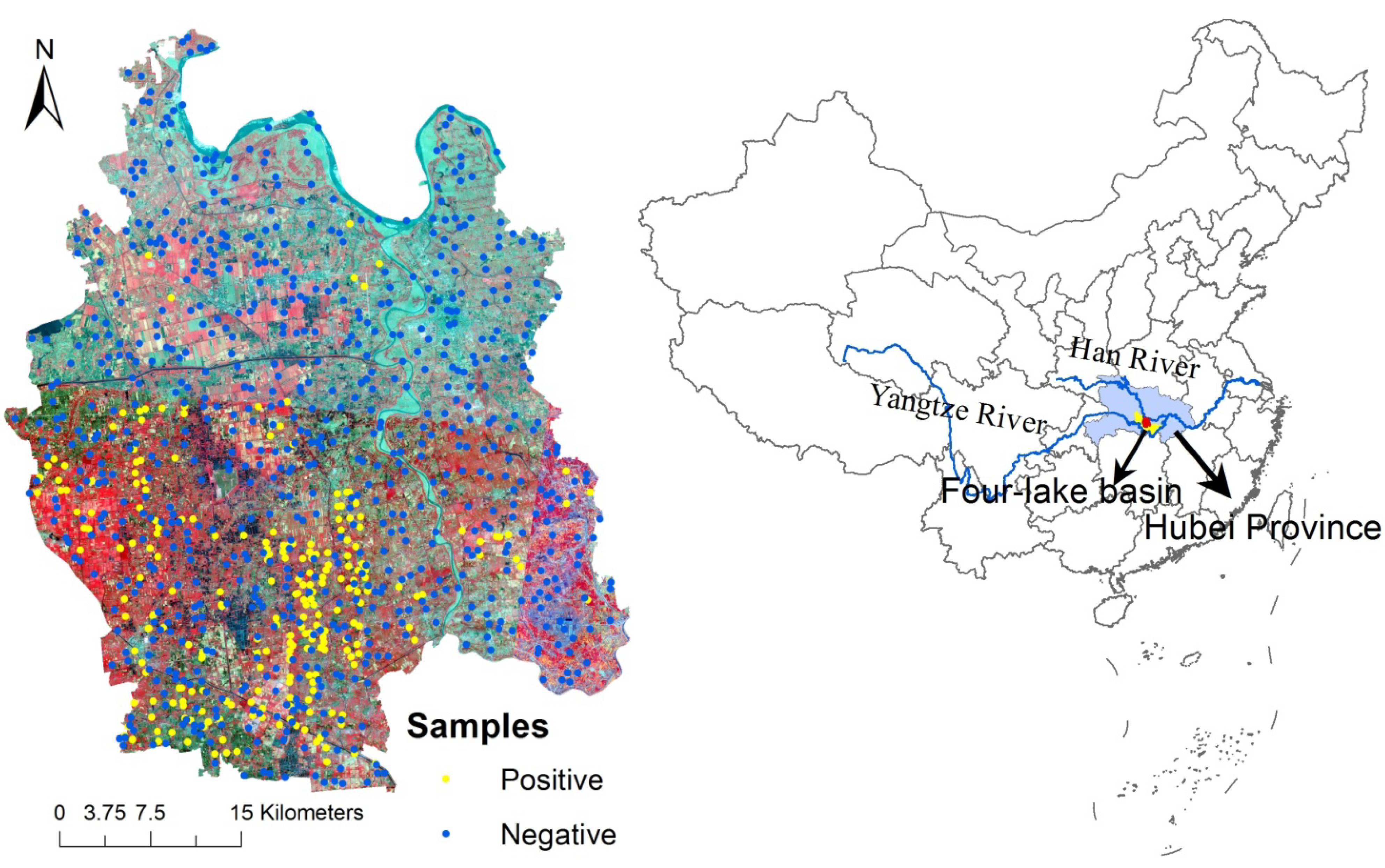
2.1. Study Area
2.2. Data Sources
2.2.1. Parasitological Data
2.2.2. Environmental Data
Land-Use Classification
Landscape Pattern
Normalized difference vegetation index (NDVI) and wetness index (WI)
Soil texture
Topological data
2.3. Statistical Analysis
3. Results
| Factors | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| B | OR (95% CI) | p value | B | OR (95% CI) | p value | |
| FD (×100) | 0.280 | 1.323 (1.272–1.377) | <0.001 | 0.307 | 1.359 (1.292–1.430) | <0.001 |
| MedPS | 0.974 | 2.648 (1.803–3.891) | <0.001 | |||
| PSCOV | −0.002 | 0.998 (0.996–1.000) | 0.040 | |||
| MPFD | −11.872 | 0.000 (0.000–0.008) | 0.001 | |||
| AWMSI | −0.450 | 0.637 (0.425–0.956) | 0.029 | |||
| Dry farm land proportion (%) | −0.010 | 0.990 (0.982–0.999) | 0.035 | |||
| Paddy farm landproportion (%) | 0.040 | 1.041 (1.031–1.051) | <0.001 | 0.054 | 1.055 (1.032–1.079) | <0.001 |
| Silt proportion (%) | 0.213 | 1.237 (1.170–1.309) | <0.001 | |||
| DEM (m) | −0.052 | 0.949 (0.928–0.971) | <0.001 | −0.121 | 0.886 (0.828–0.947) | <0.001 |
| Wetness | 0.017 | 1.017 (1.003–1.031) | 0.017 | 0.051 | 1.052 (1.017–1.089) | 0.003 |
| NDVI | −1.358 | 0.257 (0.182–0.364) | <0.001 | −1.047 | 0.351 (0.166–0.743) | 0.006 |
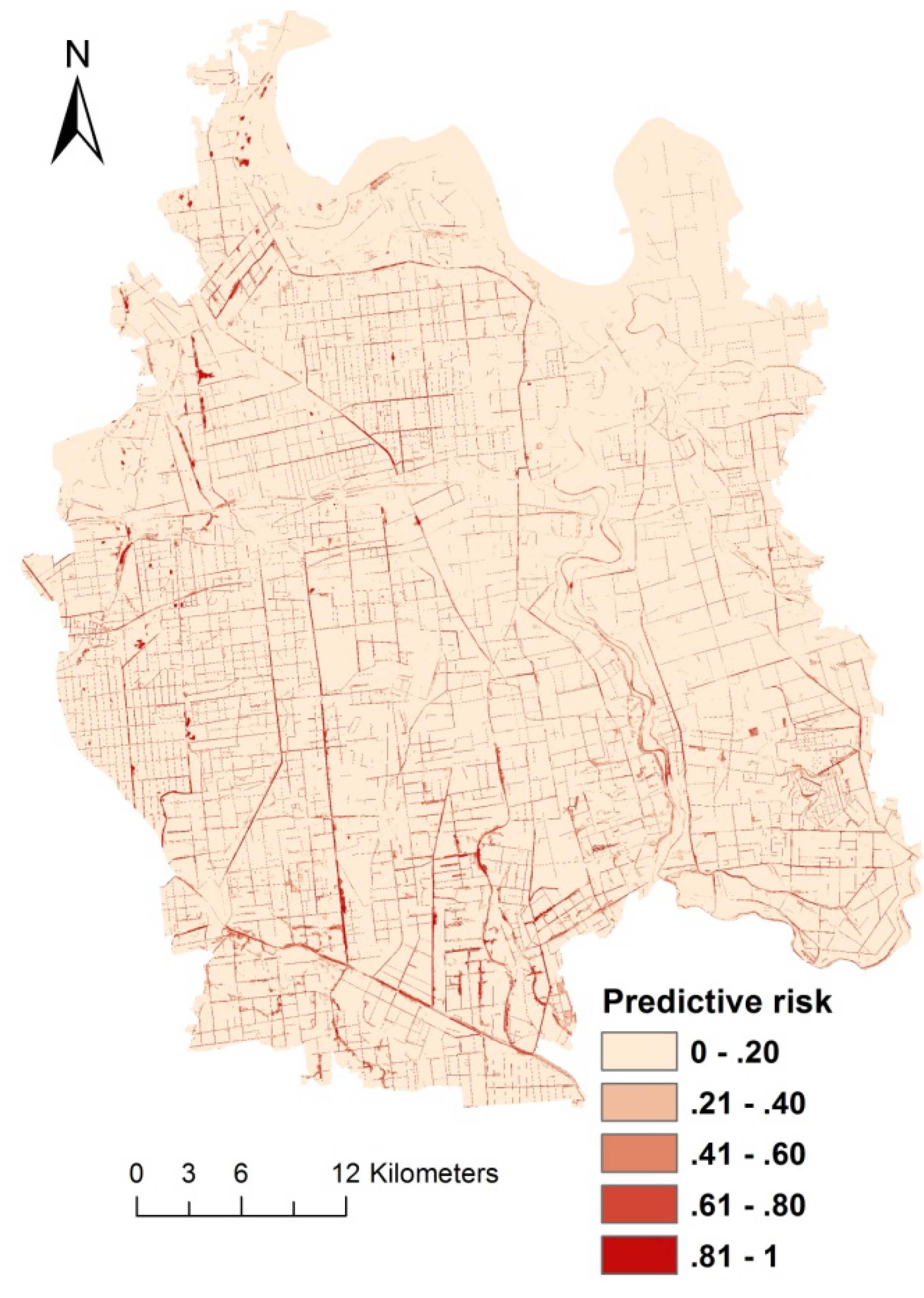
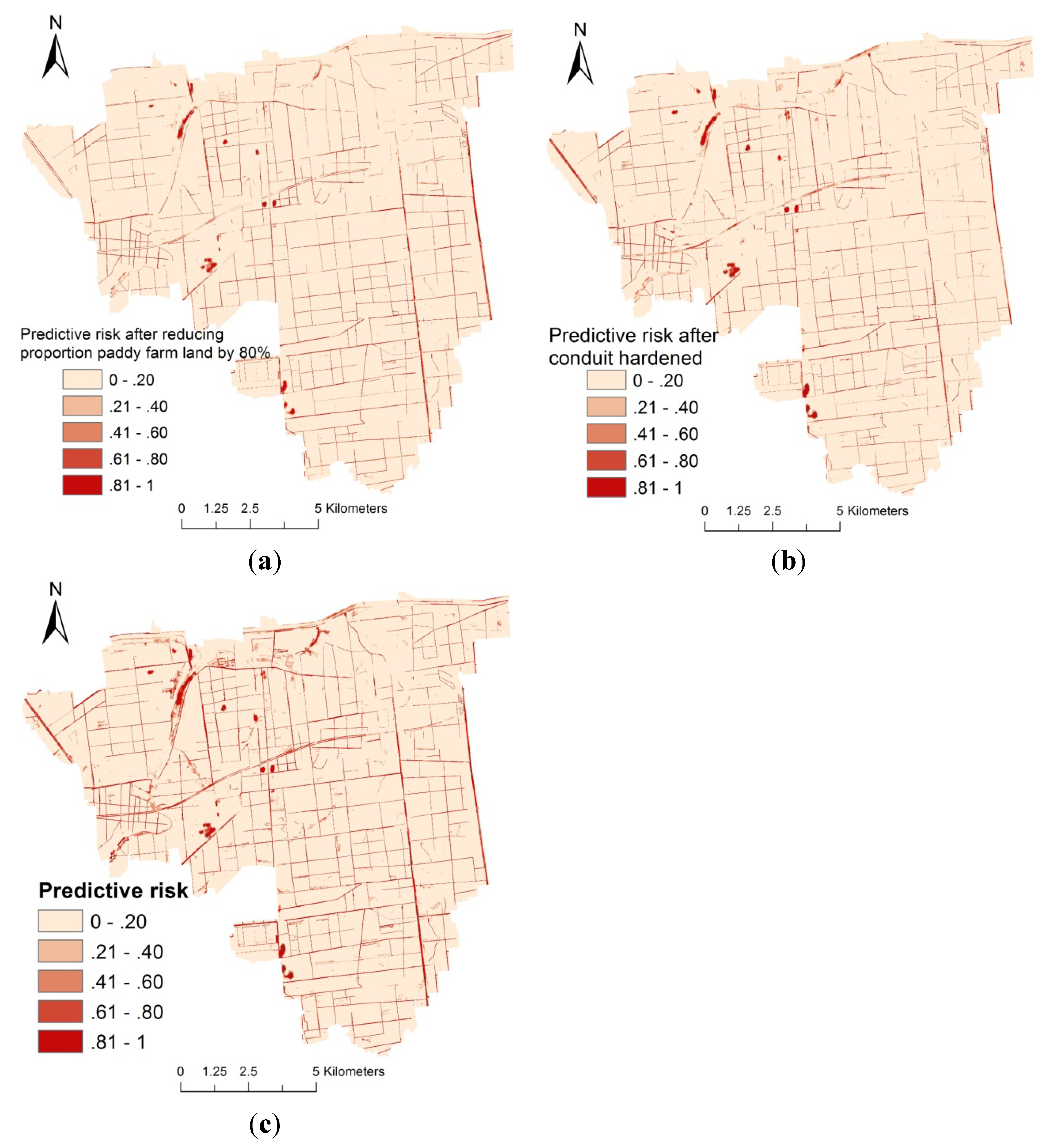
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Utzinger, J.; Zhou, X.N.; Chen, M.G.; Bergquist, R. Conquering schistosomiasis in China: The long march. Acta Trop. 2005, 96, 69–96. [Google Scholar] [CrossRef]
- Zhou, X.N.; Bergquist, R.; Leonardo, L.; Yang, G.J.; Yang, K.; Sudomo, M.; Olveda, R. Schistosomiasis japonica: Control and research needs. In Advances in Parasitology, volume72: Important Helminth Infections in Southeast Asia: Diversity and Potential for Control and Elimination, part A; Zhou, X.N., Bergquist, R., Olveda, R., Utzinger, J., Eds.; Elsevier Academic Press Inc.: San Diego, CA, USA, 2010; Volume 72, pp. 145–178. [Google Scholar]
- Zhang, Z.J.; Ong, S.; Peng, W.X.; Zhou, Y.B.; Zhuang, J.L.; Zhao, G.M.; Jiang, Q.W. A model for the prediction of Oncomelania hupensis in the lake and marshland regions, China. Parasitol. Int. 2008, 57, 121–131. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Z.J.; Chen, Y.; Wang, Z.L.; Gao, J.; Tao, B.; Jiang, Q.L.; Jiang, Q.W. Spatial pattern of schistosomiasis in Xingzi, Jiangxi Province, China: The effects of environmental factors. Parasites Vectors 2013, 6. [Google Scholar] [CrossRef]
- Zhou, X.N. Science on Oncomelania Snail; Sciences Press: Beijing, China, 2005; pp. 186–188. [Google Scholar]
- Spear, R.C.; Seto, E.; Liang, S.; Birkner, M.; Hubbard, A.; Qiu, D.C.; Yang, C.H.; Zhong, B.; Xu, F.S.; Gu, X.G.; et al. Factors influencing the transmission of Schistosoma japonicum in the mountains of Sichuan Province of China. Am. J. Trop. Med. Hyg. 2004, 70, 48–56. [Google Scholar]
- Guo, J.G.; Vounatsou, P.; Cao, C.L.; Utzinger, J.; Zhu, H.Q.; Anderegg, D.; Zhu, R.; He, Z.Y.; Li, D.; Hu, F.; et al. A geographic information and remote sensing based model for prediction of Oncomelania hupensis habitats in the Poyang lake area, China. Acta Trop. 2005, 96, 213–222. [Google Scholar] [CrossRef]
- Yang, G.J.; Vounatsou, P.; Zhou, X.N.; Tanner, M.; Utzinger, J. A Bayesian-based approach for spatio-temporal modeling of county level prevalence of Schistosoma japonicum infection in Jiangsu Province, China. Int. J. Parasitol. 2005, 35, 155–162. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Xu, D.Z.; Zhou, X.N.; Zhou, Y.; Liu, S.J. Remote sensing and spatial statistical analysis to predict the distribution of Oncomelania hupensis in the marshlands of China. Acta Trop. 2005, 96, 205–212. [Google Scholar] [CrossRef]
- Peng, W.X.; Tao, B.; Clements, A.; Jiang, Q.L.; Zhang, Z.J.; Zhou, Y.B.; Jiang, Q.W. Identifying high-risk areas of schistosomiasis and associated risk factors in the Poyang lake region, China. Parasitology 2010, 137, 1099–1107. [Google Scholar] [CrossRef]
- Zhou, X.N.; Li, S.Z. Essential issues for project management and quality control in the national schistosomiasis control programme of China. Chin. J. Schisto. Control 2012, 24, 373–375. (In Chinese) [Google Scholar]
- Zhou, X.N.; Guo, J.G.; Wu, X.H.; Jiang, Q.W.; Zheng, J.; Dang, H.; Wang, X.H.; Xu, J.; Zhu, H.Q.; Wu, G.L.; et al. Epidemiology of schistosomiasis in the People’s Republic of China, 2004. Emerg. Inf. Dis. 2007, 13, 1470–1476. [Google Scholar] [CrossRef]
- Wang, L.D.; Chen, H.G.; Guo, J.G.; Zeng, X.J.; Hong, X.L.; Xiong, J.J.; Wu, X.H.; Wang, X.H.; Wang, L.Y.; Xia, G.; et al. A strategy to control transmission of Schistosoma japonicum in China. N. Engl. J. Med. 2009, 360, U121–U144. [Google Scholar] [CrossRef]
- Liang, S.; Yang, C.H.; Zhong, B.; Qiu, D.C. Re-emerging schistosomiasis in hilly and mountainous areas of Sichuan, China. Bull. World Health Organ. 2006, 84, 139–144. [Google Scholar] [CrossRef]
- Ding, Z.J.; Wu, J.P.; Wang, D.F.; Wei, Z.Y.; He, B.; He, Y.L.; Yu, Y.S.; Wan, J. Causes of re-emergence of oncomelania snails in hardened ditches. Chin. J. Schist Control 2013, 25, 213–214, 216. (in Chinese). [Google Scholar]
- Zhang, Z.J.; Carpenter, T.E.; Lynn, H.S.; Chen, Y.; Bivand, R.; Clark, A.B.; Hui, F.M.; Peng, W.X.; Zhou, Y.B.; Zhao, G.M.; et al. Location of active transmission sites of Schistosoma japonicum in lake and marshland regions in China. Parasitology 2009, 136, 737–746. [Google Scholar]
- Reisen, W.K. Landscape epidemiology of vector-borne diseases. Annu. Rev. Entomol. 2010, 55, 461–483. [Google Scholar] [CrossRef]
- Pavlovsky, E.N. Natural Nidality of Transmissable Diseases, with Special Reference to the Landscape Epidemiology of Zooanthroponse; University of Illinois Press: Urbana, IL, USA, 1966. [Google Scholar]
- Kitron, U. Landscape ecology and epidemiology of vector-borne diseases: Tools for spatial analysis. J. Med. Entomol. 1998, 35, 435–445. [Google Scholar]
- Brownstein, J.S.; Rosen, H.; Purdy, D.; Miller, J.R.; Merlino, M.; Mostashari, F.; Fish, D. Spatial analysis of West Nile virus: Rapid risk assessment of an introduced vector-borne zoonosis. Vector Borne Zoonotic Dis. 2002, 2, 157–164. [Google Scholar] [CrossRef]
- Yang, K.; Zhou, X.N.; Wu, X.H.; Steinmann, P.; Wang, X.H.; Yang, G.J.; Utzinger, J.; Li, H.J. Landscape pattern analysis and bayesian modeling for predicting Oncomelania hupensis distribution in Eryuan county, People’s Republic of China. Am. J. Trop. Med. Hyg. 2009, 81, 416–423. [Google Scholar]
- Tu, Z.W.; Huang, X.B.; Cai, S.X.; Fan, H.P.; Liu, J.B.; Zhu, H.G.; Su, Z.M.; Liu, H.C. Evaluation of the endemic situation of schistosomiasis control in Hubei Province (2009). J. Pub. Health Prev. Med. 2010, 21, 14–17. (In Chinese) [Google Scholar]
- Xiao, X.L.; Cheng, Y.P.; Xu, Q.C. Growth and decline of oncomelania snail status of canals in Qianjiang city in four lake areas, 1990–2010. Chin. J. Schist Control 2012, 24, 619–620. (In Chinese) [Google Scholar]
- Peng, X.W.; Wang, J.S.; Rong, X.B.; Wang, S.H.; He, L.C.; Fu, Z.Y. Schistosomiasis prevalence characteristic and epidemic factors in four-lake basin of Jianghan Plain, Hubei province. J. Pub. Health Prev. Med. 2007, 18, 66–68, 70. (In Chinese) [Google Scholar]
- Zhang, H.Z.; Wang, W.L.; Tian, Z.H.; Liu, F.C. The drainage system evolutionin and snail control programinner embankment of hyper-endemic area. Hubei J. Prev. Med. 2002, 13, 36–37. (In Chinese) [Google Scholar]
- Wang, W.L. Schistosomiasis epidemic characteristics after lake beach type region evolving into irrigation network endemic area. Chin. J. Parasitol. Parasit. Dis. 1990, 8, 73. (In Chinese) [Google Scholar]
- China Center for Resources Satellite Data and Application. Available online: http://www.cresda.com (accessed on 5 February 2013).
- Trimble. Ecognition Developer 8.64.1-User Guide; Trimble Germany GmbH: Munchen, Germany, 2011; p. 242. [Google Scholar]
- Patch Analyst Version 5. Available online: http://www.cnfer.on.ca/SEP/patchanalyst/ (accessed on 10 May 2013).
- McGarigal, K.; Marks, B.J. FRAGSTATS: Spatial Pattern Analysis Program for Quantifying Landscape Structure. Available online: http://andrewsforest.oregonstate.edu/pubs/pdf/pub1538.pdf (accessed on 21 March 2014).
- Hargis, C.D.; Bissonette, J.A.; David, J.L. The behavior of landscape metrics commonly used in the study of habitat fragmentation. Landsc. Ecol. 1998, 13, 167–186. [Google Scholar] [CrossRef]
- The U.S. Geological Survey, USGS. Available online: http://glovis.usgs.gov/ (accessed on 25 February 2013).
- Guo, J.G.; Lin, D.D.; Hu, G.Q.; Ning, A.; Liu, H.Y.; Lu, S.B.; Li, D.; WU, X.H.; Wang, R.R.; Chen, M.G.; et al. Rapid identifition of Oncomelania hupensis snail habitat in the Poyang lake region by geographic information system (GIS) and remote sensing(RS). Chin. J. Epidemiol. 2002, 23, 99–101. (In Chinese) [Google Scholar]
- Wang, Z.L.; Gao, J.; Tao, B.; Jiang, Q.J.; Zhai, M.L.; Zhang, Z.j.; Jiang, Q.W. Preliminary study on applying high resolution cbers images to identify oncomelania snail habitats in lake and marshland regions. Chin. J. Schist Control 2012, 24, 640–644. (In Chinese) [Google Scholar]
- The International Scientific and Technical Data Mirror Site, Computer Network Information Center, CAS. Available online: http://www.gscloud.cn (accessed on 25 February 2013).
- Kutner, M.H.; Nachtsheim, C.J.; Neter, J.; Li, W. Applied Linear Statistical Models; McGraw-Hill/Irwin: Chicago, IL, USA, 2004; p. 1. [Google Scholar]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression; John Wiley: New York, NY, USA, 2000; p. 1. [Google Scholar]
- Greiner, M.; Pfeiffer, D.; Smith, R.D. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev. Vet. Med. 2000, 45, 23–41. [Google Scholar] [CrossRef]
- Malone, J.B.; Bergquist, N.R.; Huh, O.K.; Bavia, M.E.; Bernardi, M.; El Bahy, M.M.; Fuentes, M.V.; Kristensen, T.K.; McCarroll, J.C.; Yilma, J.M.; et al. A global network for the control of snail-borne disease using satellite surveillance and geographic information systems. Acta Trop. 2001, 79, 7–12. [Google Scholar] [CrossRef]
- Zhou, X.N.; Malone, J.B.; Kristensen, T.K.; Bergquist, N.R. Application of geographic information systems and remote sensing to schistosomiasis control in China. Acta Trop. 2001, 79, 97–106. [Google Scholar] [CrossRef]
- Xu, B.; Gong, P.; Biging, G.; Liang, S.; Seto, E.; Spear, R. Snail density prediction for schistosomiasis control using IKONOS and ASTER images. Photogramm. Eng. Remote Sens. 2004, 70, 1285–1294. [Google Scholar] [CrossRef]
- Gong, P.; Xu, B.; Liang, S. Remote sensing and geographic information systems in the spatial temporal dynamics modeling of infectious diseases. Sci. China Ser. C Life Sci. 2006, 49, 573–582. [Google Scholar] [CrossRef]
- Xu, Q.C.; Wang, W.L.; Cai, Z.W.; Xiong, Y.Q.; Liu, J.G.; Shi, J.B. Schistosomiasis survey in Qiangjiang city, 2007. J. Pub. Health Prev. Med. 2008, 19, 31–33. (In Chinese) [Google Scholar]
- Bavia, M.E.; Malone, J.B.; Hale, L.; Dantas, A.; Marroni, L.; Reis, R. Use of thermal and vegetation index data from earth observing satellites to evaluate the risk of schistosomiasis in Bahia, Brazil. Acta Trop. 2001, 79, 79–85. [Google Scholar] [CrossRef]
- Kristensen, T.K.; Malone, J.B.; McCarroll, J.C. Use of satellite remote sensing and geographic information systems to model the distribution and abundance of snail intermediate hosts in Africa: A preliminary model for biomphalaria pfeifferi in Ethiopia. Acta Trop. 2001, 79, 73–78. [Google Scholar] [CrossRef]
- Yang, K.; Wang, X.-H.; Yang, G.-J.; Wu, X.-H.; Qi, Y.-L.; Li, H.-J.; Zhou, X.-N. An integrated approach to identify distribution of Oncomelania hupensis, the intermediate host of Schistosoma japonicum, in a mountainous region in china. Int. J. Parasitol. 2008, 38, 1007–1016. [Google Scholar]
- Wu, J.G. Landscape Ecology—Pattern, Process, Scale and Hierarchy; Higher Education Press: Beijing, China, 2009. [Google Scholar]
- Wang, D.J. Effect of environmental modification combined with mollusciciding for snail control. Chin. J. Schist. Control 2009, 21, 521, 524. (In Chinese) [Google Scholar]
- Yang, R.; Zhu, Z.H.; Huang, X.B.; Tan, X.D. Performance evaluation of small snail control projects in Hubei Province in 2009 and 2010. Chin. J. Schist. Control 2012, 24, 397–403. (In Chinese) [Google Scholar]
- Zhang, Y.Q.; Zhang, J.; Shu, Y.R.; He, Q.X.; Qi, L.J.; Zhang, R.; Hu, H.J.; Sun, W.S.; Yang, H.; Zhang, W.; et al. Effect of schistosomiasis control by environment alteration and chemotherapy in lake regions. Chin. J. Schisto. Control 2003, 15, 304–305. (In Chinese) [Google Scholar]
- Wang, J.G.; Liao, H.Y.; Zhong, G.T.; Yu, B.G.; Huang, S.S. Observation on the effect of schisiosomiasis through snail control by environmental modification. Chin. J. Schist. Control 2002, 14, 200–202. (In Chinese) [Google Scholar]
- Wang, W.L.; Fang, T.Q.; Pan, D.Z.; Ca, Z.D.; Tian, Z.H.; Shu, B.X. Effect of paddy-upland rotation for snail control in irrigation network endemic area. Chin. J. Schist. Control 1998, 10, 227–228. (In Chinese) [Google Scholar]
- Liu, J.B.; Huang, S.S.; Huan, H.P.; Dai, Y.H. Analysis of the economic benefits on the snail control project through environmental modification supported by world bank loan project for schistosomiasis control in Hubei Province. J. Trop. Med. 2006, 6, 696–699. (In Chinese) [Google Scholar]
- Yu, B.G.; Huang, S.S.; Liao, H.Y.; Wang, J.G.; Deng, A.Q.; Liu, J.B. Effectiveness of snail control through envlronmental modification in World Bank loan project for schistosomiasis control in Hubei Provlnce. Chin. J. Schist. Control 2002, 14, 351–353. (In Chinese) [Google Scholar]
- Yang, M.X.; Zhou, Y.B.; Li, M.F.; Li, Z.W.; Zhou, Z.J. Observation of the effect for killing snails by hardening conduits in dongting regions. Chin. J. Schist. Control 2001, 13, 72–74. (In Chinese) [Google Scholar]
- Huang, Y.Q.; Cai, G.; Hong, Q.B.; Sun, L.P.; Wu, F.; Zhu, M.C.; Zhao, Y.J. Evaluation on the cost-effectiveness of the 243 snail control projects in Jiangsu Province. Chin. J. Schist. Control 2002, 14, 354–359. (In Chinese) [Google Scholar]
- Gosoniu, L.; Vounatsou, P.; Sogoba, N.; Smith, T. Bayesian modelling of geostatistical malaria risk data. Geosp. Health 2006, 1, 127–139. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Qiu, J.; Li, R.; Xu, X.; Yu, C.; Xia, X.; Hong, X.; Chang, B.; Yi, F.; Shi, Y. Identifying Determinants of Oncomelania hupensis Habitats and Assessing the Effects of Environmental Control Strategies in the Plain Regions with the Waterway Network of China at the Microscale. Int. J. Environ. Res. Public Health 2014, 11, 6571-6585. https://doi.org/10.3390/ijerph110606571
Qiu J, Li R, Xu X, Yu C, Xia X, Hong X, Chang B, Yi F, Shi Y. Identifying Determinants of Oncomelania hupensis Habitats and Assessing the Effects of Environmental Control Strategies in the Plain Regions with the Waterway Network of China at the Microscale. International Journal of Environmental Research and Public Health. 2014; 11(6):6571-6585. https://doi.org/10.3390/ijerph110606571
Chicago/Turabian StyleQiu, Juan, Rendong Li, Xingjian Xu, Chuanhua Yu, Xin Xia, Xicheng Hong, Bianrong Chang, Fengjia Yi, and Yuanyuan Shi. 2014. "Identifying Determinants of Oncomelania hupensis Habitats and Assessing the Effects of Environmental Control Strategies in the Plain Regions with the Waterway Network of China at the Microscale" International Journal of Environmental Research and Public Health 11, no. 6: 6571-6585. https://doi.org/10.3390/ijerph110606571





