The Association between Endometriosis, Tubal Ligation, Hysterectomy and Epithelial Ovarian Cancer: Meta-Analyses
Abstract
:1. Introduction
2. Methods
2.1. Literature Search
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
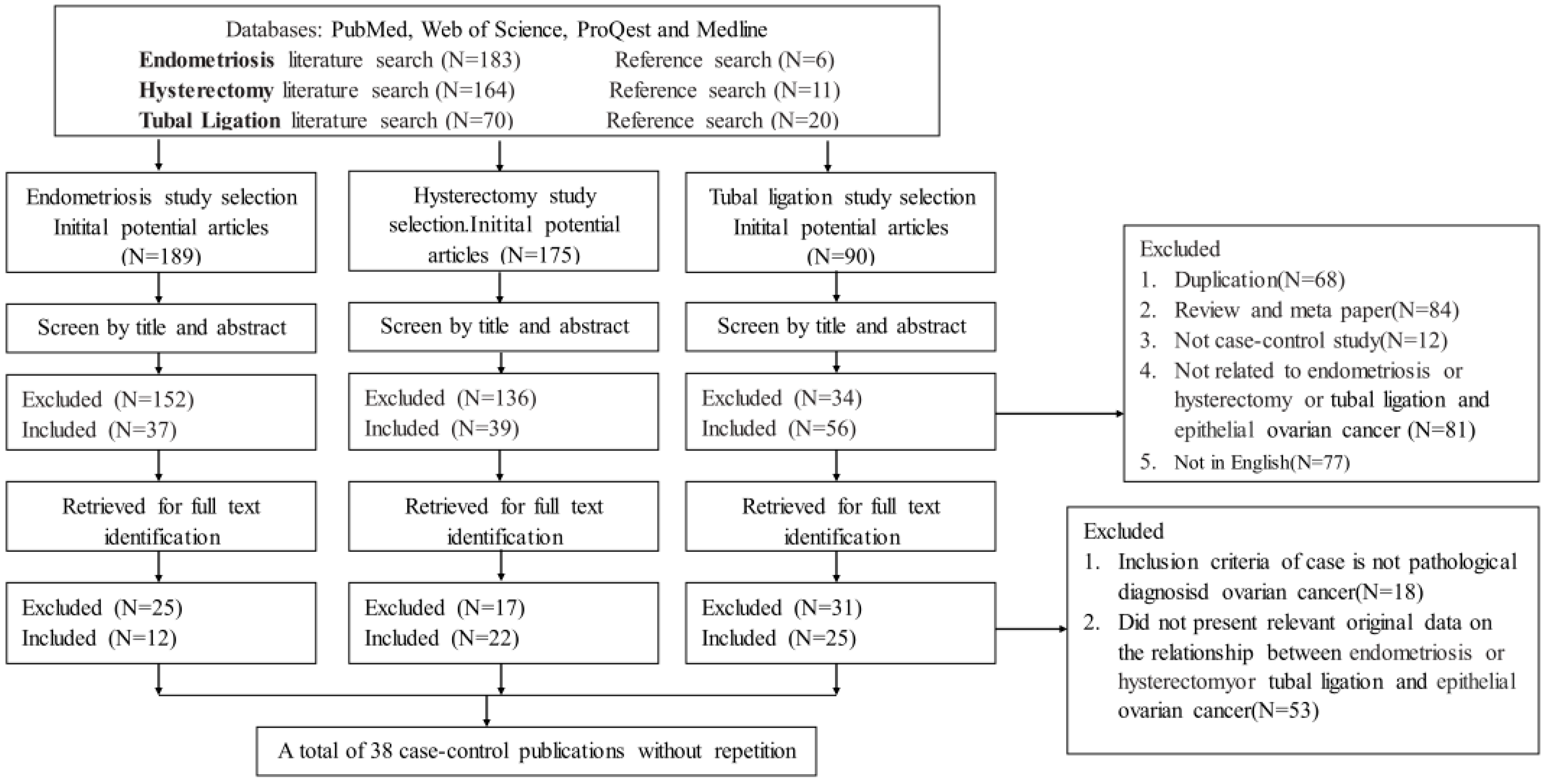
3.1. Search Results and Study Characteristics
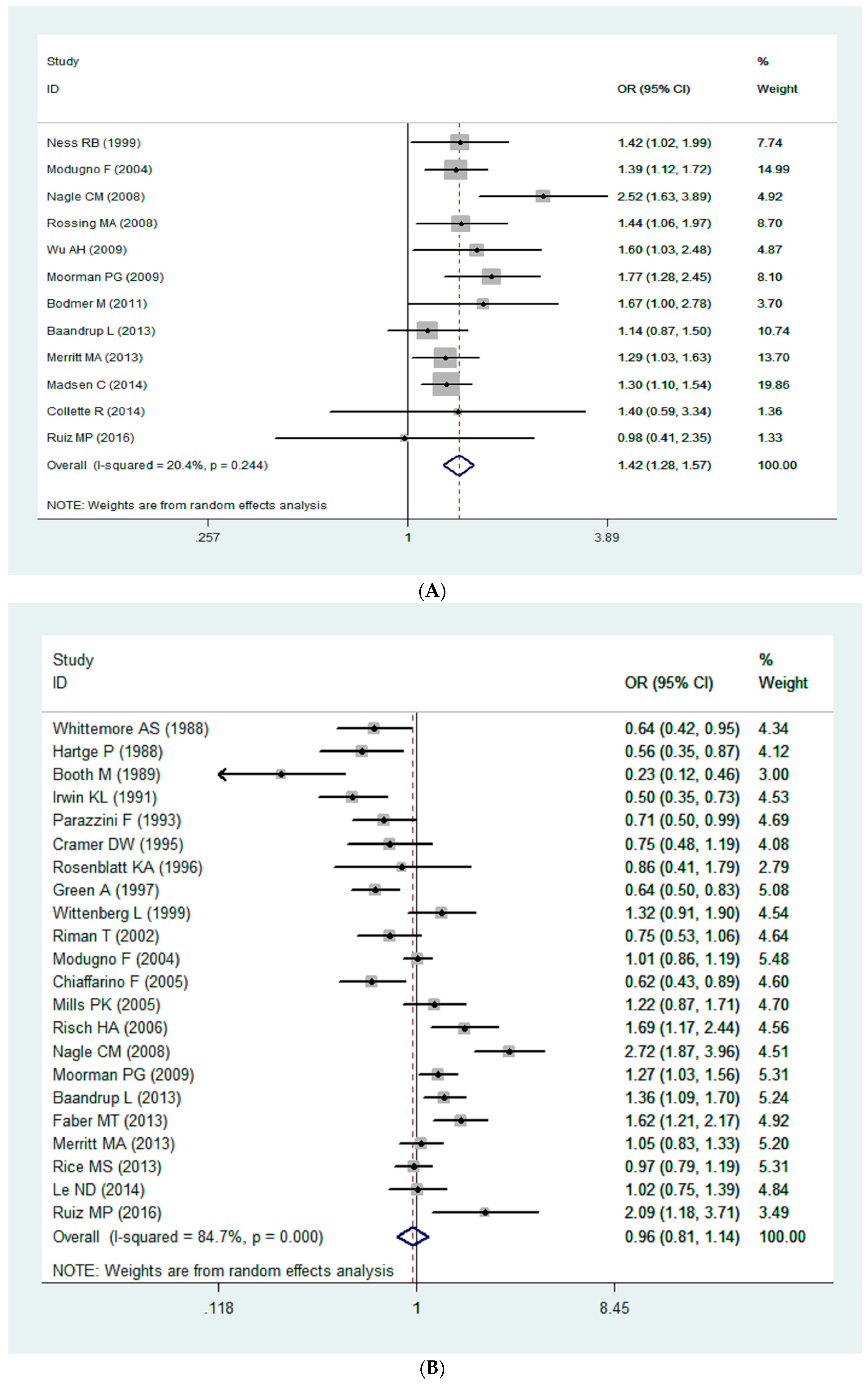
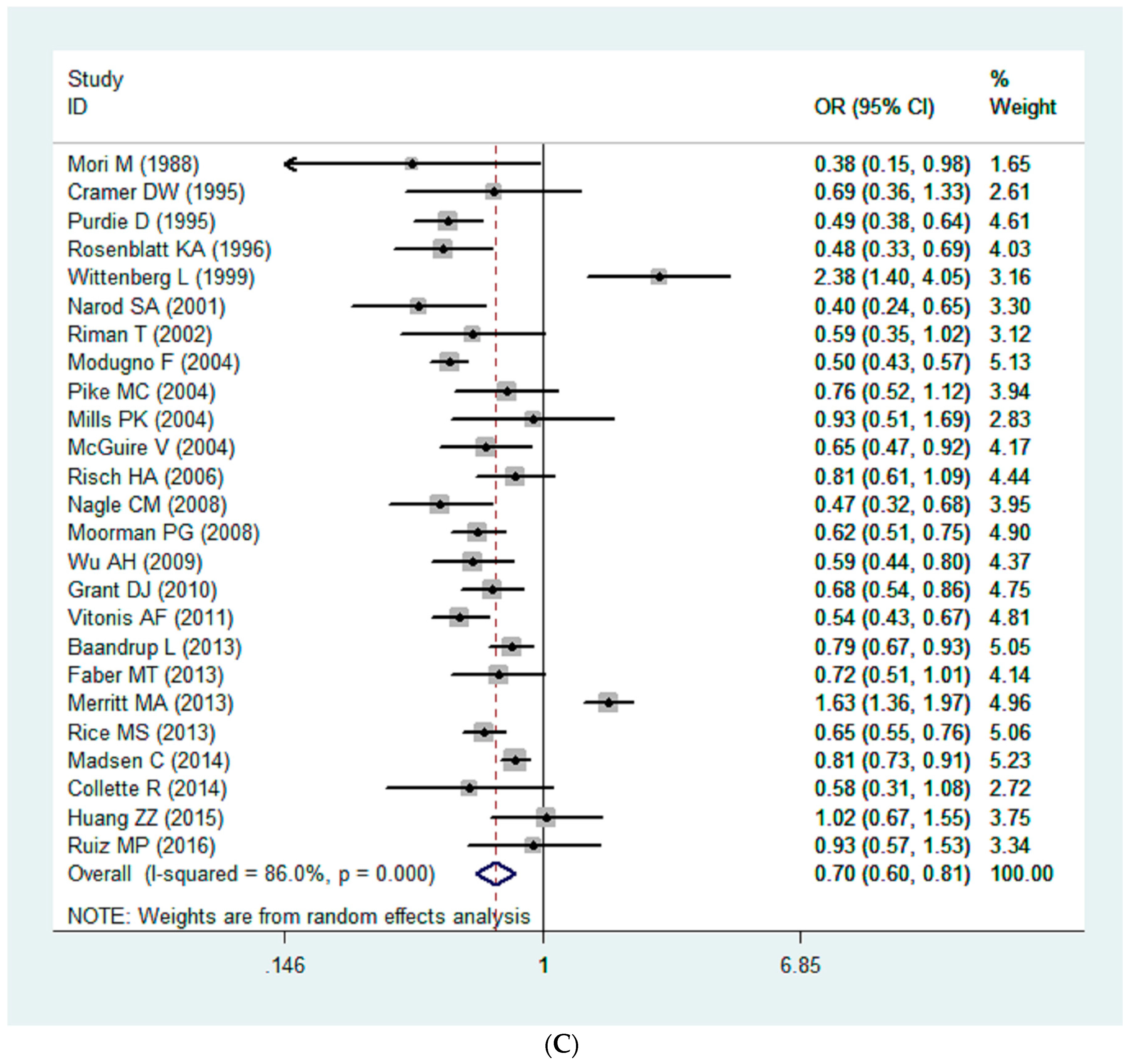
3.2. Quantitative Synthesis
3.3. Bias Diagnosis
3.4. Sensitivity Analysis
3.5. Cumulative Meta-Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: Globocan 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer statistics, 2009. CA Cancer J. Clin. 2009, 59, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Ness, R.B.; Grisso, J.A.; Cottreau, C.; Klapper, J.; Vergona, R.; Wheeler, J.E.; Morgan, M.; Schlesselman, J.J. Factors related to inflammation of the ovarian epithelium and risk of ovarian cancer. Epidemiology 2000, 11, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.; Baandrup, L.; Dehlendorff, C.; Kjaer, S.K. Tubal ligation and salpingectomy and the risk of epithelial ovarian cancer and borderline ovarian tumors: A nationwide case-control study. Acta Obstet. Gynecol. Scand. 2015, 94, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Lessard-Anderson, C.R.; Handlogten, K.S.; Molitor, R.J.; Dowdy, S.C.; Cliby, W.A.; Weaver, A.L.; Sauver, J.S.; Bakkum-Gamez, J.N. Effect of tubal sterilization technique on risk of serous epithelial ovarian and primary peritoneal carcinoma. Gynecol. Oncol. 2014, 135, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.J.; Nagle, C.M.; Coory, M.D.; Maresco, D.; Protani, M.M.; Pandeya, N.A.; Balasubramaniam, K.D.; Webb, P.M. Has the association between hysterectomy and ovarian cancer changed over time? A systematic review and meta-analysis. Eur. J. Cancer 2013, 49, 3638–3647. [Google Scholar] [CrossRef] [PubMed]
- Pike, M.C.; Pearce, C.L.; Peters, R.; Cozen, W.; Wan, P.; Wu, A.H. Hormonal factors and the risk of invasive ovarian cancer: A population-based case-control study. Fertil. Steril. 2004, 82, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Widschwendter, M.; Majek, O.; Dusek, L. Tubal ligation and the risk of ovarian cancer: Review and meta-analysis. Hum. Reprod. Update 2011, 17, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Pearce, C.L.; Templeman, C.; Rossing, M.A.; Lee, A.; Near, A.M.; Webb, P.M.; Nagle, C.M.; Doherty, J.A.; Cushing-Haugen, K.L.; Wicklund, K.G.; et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: A pooled analysis of case-control studies. Lancet. Oncol. 2012, 13, 385–394. [Google Scholar] [CrossRef]
- Le, N.D.; Leung, A.; Brooks-Wilson, A.; Gallagher, R.P.; Swenerton, K.D.; Demers, P.A.; Cook, L.S. Occupational exposure and ovarian cancer risk. Cancer Causes Control 2014, 25, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Gao, Y.; Wen, W.; Li, H.; Zheng, W.; Shu, X.O.; Beeghly-Fadiel, A. Contraceptive methods and ovarian cancer risk among Chinese women: A report from the Shanghai Women’s Health Study. Int. J. Cancer 2015, 137, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.P.; Morales-Ramirez, P.B.; Dziadek, O.L.; Algren, S.D. Epithelial ovarian cancer and type of peritoneal insult: A case-control study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 205, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.K.; Mertz, D.; Loeb, M. Newcastle-Ottawa scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.; Antman, E.M.; Jimenez-Silva, J.; Kupelnick, B.; Mosteller, F.; Chalmers, T.C. Cumulative meta-analysis of therapeutic trials for myocardial infarction. N. Engl. J. Med. 1992, 327, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Modugno, F.; Ness, R.B.; Allen, G.O.; Schildkraut, J.M.; Davis, F.G.; Goodman, M.T. Oral contraceptive use, reproductive history, and risk of epithelial ovarian cancer in women with and without endometriosis. Am. J. Obstet. Gynecol. 2004, 191, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Nagle, C.M.; Olsen, C.M.; Webb, P.M.; Jordan, S.J.; Whiteman, D.C.; Green, A.C.; Australian Ovarian Cancer Study Group. Endometrioid and clear cell ovarian cancers: A comparative analysis of risk factors. Eur. J. Cancer 2008, 44, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Rossing, M.A.; Cushing-Haugen, K.L.; Wicklund, K.G.; Doherty, J.A.; Weiss, N.S. Risk of epithelial ovarian cancer in relation to benign ovarian conditions and ovarian surgery. Cancer Causes Control 2008, 19, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.H.; Pearce, C.L.; Tseng, C.C.; Templeman, C.; Pike, M.C. Markers of inflammation and risk of ovarian cancer in Los Angeles County. Int. J. Cancer 2009, 124, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Moorman, P.G.; Palmieri, R.T.; Akushevich, L.; Berchuck, A.; Schildkraut, J.M. Ovarian cancer risk factors in African-American and white women. Am. J. Epidemiol. 2009, 170, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, M.; Becker, C.; Meier, C.; Jick, S.; Meier, C. Use of metformin and the risk of ovarian cancer: A case-control analysis. Gynecol. Oncol. 2011, 123, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Merritt, M.A.; de Pari, M.; Vitonis, A.F.; Titus, L.J.; Cramer, D.W.; Terry, K.L. Reproductive characteristics in relation to ovarian cancer risk by histologic pathways. Hum. Reprod. 2013, 28, 1406–1417. [Google Scholar] [CrossRef] [PubMed]
- Whittemore, A.S.; Wu, M.L.; Paffenbarger, R.S., Jr.; Sarles, D.L.; Kampert, J.B.; Grosser, S.; Jung, D.L.; Ballon, S.; Hendrickson, M. Personal and environmental characteristics related to epithelial ovarian cancer. II. Exposures to talcum powder, tobacco, alcohol, and coffee. Am. J. Epidemiol. 1988, 128, 1228–1240. [Google Scholar] [PubMed]
- Hartge, P.; Hoover, R.; Mcgowan, L.; Lesher, L.; Norris, H.J. Menopause and ovarian cancer. Am. J. Epidemiol. 1988, 127, 990–998. [Google Scholar] [PubMed]
- Booth, M.; Beral, V.; Smith, P. Risk factors for ovarian cancer: A case-control study. Br. J. Cancer 1989, 60, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Irwin, K.L.; Weiss, N.S.; Lee, N.C.; Peterson, H.B. Tubal sterilization, hysterectomy, and the subsequent occurrence of epithelial ovarian cancer. Am. J. Epidemiol. 1991, 134, 362–369. [Google Scholar] [PubMed]
- Parazzini, F.; Negri, E.; La Vecchia, C.; Luchini, L.; Mezzopane, R. Hysterectomy, oophorectomy, and subsequent ovarian cancer risk. Obstet. Gynecol. 1993, 81, 363–366. [Google Scholar] [PubMed]
- Cramer, D.W.; Xu, H. Epidemiologic evidence for uterine growth factors in the pathogenesis of ovarian cancer. Ann. Epidemiol. 1995, 5, 310–314. [Google Scholar] [CrossRef]
- Rosenblatt, K.A.; Thomas, D.B. Reduced risk of ovarian cancer in women with a tubal ligation or hysterectomy. The world health organization collaborative study of neoplasia and steroid contraceptives. Cancer Epidemiol. Biomark. Prev. 1996, 5, 933–935. [Google Scholar]
- Green, A.; Purdie, D.; Bain, C.; Siskind, V.; Russell, P.; Quinn, M.; Ward, B. Tubal sterilisation, hysterectomy and decreased risk of ovarian cancer. Survey of Women’s Health Study Group. Int. J. Cancer 1997, 71, 948–951. [Google Scholar]
- Wittenberg, J.; Cook, L.S.; Rossing, M.A.; Weiss, N.S. Reproductive risk factors for mucinous and non-mucinous epithelial ovarian cancer. Epidemiology 1999, 10, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Riman, T.; Dickman, P.W.; Nilsson, S.; Correia, N.; Nordlinder, H.; Magnusson, C.M.; Persson, I.R. Risk factors for invasive epithelial ovarian cancer: Results from a Swedish case-control study. Am. J. Epidemiol. 2002, 156, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Chiaffarino, F.; Parazzini, F.; Decarli, A.; Franceschi, S.; Talamini, R.; Montella, M.; La Vecchia, C. Hysterectomy with or without unilateral oophorectomy and risk of ovarian cancer. Gynecol. Oncol. 2005, 97, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Mills, P.K.; Riordan, D.G.; Cress, R.D.; Goldsmith, D.F. Hormone replacement therapy and invasive and borderline epithelial ovarian cancer risk. Cancer Detect. Prev. 2005, 29, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Risch, H.A.; Bale, A.E.; Beck, P.A.; Zheng, W. Pgr +331 A/G and increased risk of epithelial ovarian cancer. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1738–1741. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.T.; Jensen, A.; Frederiksen, K.; Glud, E.; Hogdall, E.; Hogdall, C.; Blaakaer, J.; Kjaer, S.K. Oral contraceptive use and impact of cumulative intake of estrogen and progestin on risk of ovarian cancer. Cancer Causes Control 2013, 24, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.S.; Murphy, M.A.; Vitonis, A.F.; Cramer, D.W.; Titus, L.J.; Tworoger, S.S.; Terry, K.L. Tubal ligation, hysterectomy and epithelial ovarian cancer in the new england case-control study. Int. J. Cancer 2013, 133, 2415–2421. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Miyake, H. Dietary and other risk factors of ovarian cancer among elderly women. Jpn. J. Cancer Res. 1988, 79, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Purdie, D.; Green, A.; Bain, C.; Siskind, V.; Ward, B.; Hacker, N.; Quinn, M.; Wright, G.; Russell, P.; Susil, B. Reproductive and other factors and risk of epithelial ovarian cancer: An Australian case-control study. Survey of Women’s Health Study Group. Int. J. Cancer 1995, 62, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Narod, S.A.; Sun, P.; Ghadirian, P.; Lynch, H.; Isaacs, C.; Garber, J.; Weber, B.; Karlan, B.; Fishman, D.; Rosen, B.; et al. Tubal ligation and risk of ovarian cancer in carriers of BRCA1 or BRCA2 mutations: A case-control study. Lancet 2001, 357, 1467–1470. [Google Scholar] [CrossRef]
- Mills, P.K.; Riordan, D.G.; Cress, R.D. Epithelial ovarian cancer risk by invasiveness and cell type in the central valley of California. Gynecol. Oncol. 2004, 95, 215–225. [Google Scholar] [CrossRef] [PubMed]
- McGuire, V.; Felberg, A.; Mills, M.; Ostrow, K.L.; DiCioccio, R.; John, E.M.; West, D.W.; Whittemore, A.S. Relation of contraceptive and reproductive history to ovarian cancer risk in carriers and noncarriers of BRCA1 gene mutations. Am. J. Epidemiol. 2004, 160, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Moorman, P.G.; Calingaert, B.; Palmieri, R.T.; Iversen, E.S.; Bentley, R.C.; Halabi, S.; Berchuck, A.; Schildkraut, J.M. Hormonal risk factors for ovarian cancer in premenopausal and postmenopausal women. Am. J. Epidemiol. 2008, 167, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Grant, D.J.; Moorman, P.G.; Akushevich, L.; Palmieri, R.T.; Bentley, R.C.; Schildkraut, J.M. Primary peritoneal and ovarian cancers: An epidemiological comparative analysis. Cancer Causes Control 2010, 21, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Vitonis, A.F.; Titus-Ernstoff, L.; Cramer, D.W. Assessing ovarian cancer risk when considering elective oophorectomy at the time of hysterectomy. Obstet. Gynecol. 2011, 117, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.S.; Hankinson, S.E.; Tworoger, S.S. Tubal ligation, hysterectomy, unilateral oophorectomy, and risk of ovarian cancer in the Nurses’ Health Studies. Fertil. Steril. 2014, 102, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Gaitskell, K.; Green, J.; Pirie, K.; Reeves, G.; Beral, V.; Million Women Study Collaborators. Tubal ligation and ovarian cancer risk in a large cohort: Substantial variation by histological type. Int. J. Cancer 2016, 138, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Miracle-McMahill, H.L.; Calle, E.E.; Kosinski, A.S.; Rodriguez, C.; Wingo, P.A.; Thun, M.J.; Heath, C.W., Jr. Tubal ligation and fatal ovarian cancer in a large prospective cohort study. Am. J. Epidemiol. 1997, 145, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Ness, R.B. Endometriosis and ovarian cancer: Thoughts on shared pathophysiology. Am. J. Obstet. Gynecol. 2003, 189, 280–294. [Google Scholar] [CrossRef] [PubMed]
- De la Cuesta, R.S.; Eichhorn, J.H.; Rice, L.W.; Fuller, A.F., Jr.; Nikrui, N.; Goff, B.A. Histologic transformation of benign endometriosis to early epithelial ovarian cancer. Gynecol. Oncol. 1996, 60, 238–244. [Google Scholar] [CrossRef]
- Kato, N.; Sasou, S.; Motoyama, T. Expression of hepatocyte nuclear factor-1beta (HNF-1beta) in clear cell tumors and endometriosis of the ovary. Mod. Pathol. 2006, 19, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Dubeau, L. The cell of origin of ovarian epithelial tumours. Lancet Oncol. 2008, 9, 1191–1197. [Google Scholar] [CrossRef]
| Reference | Authors | Country | Group | Year | Case | Control | Risk Factors | NOS Score |
|---|---|---|---|---|---|---|---|---|
| [5] | Ness et al. | USA | North America | 1999 | 767 | 1367 | Endometriosis | 8 |
| [6] | Madsen et al. | Denmark | Europe | 2014 | 13,241 | 194,689 | Endometriosis | 8 |
| [7] | Collette et al. | USA | North America | 2014 | 194 | 388 | Endometriosis | 7 |
| [11] | Baandrup et al. | Denmark | Europe | 2013 | 3471 | 50,576 | Endometriosis | 8 |
| [14] | Ruiz et al. | USA | North America | 2016 | 208 | 224 | Endometriosis | 8 |
| [18] | Modugno et al. | USA | North America | 2004 | 2098 | 2953 | Endometriosis | 8 |
| [19] | Nagle et al. | Australia | Oceania | 2008 | 232 | 1508 | Endometriosis | 8 |
| [20] | Rossing et al. | USA | North America | 2008 | 812 | 1313 | Endometriosis | 8 |
| [21] | Wu et al. | USA | North America | 2009 | 609 | 688 | Endometriosis | 7 |
| [22] | Moorman et al. | USA | North America | 2009 | 857 | 1057 | Endometriosis | 6 |
| [23] | Bodmer et al. | Switzerland | Europe | 2011 | 1611 | 9710 | Endometriosis | 8 |
| [24] | Merritt et al. | USA | North America | 2013 | 1571 | 2100 | Endometriosis | 7 |
| [11] | Baandrup et al. | Denmark | Europe | 2013 | 3471 | 50,576 | Hysterectomy | 8 |
| [12] | Le et al. | Canada | North America | 2014 | 607 | 334 | Hysterectomy | 8 |
| [14] | Ruiz et al. | USA | North America | 2016 | 208 | 224 | Hysterectomy | 8 |
| [18] | Modugno et al. | USA | North America | 2004 | 2098 | 2953 | Hysterectomy | 8 |
| [19] | Nagle et al. | Australia | Oceania | 2008 | 232 | 1508 | Hysterectomy | 8 |
| [22] | Moorman et al. | USA | North America | 2009 | 857 | 1057 | Hysterectomy | 6 |
| [24] | Merritt et al. | USA | North America | 2013 | 1571 | 2100 | Hysterectomy | 7 |
| [25] | Whittemore et al. | USA | North America | 1988 | 188 | 539 | Hysterectomy | 7 |
| [26] | Hartge et al. | USA | North America | 1988 | 296 | 343 | Hysterectomy | 6 |
| [27] | Booth et al. | England | Europe | 1989 | 235 | 451 | Hysterectomy | 7 |
| [28] | Irwin et al. | USA | North America | 1991 | 494 | 4238 | Hysterectomy | 8 |
| [29] | Parazzini et al. | Swizerland | Europe | 1993 | 953 | 2758 | Hysterectomy | 7 |
| [30] | Cramer et al. | USA | North America | 1995 | 450 | 454 | Hysterectomy | 7 |
| [31] | Rosenblatt et al. | USA | North America | 1996 | 393 | 2563 | Hysterectomy | 8 |
| [32] | Green et al. | Australia | Oceania | 1997 | 824 | 855 | Hysterectomy | 7 |
| [33] | Wittenberg et al. | The Netherlands | Europe | 1999 | 322 | 426 | Hysterectomy | 7 |
| [34] | Riman et al. | Sweden | Europe | 2002 | 655 | 3899 | Hysterectomy | 8 |
| [35] | Chiaffarino et al. | Italy | Europe | 2005 | 1031 | 2411 | Hysterectomy | 8 |
| [36] | Mills et al. | USA | North America | 2005 | 256 | 1122 | Hysterectomy | 7 |
| [37] | Risch et al. | USA | North America | 2006 | 490 | 534 | Hysterectomy | 7 |
| [38] | Faber et al. | Denmark | Europe | 2013 | 554 | 1564 | Hysterectomy | 7 |
| [39] | Rice et al. | USA | North America | 2013 | 2265 | 2333 | Hysterectomy | 8 |
| [6] | Madsen et al. | Denmark | Europe | 2014 | 13,241 | 194,689 | Tubal ligation | 8 |
| [7] | Collette et al. | USA | North America | 2014 | 194 | 388 | Tubal ligation | 7 |
| [9] | Pike et al. | USA | North America | 2004 | 477 | 660 | Tubal ligation | 7 |
| [11] | Baandrup et al. | Denmark | Europe | 2013 | 3471 | 50,576 | Tubal ligation | 8 |
| [13] | Huang et al. | China | China | 2015 | 174 | 70,085 | Tubal ligation | 7 |
| [14] | Ruiz et al. | USA | North America | 2016 | 208 | 224 | Tubal ligation | 8 |
| [18] | Modugno et al. | USA | North America | 2004 | 2098 | 2953 | Tubal ligation | 8 |
| [19] | Nagle et al. | Australia | Oceania | 2008 | 232 | 1508 | Tubal ligation | 8 |
| [21] | Wu et al. | USA | North America | 2009 | 609 | 688 | Tubal ligation | 7 |
| [24] | Merritt et al. | USA | North America | 2013 | 1571 | 2100 | Tubal ligation | 7 |
| [30] | Cramer et al. | USA | North America | 1995 | 450 | 454 | Tubal ligation | 7 |
| [31] | Rosenblatt et al. | USA | North America | 1996 | 393 | 2563 | Tubal ligation | 8 |
| [33] | Wittenberg et al. | The Netherlands | Europe | 1999 | 322 | 426 | Tubal ligation | 7 |
| [34] | Riman et al. | Sweden | Europe | 2002 | 655 | 3899 | Tubal ligation | 8 |
| [37] | Risch et al. | USA | North America | 2006 | 490 | 534 | Tubal ligation | 7 |
| [38] | Faber et al. | Denmark | Europe | 2013 | 554 | 1564 | Tubal ligation | 7 |
| [39] | Rice et al. | USA | North America | 2013 | 2265 | 2333 | Tubal ligation | 8 |
| [40] | Mori et al. | Japan | Asia | 1988 | 56 | 112 | Tubal ligation | 8 |
| [41] | Purdie et al. | Australia | Oceania | 1995 | 824 | 860 | Tubal ligation | 7 |
| [42] | Narod et al. | Canada | North America | 2001 | 232 | 232 | Tubal ligation | 8 |
| [43] | Mills et al. | USA | North America | 2004 | 256 | 1122 | Tubal ligation | 7 |
| [44] | McGuire et al. | USA | North America | 2004 | 417 | 568 | Tubal ligation | 7 |
| [45] | Moorman et al. | USA | North America | 2008 | 896 | 967 | Tubal ligation | 7 |
| [46] | Grant et al. | USA | North America | 2010 | 495 | 1086 | Tubal ligation | 8 |
| [47] | Vitonis et al. | USA | North America | 2011 | 1098 | 1363 | Tubal ligation | 8 |
| Risk Factors | N | OR | 95% CI | I2 (%) | p | E-T2 |
|---|---|---|---|---|---|---|
| Endometriosis | ||||||
| All | 12 | 1.42 | 1.28–1.57 | 20.40 | ˂0.01 | 0.24 |
| Area | ||||||
| North America | 7 | 1.42 | 1.27–1.60 | 0.00 | ˂0.01 | |
| Europe | 4 | 1.28 | 1.12–1.47 | 0.00 | ˂0.01 | |
| Oceania | 1 | 2.52 | 1.63–3.90 | -- | ˂0.01 | |
| Hysterectomy | ||||||
| All | 22 | 0.97 | 0.81–1.14 | 84.70 | 0.68 | 0.31 |
| Area | ||||||
| North America | 13 | 1.00 | 0.93–1.09 | 74.60 | 0.94 | |
| Europe | 7 | 0.97 | 0.86–1.10 | 88.30 | 0.63 | |
| Oceania | 2 | 0.98 | 0.79–1.21 | 97.40 | 0.84 | |
| Tubal ligation | ||||||
| All | 25 | 0.68 | 0.59–0.79 | 90.50 | ˂0.01 | 0.64 |
| Area | ||||||
| Asia | 2 | 0.85 | 0.58–1.24 | 71.80 | 0.06 | |
| North America | 16 | 0.68 | 0.64–0.72 | 87.90 | ˂0.01 | |
| Europe | 5 | 0.82 | 0.75–0.89 | 77.30 | ˂0.01 | |
| Oceania | 2 | 0.48 | 0.39–0.60 | 0.00 | ˂0.81 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Liang, Z.; Liu, X.; Zhang, Q.; Li, S. The Association between Endometriosis, Tubal Ligation, Hysterectomy and Epithelial Ovarian Cancer: Meta-Analyses. Int. J. Environ. Res. Public Health 2016, 13, 1138. https://doi.org/10.3390/ijerph13111138
Wang C, Liang Z, Liu X, Zhang Q, Li S. The Association between Endometriosis, Tubal Ligation, Hysterectomy and Epithelial Ovarian Cancer: Meta-Analyses. International Journal of Environmental Research and Public Health. 2016; 13(11):1138. https://doi.org/10.3390/ijerph13111138
Chicago/Turabian StyleWang, Chunpeng, Zhenzhen Liang, Xin Liu, Qian Zhang, and Shuang Li. 2016. "The Association between Endometriosis, Tubal Ligation, Hysterectomy and Epithelial Ovarian Cancer: Meta-Analyses" International Journal of Environmental Research and Public Health 13, no. 11: 1138. https://doi.org/10.3390/ijerph13111138






