The Association between Environmental Factors and Scarlet Fever Incidence in Beijing Region: Using GIS and Spatial Regression Models
Abstract
:1. Introduction
2. Methods
2.1. Study Area and Design
2.2. Data Source of Scarlet Fever
2.3. Ethical Approval
2.4. Air Pollutants and Meteorological Data
2.5. Statistical Analysis
2.5.1. Spatial Analysis
2.5.2. Global Spatial Autocorrelation Analysis
2.5.3. Spatial Regression Analysis
3. Results
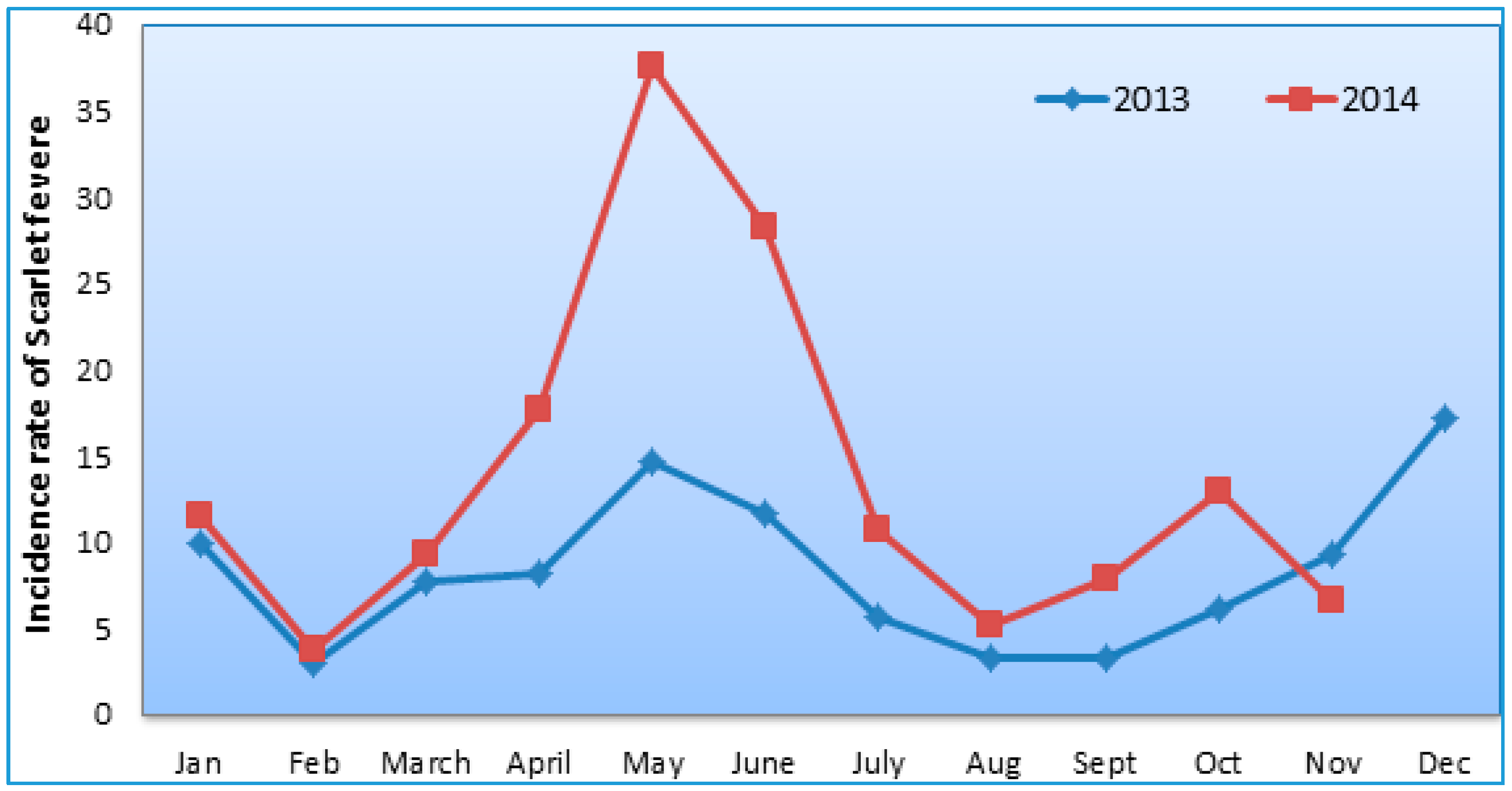
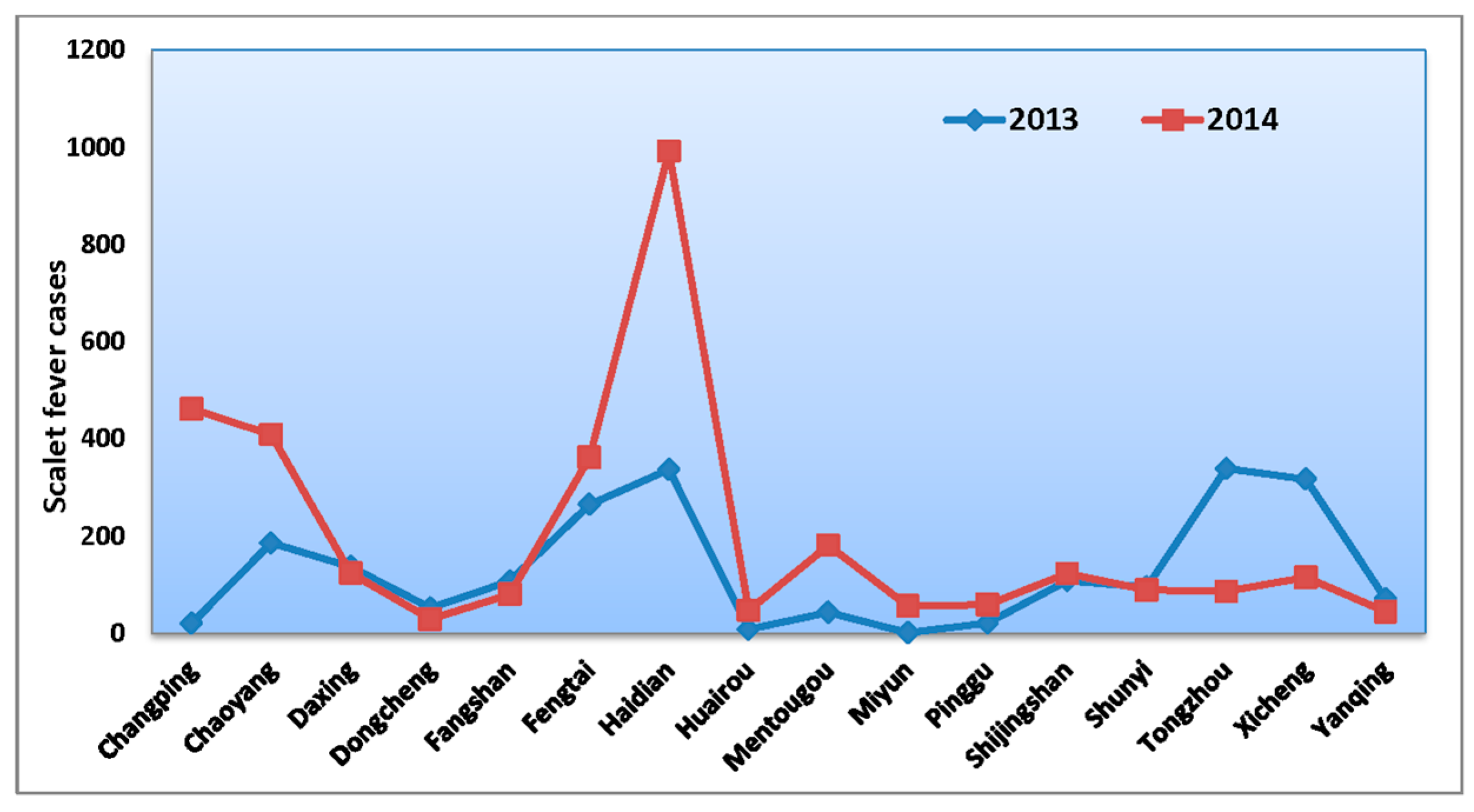
3.1. Basic Characteristics
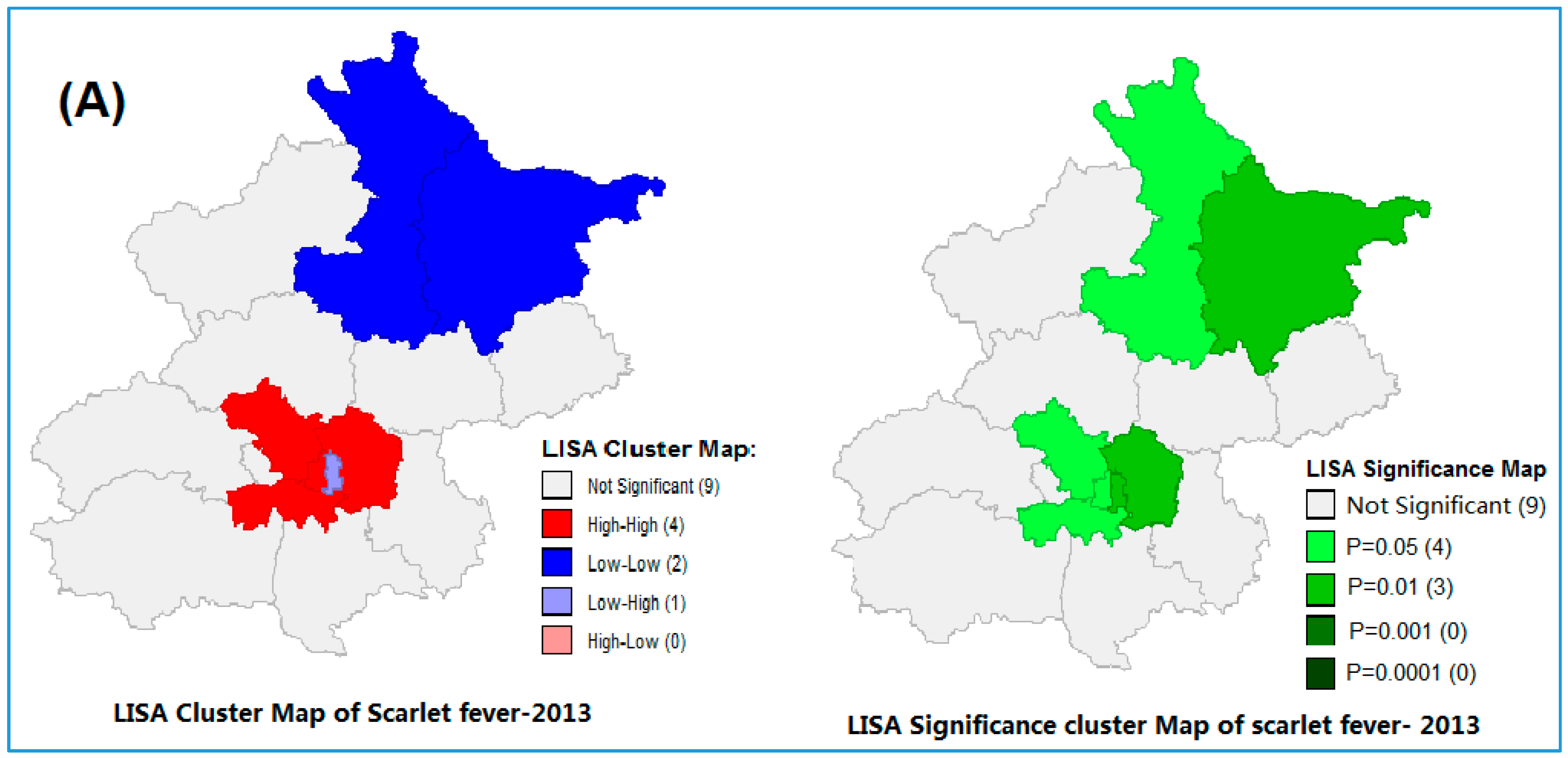
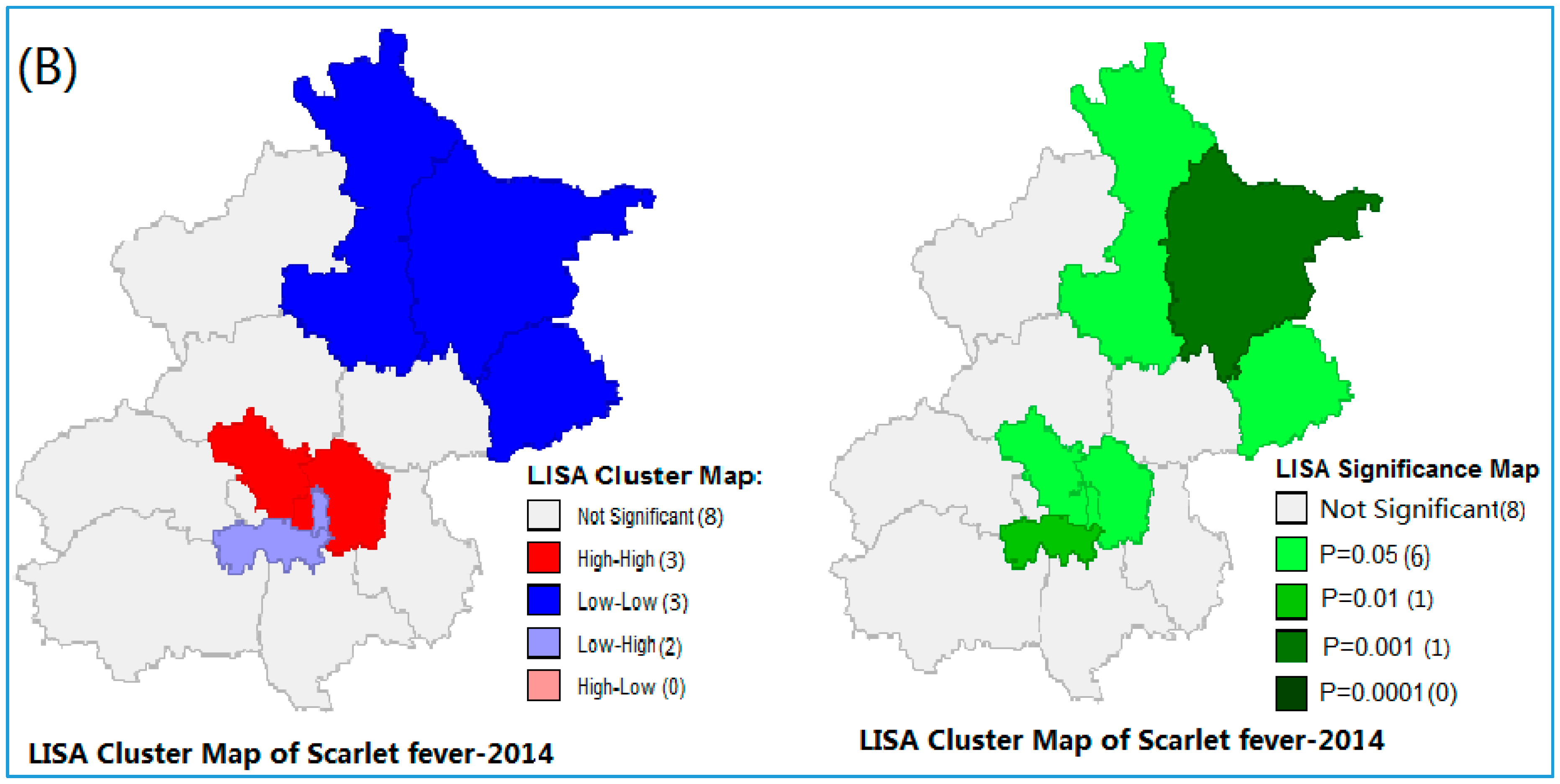
3.2. Spatial Autocorrelation of Scarlet Fever Incidence
3.3. Spatial Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AAP | average atmospheric pressure |
| ARH | average relative humidity |
| AT | average temperature |
| ARH | average relative humidity |
| MRF | monthly rainfall |
| ASH | average sunshine hour |
| AWS | average wind speed |
| PM | particulate matter |
| China CDC | China Center for Disease Control and Prevention |
| GWR | geographically-weighted regression models |
| LM | Lagrange multiplier |
| LLR | Log likelihood ratio |
| Robust LM | robust Lagrange multiplier |
| AIC | Akaike Information Criterion |
References
- World Health Organization (WHO). Western Pacific Region, China. Scarlet Fever: Factsheet of Scarlet Fever in China. Available online: http://www.wpro.who.int/china/mediacentre/factsheets/scarlet_fever/en/ (accessed on 5 January 2016).
- Centers for Disease Control and Prevention (CDC). Scarlet Fever: A Group A Streptococcal Infection. Available online: http://www.cdc.gov/features/scarletfever/ (accessed on 9 January 2016).
- Sotoodian, B. Scarlet Fever. Medscape.com, 2016. Available online: http://emedicine.medscape.com/article/1053253-overview (accessed on 17 April 2016).
- Cunningham, W. Madeleine. Pathogenesis of Group A streptococcal infections. Clin. Microbiol. Rev. 2000, 13, 470–511. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.G.; Dong, H.J.; Li, F.R.; Xie, S.Y.; Cao, H.C.; Xia, S.C.; Yu, Z.; Li, L.J. Report and analysis of a scarlet fever outbreak among adults through food-borne transmission in China. J. Infect. 2007, 55, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Eyler, J.M. Epidemiology of milk-born scarlet fever: The case of Edwardian Brighton. Am. J. Public Health 1986, 76, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health of China. Incidence of Notifiable Infectious Disease in China. Available online: www.moh.gov.cn/public files (accessed on 27 September 2015).
- Mahara, G.; Chhetri, J.K.; Guo, X. Increasing prevalence of scarlet fever in China. Br. Med. J. 2016. [Google Scholar] [CrossRef] [PubMed]
- Government of United Kingdom (GOV UK). Update on Rising Scarlet Fever across England. Available online: https://www.gov.uk/government/news/update-on-rising-scarlet-fever-across-england (accessed on 17 April 2015).
- Staszewska, E.; Kondej, B.; Czarkowski, M.P. Scarlet fever in Poland in 2012. Prz. Epidemiol. 2014, 68, 209–212. [Google Scholar]
- Wong, S.S.; Yuen, K.Y. Streptococcus pyogenes and re-emergence of scarlet fever as a public health problem. Emerg. Microbes Infect. 2012. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, P.; Feng, G. The relationship between incidence of scarlet fever and meteorological factors in Shandong Province. China Prev. Med. 2011, 02, 015. (In Chinese) [Google Scholar]
- Li, X.; Wang, Q.; Gao, T.; Jia, L.; Pang, X. Correlation study on association between meteorological factors and incidence of scarlet fever in Beijing. Pract. Prev. Med. 2007, 5, 039. (In Chinese) [Google Scholar]
- Tang, Q.L.; Ma, S.L.; Liu, H.W.; Gao, S.H.; He, J. Correlation between attack of scarlet fever and six qi or meteorological factors in Beijing from 1979 to 2004. J. Beijing Univ. Tradit. Chin. Med. 2013, 05, 0011. (In Chinese) [Google Scholar]
- Duan, Y.; Huang, X.L.; Wang, Y.J.; Zhang, J.Q.; Zhang, Q.; Dang, Y.W.; Wang, J. Impact of meteorological changes on the incidence of scarlet fever in Hefei city China. Int. J. Biometeorol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.X.; Peng, W.J.; Yan, J.W.; Wan, Y.N.; Wang, J. Negative binomial regression analysis of the scarlet fever incidence and meteorological factors. China J. Dis. Control 2014, 02, 166–168. (In Chinese) [Google Scholar]
- Zhang, T.; Yang, M.; Xiao, X.; Feng, Z.; Li, C.; Zhou, Z.; Ren, Q.; Li, X. Spectral analysis based on fast Fourier transformation (FFT) of surveillance data: The case of scarlet fever in China. Epidemiol. Infect. 2014, 142, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Yang, P.; Zhang, Y.; Wang, X.; Duan, W.; Wang, Q. Spatial-temporal scan statistic on scarlet fever cases in Beijing, 2005–2010. Dis. Surveill. 2011, 26, 435–438. [Google Scholar]
- Wang, W.; Gao, L. Analysis on epidemic characteristics of scarlet fever in Zhengzhou from 2004 to 2010. Henan J. Prev. Med. 2012, 02, 0123. (In Chinese) [Google Scholar]
- Shen, Y.H.; Jiang, C.; Dun, Z.; Wang, Q.Y.; Wu, J.; Sun, S.G.; Pu, Y.L. Analysis of epidemiological trend and prediction of scarlet fever from 1957 to 2004 in the Downtown areas of Beijing. Mod. Prev. Med. 2008, 07, 011. (In Chinese) [Google Scholar]
- Liu, Z.; Wang, B.X.; Wang, S.C. Epidemiological tendency of scarlet fever in China, 2003–2008. J. Public Health Prev. Med. 2009, 05, 0021. (In Chinese) [Google Scholar]
- Chu, Y.; Liu, X.; Wang, H.; Sun, Q.; Zhang, Z. Epidemiological survey of scarlet infection in a primary school. China Trop. Med. 2012, 20, 127–128. (In Chinese) [Google Scholar]
- Sun, W.; Gong, J.; Zhou, J.; Zhao, Y.; Tan, J.; Ibrahim, A.N.; Zhou, Y. A spatial, social and environmental study of tuberculosis in China using statistical and GIS technology. Int. J. Environ. Res. Public Health 2015, 12, 1425–1448. [Google Scholar] [CrossRef] [PubMed]
- Uthman, O.A. Spatial and temporal variations in incidence of tuberculosis in Africa, 1991 to 2005. World Health Popul. 2008, 10, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.; Wang, W.; Li, Z.; Hou, M.; He, Y.; Wu, W.; Wang, H.; Liang, H.; Guo, X. Investigation of space-time clusters and geospatial hot spots for the occurrence of tuberculosis in Beijing. Int. J. Tuberc. Lung Dis. 2012. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.N.; Malone, J.B.; Kristensen, T.K.; Bergquist, R.N. Application of geographic information systems and remote sensing to schistosomiasis control in China. Acta Trop. 2001, 79, 97–106. [Google Scholar] [CrossRef]
- Caprarelli, G.; Fletcher, S. A brief review of spatial analysis concepts and tools used for mapping, containment and risk modelling of infectious diseases and other illnesses. Parasitology 2014, 141, 581–601. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.C.; King, C.C. Surveillance and epidemiology of infectious diseases using spatial and temporal clustering methods. In Infectious Disease Informatics and Biosurveillance: Research, Systems and Case Studies; Carlos Castillo-Chavez, H.C., Lober William, B., Thurmond, M., Zeng, D., Eds.; Springer: New York, NY, USA, 2010; pp. 208–234. [Google Scholar]
- Esri. Common Regression Problems, Consequences, and Solutions in Spatial Regression Analysis Basic. 2011. Available online: http://help.arcgis.com/en/arcgisdesktop/10.0/help/index.html#//005p00000023000000.html (accessed on 17 March 2016).
- Fotheringham, A.S.; Charlton, M.E.; Brunsdon, C. Geographically weighted regression: A natural evolution of the expansion method for spatial data analysis. Environ. Plan. A 1998, 30, 1905–1927. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L. Haze in China: Current and future challenges. Environ. Pollut. 2014, 189, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ying, Q.; Hu, J.; Zhang, H. Spatial and temporal variations of six criteria air pollutants in 31 provincial capital cities in China during 2013–2014. Environ. Int. 2014, 73, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Beijing Municipal Bureau of Statistics (BMBS). Available online: http://www.bjstats.gov.cn/esite/ (accessed on 15 March 2016).
- National Bureau of Statistics of the People’s Republic of China. Available online: http://www.stats.gov.cn/tjsj/tjgb/rkpcgb/dfrkpcgb/ (accessed on 14 March 2016).
- Mahara, G.; Wang, C.; Huo, D.; Xu, Q.; Huang, F.; Tao, L.; Guo, J.; Cao, K.; Long, L.; Chhetri, J.K.; et al. Spatiotemporal Pattern Analysis of Scarlet Fever Incidence in Beijing, China, 2005–2014. Int. J. Environ. Res. Public Health 2016, 13, 131. [Google Scholar] [CrossRef] [PubMed]
- China Meteorological Data Sharing Service System. Available online: http://cdc.nmic.cn/home.do (accessed on 17 December 2015).
- Beijing Environmental Protection Bureau, Ambient Air Quality Monitoring center, Beijing, China. Available online: www.bjmemc.com.cn (accessed on 21 March 2016).
- Kelly-Hope, L.A.; Alonso, W.J.; Thiem, V.D.; Anh, D.D.; Canh, D.G.; Lee, H.; Smith, D.L.; Miller, M.A. Geographical distribution and risk factors associated with enteric diseases in Vietnam. Am. J. Trop. Med. Hyg. 2007, 76, 706–712. [Google Scholar] [PubMed]
- Lee, J.; Wing, D.; Wong, S. Statistical Analysis with ArcView GIS; John Wiley and Sons: New York, NY, USA, 2000. [Google Scholar]
- Alonso, W.J.; Acuna-Soto, R.; Giglio, R.; Nuckols, J.; Leyk, S.; Schuck-Paim, C.; Viboud, C.; Miller, M.A.; McCormick, B.J. Spatio-temporal patterns of diarrhoeal mortality in Mexico. Epidemiol. Infect. 2012, 140, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xue, F.; Chen, Y.; Ma, Y.; Liu, Y. The spatial epidemiology of tuberculosis in Linyi City, China, 2005–2010. BMC Public Health 2012. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X. Spatial Epidemiology; Science Press: Beijing, China, 2009. [Google Scholar]
- Data Sharing Infrastructure, Base Map of Beijing. Available online: www.geoda.cn/portal (accessed on 14 February 2016).
- Anselin, L. Local indicators of spatial association—LISA. Geogr. Anal. 1995, 27, 93–115. [Google Scholar] [CrossRef]
- Pfeiffer, D.U.; Robinson, T.P.; Stevenson, M.; Stevens, K.B.; Rogers, D.J.; Clements, A.C. Spatial Analysis in Epidemiology; Oxford University Press: London, UK, 2008. [Google Scholar]
- GIS Geography. Spatial Autocorrelation and Moran’s I in GIS. Available online: http://gisgeography.com/spatial-autocorrelation-moran-i-gis/ (accessed on 27 March 2016).
- How Spatial Autocorrelation: Moran’s I (Spatial Statistics) Works. Available online: http://desktop.arcgis.com/en/arcmap/10.3/tools/spatial-statistics-toolbox/h-how-spatial-autocorrelation-moran-s-i-spatial-st.htm (accessed on 28 December 2015).
- Spatial Autocorrelation (Moran’s I) (Spatial Statistic). Available online: webhelp.esri.com/arcgisdesktop/9.2/index.cfm?TopicName=Spatial Autocorrelation (accessed on 23 November 2015).
- ArcGIS Software for Geographic Study Version 10.1. Available online: www.ersi.com (accessed on 27 August 2015).
- Anselin, L. Exploring Spatial Data with GeoDaTM: A Workbook. Center for Spatially Integrated Social Science, 2005. Available online: https://geodacenter.asu.edu/og_tutorials (accessed on 27 March 2016).
- Kelejian, H.H.; Robinson, D.P. Spatial correlation: A suggested alternative to the autoregressive model. In New Directions in Spatial Econometrics; Anselin, L., Florax, R.J., Eds.; Springer: Berlin, Germany, 1995. [Google Scholar]
- Kumar, C.; Singh, P.K.; Rai, R.K. Under-Five mortality in high focus states in India: A district level geospatial analysis. PLoS ONE 2012. [Google Scholar] [CrossRef] [PubMed]
- Anselin, L.; Syabri, I.; Kho, Y. GeoDa: An introduction to spatial data analysis. Geogr. Anal. 2006, 38, 5–22. [Google Scholar] [CrossRef]
- Yang, P.; Peng, X.; Zhang, D.; Wu, S.; Liu, Y.; Cui, S.; Lu, G.; Duan, W.; Shi, W.; Liu, S.; et al. Group A Streptococcus strains circulating during scarlet fever epidemic, Beijing, China-2011. Emerg. Infect. Dis. 2013, 19, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Luk, E.Y.Y.; Lo, J.Y.C.; Li, A.Z.L.; Lau, M.C.K.; Cheung, T.K.M.; Wong, A.Y.M.; Wong, M.M.H.; Wong, C.W.; Chuang, S.; Tsang, T. Scarlet fever epidemic, Hong Kong, 2011. Emerg. Infect. Dis. 2012, 18, 1658–1661. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.Q.; Pan, H.F.; Zhu, Y.; He, Q. Epidemiological investigation of scarlet fever in Hefei City, China, from 2004 to 2008. Trop. Dr. 2010, 40, 4225–4226. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yao, W.; Wang, X.; Li, Y.; Chen, M.; Wang, G.; Zhang, X.; Pan, H.; Hu, J.; Zeng, M. Outbreak of scarlet fever associated with emm12 type group A Streptococcus in 2011 in Shanghai, China. Pediatr. Infect. Dis. J. 2012, 31, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Lamagni, T.; Dennis, J.; George, R.; Efstratiou, A. Analysis of Epidemiological Patterns during a Century of Scarlet Fever. 2008. Available online: www.hpa.org.uk/webc/hpawebfile/hpaweb (accessed on 27 March 2016).
- Cruickshank, J.G.; Lightfoot, N.F.; Sugars, K.H.; Colman, G.; Simmons, M.D.; Tolliday, J.; Oakley, E.H.N. A large outbreak of streptococcal pyoderma in a military training establishment. J. Hyg. 1982, 89, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, J. The relationship between rainfall and scarlet fever. Proc. R. Soc. Med. 1923, 16, 30–34. [Google Scholar] [PubMed]
- Duncan, C.J.; Duncan, S.R.; Scott, S. The dynamics of scarlet fever epidemics in England and Wales in the 19th century. Epidemiol. Infect. 1996, 117, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Li, X.; Wang, S.; Wang, C.; Huang, F.; Gao, Q.; Wu, L.; Tao, L.; Guo, J.; Wang, W.; et al. Fine particulate air pollution and hospital emergency room visits for respiratory disease in urban areas in Beijing, China, in 2013. PLoS ONE 2016. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, D.J. Study on an air quality evaluation model for Beijing City under haze-fog pollution based on new ambient air quality standards. Int. J. Environ. Res. Public Health 2014, 11, 8909–8923. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency (EPA). Health and Nitrogen Dioxide. Available online: https://www3.epa.gov/airquality/nitrogenoxides/health.html (accessed on 25 February 2016).
- Andrey, D.O.; Posfay-Barbe, K.M. Re-emergence of scarlet fever: Old players return? Expert Rev. Anti-Infect. Ther. 2016, 14, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.V.; Jones, K.; Kaddour, A.; Krieger, N. Revisiting Robinson: The perils of individualistic and ecologic fallacy. Int. J. Epidemiol. 2009, 38, 342–360. [Google Scholar] [CrossRef] [PubMed]
| Variables | Mean | SD | Percentiles | ||
|---|---|---|---|---|---|
| 25% | Median | 75% | |||
| Cases | 33.00 | 47.26 | 4.75 | 16.00 | 43.00 |
| PM2.5 (µg/m3) | 91.71 | 35.32 | 68.63 | 82.80 | 105.7 |
| PM10 (µg/m3) | 121.77 | 43.40 | 92.4 | 113.95 | 144.27 |
| SO2 (µg/m3) | 28.06 | 26.12 | 9.0 | 18.40 | 41.10 |
| NO2 (µg/m3) | 52.75 | 18.99 | 39.50 | 51.85 | 66.57 |
| O3 (µg/m3) | 117.40 | 55.88 | 62.62 | 118.25 | 169.05 |
| CO (mg/m3) | 1.73 | 55.88 | 1.20 | 1.50 | 2.00 |
| MRF (inches) | 1.46 | 1.70 | 0.10 | 0.60 | 2.90 |
| AAP (hPa) | 992.11 | 16.29 | 982.07 | 992.10 | 1004.27 |
| AT (°C) | 12.26 | 10.70 | 3.90 | 12.90 | 21.87 |
| ARH (%) | 57.06 | 11.85 | 46.10 | 57.30 | 68.40 |
| AWS (km/h) | 2.10 | 0.42 | 1.80 | 2.00 | 2.30 |
| ASH (h) | 6.54 | 1.34 | 5.80 | 2.80 | 7.60 |
| Variable | Ordinary Least Squares Model | Spatial Lag Model | Spatial Error Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | St-Error | T-Stat | p-Value | Coefficient | St-Error | Z-Value | p-Value | Coefficient | St-Error | Z-Value | p-Value | |
| PM2.5 | 0.04012 | 0.1249 | 0.3210 | 0.748 | 0.04475 | 0.1224 | 0.3655 | 0.715 | 0.04227 | 0.1228 | 0.3441 | 0.730 |
| PM10 | 0.08048 | 0.0866 | 0.9293 | 0.353 | 0.09361 | 0.0849 | 1.1025 | 0.270 | 0.08502 | 0.0852 | 0.9974 | 0.318 |
| SO2 | 0.0004 | 0.1095 | 0.0040 | 0.996 | 0.00112 | 0.1073 | 0.0105 | 0.992 | 0.00122 | 0.1075 | 0.0113 | 0.991 |
| NO2 | 0.4514 | 0.1605 | 2.8115 | 0.005 | 0.35493 | 0.1609 | 2.2053 | 0.027 | 0.42494 | 0.1604 | 2.6485 | 0.008 |
| O3 | 0.0547 | 0.0842 | 0.6499 | 0.516 | 0.05085 | 0.0825 | 0.6165 | 0.537 | 0.05213 | 0.0827 | 0.6303 | 0.528 |
| CO | −7.5136 | 6.8550 | −1.0961 | 0.273 | −7.96663 | 6.7180 | −1.1859 | 0.235 | −7.64643 | 6.7307 | −1.1361 | 0.255 |
| ARF | 5.9287 | 2.6839 | 2.2090 | 0.027 | 5.49800 | 2.6347 | 2.0868 | 0.036 | 5.81891 | 2.6381 | 2.2057 | 0.027 |
| AAP | 0.0938 | 0.1490 | 0.6292 | 0.529 | 0.05512 | 0.1481 | 0.3722 | 0.709 | 0.09062 | 0.1476 | 0.6140 | 0.539 |
| AT | −0.4282 | 0.6074 | −0.7050 | 0.481 | −0.48583 | 0.5967 | −0.8143 | 0.415 | −0.42938 | 0.5983 | −0.7176 | 0.473 |
| ARH | −0.7329 | 0.3277 | −2.2366 | 0.025 | −0.68165 | 0.3215 | −2.1201 | 0.034 | −0.71913 | 0.3223 | −2.2312 | 0.025 |
| AWS | −10.0891 | 6.4000 | −1.5764 | 0.116 | −9.69804 | 6.2731 | −1.5460 | 0.122 | −10.01692 | 6.3059 | −1.5885 | 0.112 |
| ASH | 3.9395 | 1.9747 | 1.9950 | 0.047 | 3.81360 | 1.9360 | 1.9698 | 0.048 | 3.92472 | 1.9432 | 2.0197 | 0.043 |
| LAMDA (λ) | 0.092419 | 0.3795 | 0.2435 | 0.807 | ||||||||
| Rho (ρ) | 0.3616 | |||||||||||
| R2 | 0.0741 | 0.0786 | 0.0743 | |||||||||
| LLR | −1819.69 | −1819.04 | −1819.67 | |||||||||
| AIC | 3665.38 | 3665.08 | 3665.36 | |||||||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahara, G.; Wang, C.; Yang, K.; Chen, S.; Guo, J.; Gao, Q.; Wang, W.; Wang, Q.; Guo, X. The Association between Environmental Factors and Scarlet Fever Incidence in Beijing Region: Using GIS and Spatial Regression Models. Int. J. Environ. Res. Public Health 2016, 13, 1083. https://doi.org/10.3390/ijerph13111083
Mahara G, Wang C, Yang K, Chen S, Guo J, Gao Q, Wang W, Wang Q, Guo X. The Association between Environmental Factors and Scarlet Fever Incidence in Beijing Region: Using GIS and Spatial Regression Models. International Journal of Environmental Research and Public Health. 2016; 13(11):1083. https://doi.org/10.3390/ijerph13111083
Chicago/Turabian StyleMahara, Gehendra, Chao Wang, Kun Yang, Sipeng Chen, Jin Guo, Qi Gao, Wei Wang, Quanyi Wang, and Xiuhua Guo. 2016. "The Association between Environmental Factors and Scarlet Fever Incidence in Beijing Region: Using GIS and Spatial Regression Models" International Journal of Environmental Research and Public Health 13, no. 11: 1083. https://doi.org/10.3390/ijerph13111083







