Association between the Angiotensin-Converting Enzyme (ACE) Genetic Polymorphism and Diabetic Retinopathy—A Meta-Analysis Comprising 10,168 Subjects
Abstract
:1. Introduction
2. Methods
2.1. Identification and Eligibility of Relevant Studies
2.2. Data Extraction and Conversion
2.3. Quality Assessment and Study Stratification
2.4. Meta-Analysis
3. Results
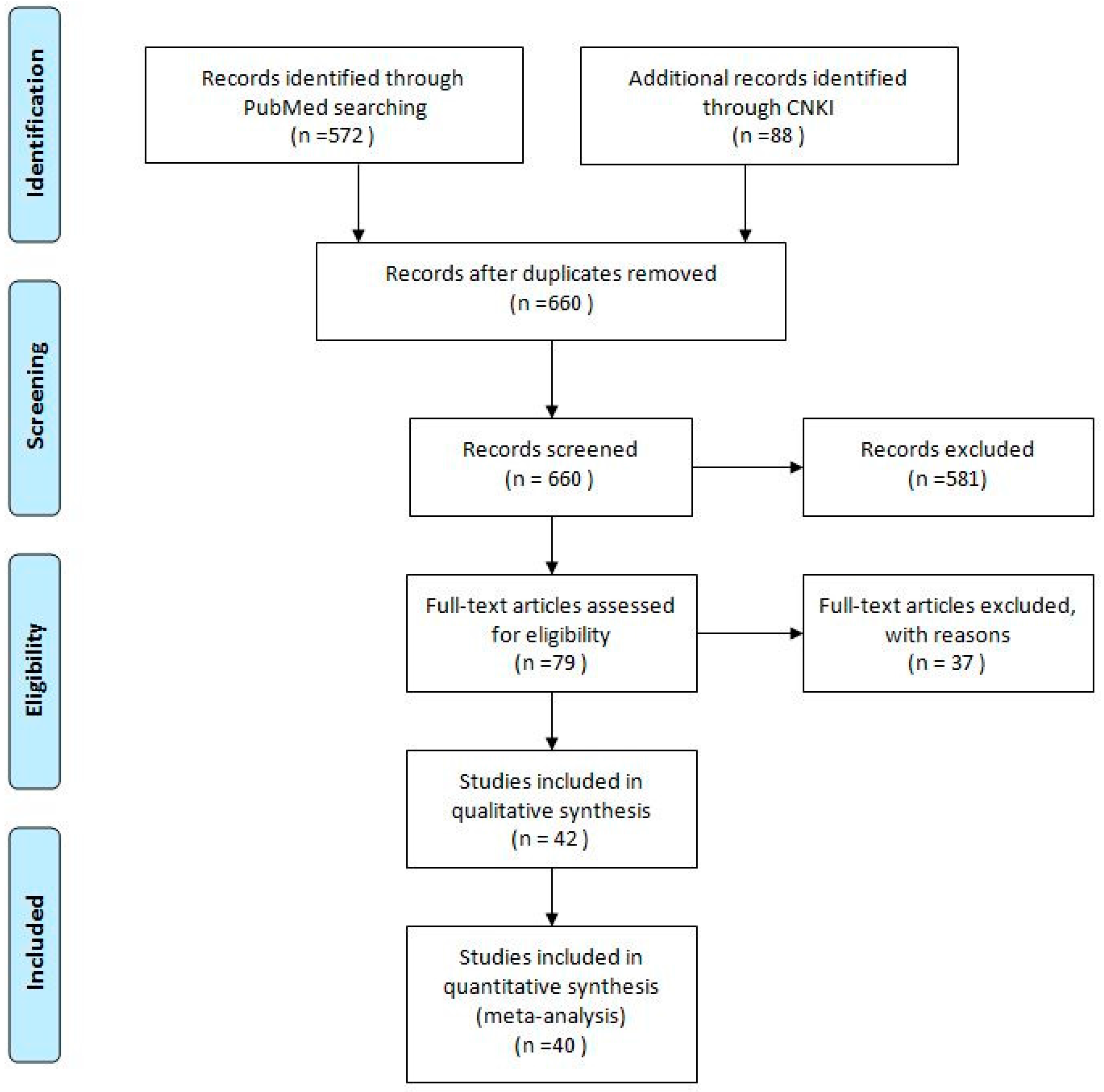
3.1. Literature Search
3.2. Eligible Studies and Study Characteristics
3.3. Summary Statistics
3.4. Main Results, Stratification, and Sensitivity Analyses
3.5. Source of Heterogeneity and Publication Bias
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Bourne, R.R.; Stevens, G.A.; White, R.A.; Smith, J.L.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; et al. Causes of vision loss worldwide, 1990–2010: A systematic analysis. Lancet Glob. Health 2013, 1, e339–e349. [Google Scholar] [CrossRef]
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, R.; Klein, B.E.; Moss, S.E.; Linton, K.L. The Beaver Dam Eye Study. Retinopathy in adults with newly discovered and previously diagnosed diabetes mellitus. Ophthalmology 1992, 99, 58–62. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.; Moss, S.E.; Cruickshanks, K.J. Relationship of hyperglycemia to the long-term incidence and progression of diabetic retinopathy. Arch. Intern. Med. 1994, 154, 2169–2178. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E.; Moss, S.E.; Cruickshanks, K.J. The Wisconsin epidemiologic study of diabetic retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology 1998, 105, 1801–1815. [Google Scholar] [CrossRef]
- Nathan, D.M. Long-term complications of diabetes mellitus. N. Engl. J. Med. 1993, 328, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Sobrin, L. Genetics of diabetic retinopathy. Curr. Diabetes Rep. 2014, 14, 515. [Google Scholar] [CrossRef] [PubMed]
- Danser, A.H.; Schalekamp, M.A.; Bax, W.A.; van den Brink, A.M.; Saxena, P.R.; Riegger, G.A.; Schunkert, H. Angiotensin-converting enzyme in the human heart. Effect of the deletion/insertion polymorphism. Circulation 1995, 92, 1387–1388. [Google Scholar] [CrossRef] [PubMed]
- Migdalis, I.N.; Iliopoulou, V.; Kalogeropoulou, K.; Koutoulidis, K.; Samartzis, M. Elevated serum levels of angiotensin-converting enzyme in patients with diabetic retinopathy. South. Med. J. 1990, 83, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Rigat, B.; Hubert, C.; Alhenc-Gelas, F.; Cambien, F.; Corvol, P.; Soubrier, F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J. Clin. Investig. 1990, 86, 1343–1346. [Google Scholar] [CrossRef] [PubMed]
- Tiret, L.; Rigat, B.; Visvikis, S.; Breda, C.; Corvol, P.; Cambien, F.; Soubrier, F. Evidence, from combined segregation and linkage analysis, that a variant of the angiotensin I-converting enzyme (ACE) gene controls plasma ACE levels. Am. J. Hum. Genet. 1992, 51, 197–205. [Google Scholar] [PubMed]
- Crisan, D.; Carr, J. Angiotensin I-converting enzyme: Genotype and disease associations. J. Mol. Diagn. 2000, 2, 105–115. [Google Scholar] [CrossRef]
- Marre, M.; Bernadet, P.; Gallois, Y.; Savagner, F.; Guyene, T.T.; Hallab, M.; Cambien, F.; Passa, P.; Alhenc-Gelas, F. Relationships between angiotensin I converting enzyme gene polymorphism, plasma levels, and diabetic retinal and renal complications. Diabetes 1994, 43, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, T.; Ikegami, H.; Shen, G.Q.; Yamato, E.; Takekawa, K.; Nakagawa, Y.; Hamada, Y.; Ueda, H.; Rakugi, H.; Higaki, J. Angiotensin I-converting enzyme gene polymorphism is associated with myocardial infarction, but not with retinopathy or nephropathy, in NIDDM. Diabetes Care 1995, 18, 983–985. [Google Scholar] [CrossRef] [PubMed]
- Tarnow, L.; Cambien, F.; Rossing, P.; Nielsen, F.S.; Hansen, B.V.; Lecerf, L.; Poirier, O.; Danilov, S.; Parving, H.H. Lack of relationship between an insertion/deletion polymorphism in the angiotensin I-converting enzyme gene and diabetic nephropathy and proliferative retinopathy in IDDM patients. Diabetes 1995, 44, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Nagi, D.K.; Mansfield, M.W.; Stickland, M.H.; Grant, P.J. Angiotensin converting enzyme (ACE) insertion/deletion (I/D) polymorphism, and diabetic retinopathy in subjects with IDDM and NIDDM. Diabet Med. 1995, 12, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y.; Yoshizumi, H.; Yoshinari, M.; Iino, K.; Yamamoto, M.; Ichikawa, K.; Iwase, M.; Fujishima, M. Association between a polymorphism in the angiotensin-converting enzyme gene and microvascular complications in Japanese patients with NIDDM. Diabetologia 1996, 39, 97–102. [Google Scholar] [PubMed]
- Yoshida, H.; Kuriyama, S.; Atsumi, Y.; Tomonari, H.; Mitarai, T.; Hamaguchi, A.; Kubo, H.; Kawaguchi, Y.; Kon, V.; Matsuoka, K.; et al. Angiotensin I converting enzyme gene polymorphism in non-insulin dependent diabetes mellitus. Kidney Int. 1996, 50, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, C.; Vendrell, J.; Pastor, R.; Llor, C.; Aguilar, C.; Broch, M.; Richart, C. Angiotensin I-converting enzyme and angiotensinogen gene polymorphisms in non-insulin-dependent diabetes mellitus. Lack of relationship with diabetic nephropathy and retinopathy in a Caucasian Mediterranean population. Metabolism 1997, 46, 976–980. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, C.; Guan, T.; Chen, H.; Li, L. The relationship between angiotensin converting enzyme gene polymorphism and diabetic nephropathy and diabetic mellitus. J. Kidney Dis. Dial. Kidney Transplant. 1997, 6, 407–410. [Google Scholar]
- Hu, W.; Wang, L.; Liu, C. Association between ACE gene polymorphism and diabetic retinopathy. J. Cap. Univ. Med. Sci. 1998, 19, 41–44. [Google Scholar]
- Hanyu, O.; Hanawa, H.; Nakagawa, O.; Tani, N.; Andou, N.; Aizawa, Y.; Shibata, A. Polymorphism of the angiotensin I-converting enzyme gene in diabetic nephropathy in type II diabetic patients with proliferative retinopathy. Ren. Fail 1998, 20, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Frost, D.; Pfohl, M.; Clemens, P.; Haring, H.U.; Beischer, W. Evaluation of the insertion/deletion ACE gene polymorphism as a risk factor for carotid artery intima-media thickening and hypertension in young type 1 diabetic patients. Diabetes Care 1998, 21, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Gejyo, F.; Suzuki, Y.; Suzuki, S.; Miyazaki, R.; Arakawa, M. Polymorphisms of angiotensin converting enzyme and plasminogen activator inhibitor-1 genes in diabetes and macroangiopathy1. Kidney Int. 1998, 54, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Rabensteiner, D.; Abrahamian, H.; Irsigler, K.; Hermann, K.M.; Kiener, H.P.; Mayer, G.; Kaider, A.; Prager, R. ACE gene polymorphism and proliferative retinopathy in type 1 diabetes: Results of a case-control study. Diabetes Care 1999, 22, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Solini, A.; Giacchetti, G.; Sfriso, A.; Fioretto, P.; Sardu, C.; Saller, A.; Tonolo, G.; Maioli, M.; Mantero, F.; Nosadini, R. Polymorphisms of angiotensin-converting enzyme and angiotensinogen genes in type 2 diabetic sibships in relation to albumin excretion rate. Am. J. Kidney Dis. 1999, 34, 1002–1009. [Google Scholar] [CrossRef]
- Liao, L.; Lei, M.; Chen, H.; Han, X.; Fan, C. Studies on ACE gene insertion/deletion polymorphism, serum ACE activity, and diabetic retinopathy in type II diabetic patients. Hunan Yi Ke Da Xue Xue Bao 1999, 24, 33–36. [Google Scholar] [PubMed]
- Xiang, K.; Zhen, T.; Sun, D.; Wen, Q.; Xu, J.; Li, J. The association of three genes of renin-angiotensin system with diabetic retinopathy. Chin. J. Diabetes 1999, 7, 5–8. [Google Scholar]
- Wang Dawang, Y.Z.; Zhang, X.; Zhen, J.; Xu, Y.; Lin, X.; Yu, Z.; Shen, F.; Fen, W.; Chen, X.; Zhu, H. Angiotensin converting enzyme gene polymorphism and diabetic retinopathy in type 2 diabetic patients. Chin. J. Diabetes 1999, 7, 299–300. [Google Scholar]
- Liu, J.; Jin, H.; Zhang, W.; Zhou, Y.; Liao, S.; Yang, L.; Huang, Q. Study on the relationship between angiotensin converting enzyme gene polymorphism and diabetic retinopathy. Chin. J. Ocul. Fundus Dis. 1999, 15, 37–38. [Google Scholar]
- Van Ittersum, F.J.; de Man, A.M.; Thijssen, S.; de Knijff, P.; Slagboom, E.; Smulders, Y.; Tarnow, L.; Donker, A.J.; Bilo, H.J.; Stehouwer, C.D. Genetic polymorphisms of the renin-angiotensin system and complications of insulin-dependent diabetes mellitus. Nephrol. Dial. Transplant. 2000, 15, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Iwashima, Y.; Abiko, A.; Morikawa, A.; Sekiguchi, M.; Eto, M.; Makino, I. Detection of the association between a deletion polymorphism in the gene encoding angiotensin I-converting enzyme and advanced diabetic retinopathy. Diabetes Res. Clin. Pract. 2000, 50, 195–202. [Google Scholar] [CrossRef]
- Kankova, K.; Muzik, J.; Karaskova, J.; Beranek, M.; Hajek, D.; Znojil, V.; Vlková, E.; Vácha, J. Duration of non-Insulin-dependent diabetes mellitus and the TNF-beta NcoI genotype as predictive factors in proliferative diabetic retinopathy. Ophthalmologica 2001, 215, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Yunhua, L. The Relationship between Angiotensin I Converting Enzyme Gene Polymorphism and Type 2 Diabetes or Vascular Complication of Diabetes in Guangxi Region; Guangxi Medical University: Nanning, China, 2000. [Google Scholar]
- Yang, X.; Li, X.; Liu, J.; Chen, S.; Luo, H. Study of angiotensin converting enzyme gene polymorphism and non-insulin-dependent diabetes mellitus with microangiopathy correlation. Chin. J. Pract. Intern. Med. 2000, 20, 487–488. [Google Scholar]
- Araz, M.; Yilmaz, N.; Gungor, K.; Okan, V.; Kepekci, Y.; Sükrü Aynacioglu, A. Angiotensin-converting enzyme gene polymorphism and microvascular complications in Turkish type 2 diabetic patients. Diabetes Res. Clin. Pract. 2001, 54, 95–104. [Google Scholar] [CrossRef]
- Viswanathan, V.; Zhu, Y.; Bala, K.; Dunn, S.; Snehalatha, C.; Ramachandran, A.; Jayaraman, M.; Sharma, K. Association between ACE gene polymorphism and diabetic nephropathy in South Indian patients. JOP 2001, 2, 83–87. [Google Scholar] [PubMed]
- Globocnik-Petrovic, M.; Hawlina, M.; Peterlin, B.; Petrovic, D. Insertion/deletion plasminogen activator inhibitor 1 and insertion/deletion angiotensin-converting enzyme gene polymorphisms in diabetic retinopathy in type 2 diabetes. Ophthalmologica 2003, 217, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.K.; Park, H.C.; Park, H.S.; Kang, B.S.; Lee, T.H.; Hwang, H.J.; Kim, S.J.; Kim, D.H.; Kang, S.W.; Choi, K.H.; et al. ACE gene polymorphism and progression of diabetic nephropathy in Korean type 2 diabetic patients: Effect of ACE gene DD on the progression of diabetic nephropathy. Am. J. Kidney Dis. 2003, 41, 943–949. [Google Scholar] [CrossRef]
- Crook, E.D.; Genous, L.; Oliver, B. Angiotensin-converting enzyme genotype in blacks with diabetic nephropathy: Effects on risk of diabetes and its complications. J. Investig. Med. 2003, 51, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Agardh, E.; Gaur, L.K.; Lernmark, Å.; Agardh, C.-D. HLA-DRB1, -DQA1, and -DQB1 subtypes or ACE gene polymorphisms do not seem to be risk markers for severe retinopathy in younger Type 1 diabetic patients. J. Diabetes Complicat. 2004, 18, 32–36. [Google Scholar] [CrossRef]
- Xu, L.; Deng, Z.; Wu, Z.; He, R.; Tang, J.; Mu, H.; Bian, R.; Gu, H.; Wang, X.; Jiang, Y. The relationship of angiotenis converting enzyme (ACE) gene polymorphism and serum ACE levels with retinopathy in patients with type 2 diabetes mellitus. Chin. J. Diabetes 2003, 11, 344–347. [Google Scholar]
- Wu, S.S.; Guo, Q.M.; Liu, G.L.; Zhang, J.; Zhao, C.F.; Ning, S.C.; Zhao, L.N.; Yu, F.; Yi, H.L. The relationship of angiotensin I-converting enzyme gene polymorphism with diabetic retinopathy and diabetes myocardial infarction. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2004, 21, 283–285. [Google Scholar] [PubMed]
- Liao, L.; Lei, M.X.; Chen, H.L.; Guo, L.J.; Han, X.Y. Angiotensin converting enzyme gene polymorphism and type 2 diabetic retinopathy. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2004, 29, 410–413. [Google Scholar] [PubMed]
- Degirmenci, I.; Kebapci, N.; Basaran, A.; Efe, B.; Gunes, H.V.; Akalin, A.; Kurt, H.; Urhan, M.; Demirustu, C. Frequency of angiotensin-converting enzyme gene polymorphism in Turkish type 2 diabetic patients. Int. J. Clin. Pract. 2005, 59, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shen, M.; Wang, J.; Wu, S.; Xie, Y.; Zhang, Y.; Ye, X.; Gao, J.; Li, D. Correlation of gene polymorphisms of angiotensin converting enzyme and type 2 diabetic retinopathy. Clin. Med. China 2005, 21, 608–610. [Google Scholar]
- Lee, S.J.; Choi, M.G. Association of manganese superoxide dismutase gene polymorphism (V16A) with diabetic macular edema in Korean type 2 diabetic patients. Metabolism 2006, 55, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Bian, C.; Guan, H.; Chen, H. Detection of angiotensin converting enzyme gene insertion/deletion polymorphism and its relationship to diabetic retinopahty. Chin. Ophthalmic Res. 2006, 24, 654–656. [Google Scholar]
- Nikzamir, A.; Rashidi, A.; Esteghamati, A.; Nakhjavani, M.; Golmohammadi, T.; Khalilzadeh, O. The relationship between ACE gene insertion/deletion polymorphism and diabetic retinopathy in Iranian patients with type 2 diabetes. Ophthalmic Genet. 2010, 31, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, X.F.; Gu, H.; Deng, Y.; Xu, J.; Ma, K.; Liu, N.P. Relationship of angiotensin converting enzyme gene polymorphism with diabetic retinopathy. Zhonghua Yan Ke Za Zhi 2013, 49, 52–57. [Google Scholar] [PubMed]
- Saleem, S.; Azam, A.; Maqsood, S.I.; Muslim, I.; Bashir, S.; Fazal, N.; Riaz, M.; Ali, S.H.B.; Niazi, M.K.; Ishaq, M.; et al. Role of ACE and PAI-1 polymorphisms in the development and progression of diabetic retinopathy. PLoS ONE 2015, 10, e0144557. [Google Scholar] [CrossRef] [PubMed]
- Settin, A.; El-Baz, R.; Ismaeel, A.; Tolba, W.; Allah, W.A. Association of ACE and MTHFR genetic polymorphisms with type 2 diabetes mellitus: Susceptibility and complications. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Narne, P.; Ponnaluri, K.C.; Siraj, M.; Ishaq, M. Association analysis of polymorphisms in genes related to oxidative stress in south indian type 2 diabetic patients with retinopathy. Ophthalmic Genet. 2016, 37, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.N.; Critchley, J.A.; Tomlinson, B.; Yeung, V.T.; Lam, D.; Cockram, C.S.; Chan, J.C.N. Renin-angiotensin system gene polymorphisms and retinopathy in chinese patients with type 2 diabetes. Diabetes Care 2003, 26, 1643–1644. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.B.; Yang, J.K. Angiotensin-converting enzyme gene polymorphism is associated with proliferative diabetic retinopathy: A meta-analysis. Acta Diabetol. 2010, 47, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ge, Y.; Hu, Q.; Shi, Y.; Xue, C.; Shi, Y.; Chen, S.; Huang, Z. Association between angiotensin-converting enzyme gene polymorphism and diabetic retinopathy in the Chinese population. J. Renin Angiotensin Aldosterone Syst. 2012, 13, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, Y.; Cao, X.; Liu, C.; Xie, Y. Association between plasma homocysteine status and hypothyroidism: A meta-analysis. Int. J. Clin. Exp. Med. 2014, 7, 4544–4553. [Google Scholar] [PubMed]
- Zintzaras, E.; Lau, J. Synthesis of genetic association studies for pertinent gene-disease associations requires appropriate methodological and statistical approaches. J. Clin. Epidemiol. 2008, 61, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Fang, Q.; Yu, N.; Zhao, D.; Zhang, Y.; Wang, J.; Wang, Q.; Zhou, X.; Cao, X.; Fan, X. Association between genetic polymorphism of the angiotensin-converting enzyme and diabetic nephropathy: A meta-analysis comprising 26,580 subjects. J. Renin Angiotensin Aldosterone Syst. 2012, 13, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Ritz, E. Genetics of the renin-angiotensin system and renal disease: A progress report. Curr. Opin. Nephrol. Hypertens. 1997, 6, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.D. Angiotensin II and its receptors in the diabetic kidney. Am. J. Kidney Dis. 2000, 36, 449–467. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Iwao, H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol. Rev. 2000, 52, 11–34. [Google Scholar] [PubMed]
- Ye, S.; Dhillon, S.; Seear, R.; Dunleavey, L.; Day, L.B.; Bannister, W.; Day, I.N.; Simpson, I. Epistatic interaction between variations in the angiotensin I converting enzyme and angiotensin II type 1 receptor genes in relation to extent of coronary atherosclerosis. Heart 2003, 89, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.T.; Hwang, J.J.; Ritchie, M.D.; Moore, J.H.; Chiang, F.T.; Lai, L.P.; Hsu, K.L.; Tseng, C.D.; Lin, J.L.; Tseng, Y.Z. Renin-angiotensin system gene polymorphisms and coronary artery disease in a large angiographic cohort: Detection of high order gene-gene interaction. Atherosclerosis 2007, 195, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Cordonnier, D.J.; Zaoui, P.; Halimi, S. Role of ACE inhibitors in patients with diabetes mellitus. Drugs 2001, 61, 1883–1892. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.E.; Krum, H.; Wilkinson-Berka, J.; Kelly, D.J. The renin-angiotensin system and the long-term complications of diabetes: Pathophysiological and therapeutic considerations. Diabet. Med. 2003, 20, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Zellweger-Zahner, T.; Schneider, M.; Junker, C.; Lengeler, C.; Antes, G. Language bias in randomised controlled trials published in English and German. Lancet 1997, 350, 326–329. [Google Scholar] [CrossRef]

| Author (Reference) | Year | Country | Design | Case | Control | HWE # | MAF * | NOS (Stars *) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Size | Age (Year) | DM Duration (Year) | Definition | Sample Size | Age (Year) | DM Duration (Year) | Definition | |||||||
| Marre et al. [14]. | 1994 | France | CC | 52 | 39.0 ± 14.0 | 20.0 ± 11.0 | PDR | 32 | 43.0 ± 18.0 | 22.0 ± 12.0 | IDDM | 0.38 | 0.64 | 6 |
| Fujisawa et al. [15]. | 1995 | Japan | CC | 222 | NR | NR | DR | 45 | NR | NR | NIDDM | 0.84 | 0.36 | 5 |
| Tarnow et al. [16]. | 1995 | Denmark | CC | 155 | 40.9 ± 9.6 | 26.7 ± 7.9 | PDR | 67 | 42.7 ± 10.2 | 25.8 ± 8.5 | IDDM | 0.05 | 0.57 | 6 |
| Nagi et al. [17]. | 1995 | Britain | CC | 271 | 50.6 ± 14.3 for IDDM 66.8 ± 10.4 for NIDDM | 27 (12–66) for IDDM 11 (1–36) for NIDDM | DR | 376 | 38.3 ± 14.6 for IDDM 69.5 ± 11.1 for NIDDM NA for Healthy | 16 (1–56) for IDDM 7 (1–45) for NIDDM NA for Healthy | Healthy + IDDM + NIDDM | 0.71 | 0.52 | 7 |
| Doi et al. [18]. | 1995 | Japan | CC | 362 | 61.8 (30–79) | >10 | DR | 105 | NA | NA | Healthy | 0.25 | 0.34 | 4 |
| Yoshida et al. [19]. | 1996 | Japan | CS | 118 | NA | NA | DR | 50 | NA | NA | NIDDM | 0.59 | 0.31 | 4 |
| Gutie’rrez et al. [20]. | 1997 | Spain | CC | 68 | 61.9 ± 9.1 | 14.8 ± 5.7 | DR | 92 | 59.6 ± 10.3 | 12.1 ± 6.3 | NIDDM | 0.97 | 0.61 | 6 |
| Liu et al. [21]. | 1997 | China | CC | 30 | NA | NA | DR | 198 | NA for NDR 34. 8 ± 5. 9 for Healthy | NA | Healthy + NIDDM | 0.92 | 0.27 | 4 |
| Hu et al. [22]. | 1998 | China | CC | 56 | 62.07 ± 1.21 | 11.68 ± 0.91 | DR | 81 | 56 .06 ± 1 .97 for NDR 56 .86 ± 1 .46 for Healthy | 4 .23 ± 0 .47 for NDR | Healthy + NIDDM | 0.02 | 0.35 | 7 |
| Hanyu et al. [23]. | 1998 | Japan | CC | 45 | 60.0 ± 8.8 without DN 56.1 ± 10.5 with DN | 18.2 ± 5.7 without DN 17.0 ± 6.0 with DN | DR | 57 | 56.4 ± 5.1 | NR | Healthy | 0.72 | 0.46 | 6 |
| Frost et al. [24]. | 1998 | Germany | CS | 79 | 30.1 ± 6.6 | 13.1 ± 8.1 | DR | 69 | 30.1 ± 6.6 | 13.1 ± 8.1 | T1DM | 0.87 | 0.67 | 5 |
| Kimura et al. [25]. | 1998 | Japan | CC | 114 | NA | NA | PDR | 94 | 43.7 ± 15.4 | NR | Healthy | 0.14 | 0.39 | 6 |
| Rabensteiner et al. [26]. | 1999 | Austria | CC | 94 | 47.2 ± 9.9 | 31.5 ± 8.2 | PDR | 81 | 47.7 ± 11.5 | 29.7 ± 8.8 | T1DM | 0.37 | 0.44 | 6 |
| Solini et al. [27]. | 1999 | Italy | CS | 21 | NA | NA | DR | 181 | NA | NA | T2DM | 0.11 | 0.67 | 4 |
| Liao et al. [28]. | 1999 | China | CC | 68 | 51.9 ± 11.1 for BDR 53.1 ± 8.8 for PDR | 9.35 ± 3.87 for BDR 9.46 ± 5.11 for PDR | BDR+PDR | 76 | 53.2 ± 8.7 for NDR 52.3 ± 9.9 for Healthy | 9.29 ± 5.17 for NDR | Healthy + T2DM | 0.02 | 0.37 | 7 |
| Xiang et al. [29]. | 1999 | China | CC | 49 | 61.1 ± 10.5 | 7.1 ± 8.2 | DR | 162 | 53.2 ± 8.7 for NDR 52.3 ± 9.9 for Healthy | 9.29 ± 5.17 for NDR | Healthy + T2DM | 0.28 | 0.38 | 7 |
| Wang et al. [30]. | 1999 | China | CC | 23 | 58.26 ± 9.57 | 5.21 ± 5.7 | DR | 172 | 59.0 ± 10.0 for NDR 64.9 ± 10.0 for Healthy | 4.0 ± 5.1 for NDR | Healthy + T2DM | 0.00 | 0.39 | 7 |
| Liu et al. [31]. | 1999 | China | CC | 100 | 55 (36–90) | 8.8 (0.5–18) | DR | 164 | 53 (38–72) for NDR 35 (20–58) for Healthy | NA | Healthy + DM | 0.21 | 0.40 | 5 |
| Van Ittersum et al. [32]. | 2000 | New Zealand | CC | 101 | NA | NA | DR | 151 | NA | NA | IDDM | 0.61 | 0.46 | 4 |
| Matsumoto et al. [33]. | 2000 | Japan | CC | 120 | 63.2 ± 10.4 for SDR 56.8 ± 11.9 for ADR | 16.7 ± 7.6 for SDR 16.2 ± 9.1 for ADR | SDR+ADR | 190 | 58.9 ± 12.1 for NDR 52.0 ± 1.0 for Healthy | 15.0 ± 6.6 for NDR | Healthy + T2DM | 0.74 | 0.38 | 7 |
| Kankova et al. [34]. | 2000 | Czech | CH | 74 | NA | NA | PDR | 348 | 63.6 ± 13.4 for Healthy | NA | Healthy + NIDDM | 0.19 | 0.52 | 5 |
| Liao et al. [35]. | 2000 | China | CC | 42 | NA | NA | DR | 178 | 54.83 ± 13.71 for NDR 48.71 ± 15.12 for Healthy | 0.5–30 for NDR | Healthy + T2DM | 0.01 | 0.54 | 7 |
| Yang et al. [36]. | 2000 | China | CC | 60 | NA | NA | DR | 137 | NA | NA | Healthy + NIDDM | 0.21 | 0.32 | 4 |
| Araz et al. [37]. | 2001 | Turkey | CS/CC | 120 | 55.0 ± 8.0 | 11.2 ± 6.5 | DR | 257 | 51.0 ± 9.0 for NDR NA for Healthy | 5.2 ± 5.1 for NDR | Healthy + T2DM | 0.98 | 0.60 | 7 |
| Viswanathan et al. [38]. | 2001 | India | CC | 86 | 56.7 + 8.9 | 13.4 + 6.9 | DR | 23 | 56.7 + 9.3 | 13.2 + 5.1 | T2DM | 0.01 | 0.46 | 6 |
| Petrovic et al. [39]. | 2003 | Slovenia | CC | 124 | 65.6 ± 9.7 | 18.7 ± 9.1 | DR | 80 | 71.3 ± 7.0 | 16.8 ± 6.8 | T2DM | 0.07 | 0.51 | 6 |
| Ha et al. [40]. | 2003 | Korea | CS | 180 | NA | NA | DR | 59 | NA | NA | T2DM | 0.07 | 0.37 | 4 |
| Crook et al. [41]. | 2003 | USA | CH | 46 | NA | NA | DR | 10 | NA | NA | T2DM | 0.24 | 0.80 | 4 |
| Agardh et al. [42]. | 2003 | USA | CC | 24 | 32 (24–37) | 23 (16–31) | SDR | 24 | 28.5 (22–57) | 19.5 (10–56) | T1DM | 0.74 | 0.56 | 6 |
| Xu et al. [43]. | 2003 | China | CC | 58 | 62 ± 10 | 8 ± 6 | DR | 142 | 60 ± 12 for NDR 59 ± 12 for Healthy | 8 ± 7 for NDR | Healthy + T2DM | 0.03 | 0.35 | 7 |
| Thomas et al. [55]. | 2003 | China/Asia | CC | 326 | 59.8 ± 11.4 | 6.3 (5.6–7.0) | DR | 501 | 60.4 ± 9.3 for T2DM | 6.0 (5.6– 6.3) | T2DM | 0.38 | 0.33 | 6 |
| Wu et al. [44]. | 2004 | China | CH | 90 | 30.5 ± 4.3 for T1DR 60.2 ± 8.3 for T2DR | 11.8 ± 2.4 for T1DR 15.1 ± 4.7 for T2DR | DR | 294 | 36.8 ± 6.6 for T1DM 65.2 ± 3.2 for T2DM MI 59.5 ± 1.2 for T2DM NMI | 24.3 ± 9.8 for T1DM 15.1 ± 5.0 for T2DM MI 12.3 ± 3.3 for T2DM NMI | T1DM + T2DM | 0.22 | 0.57 | 8 |
| Liao et al. [45]. | 2004 | China | CC | 44 | NA | NA | BDR + PDR | 21 | NA | NA | T2DM | 0.16 | 0.40 | 4 |
| Degirmenci et al. [46]. | 2005 | Turkey | CC | 57 | NA | NA | DR | 83 | NA | NA | T2DM | 0.61 | 0.54 | 4 |
| Chen et al. [47]. | 2005 | China | CC | 27 | 58.39 ± 9.47 | NA | DR | 319 | 55.43 ± 8.31 for NDR NA for Healthy | NA | Healthy + T2DM | 0.39 | 0.63 | 5 |
| Lee et al. [48]. | 2006 | Korea | CC | 130 | 53.1 ± 12.3 | 11.4 ± 3.7 | DR | 174 | 53.7 ± 12.9 | 9.4 ± 2.8 | T2DM | 0.01 | 0.42 | 6 |
| Liang et al. [49]. | 2006 | China | CC | 82 | 63.41 ± 11.22 | 8.34 ± 6.36 | DR | 153 | 62.98 ± 11.87 for NDR 65.31 ± 9.77 for Healthy | 4.91 ± 4.76 for NDR | Healthy + T2DM | 0.54 | 0.32 | 7 |
| Nikzamir et al. [50]. | 2010 | Iran | CC | 178 | 59.0 ± 8.7 | 13 (4–30) | DR | 206 | 59.5 ± 8.2 | 11 (1–30) | T2DM | 0.29 | 0.46 | 6 |
| Li et al. [51]. | 2013 | China | CC | 207 | 62.4 ± 7.8 | 14.6 ± 7.5 | DR | 302 | 59.5 ± 8.2 for NDR 75.5 ± 2.8 for Healthy | 15.0 ± 4.3 for NDR | Healthy + T2DM | 0.02 | 0.50 | 7 |
| Narne et al. [54]. | 2016 | India | CC | 149 | 52.7 ± 7.3 | 14.7 ± 4.7 | DR | 162 | 53.4 ± 5.4 | 15.9 ± 5.6 | T2DM | 0.05 | 0.40 | 6 |
| Author (Reference) | Prevalence of ACE I/D Genotype | Prevalence of Allele Frequency | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| II | ID | DD | I | D | ||||||
| Case | Control | Case | Control | Case | Control | Case | Control | Case | Control | |
| Marre et al. [14]. | 8 | 3 | 28 | 17 | 16 | 12 | 44 | 23 | 60 | 41 |
| Fujisawa et al. [15]. | 87 | 19 | 102 | 20 | 33 | 6 | 276 | 58 | 168 | 32 |
| Tarnow et al. [16]. | 29 | 16 | 74 | 25 | 52 | 26 | 132 | 57 | 178 | 77 |
| Nagi et al. [17]. | 74 | 88 | 120 | 184 | 77 | 104 | 268 | 360 | 274 | 392 |
| Doi et al. [18]. | 132 | 48 | 179 | 42 | 51 | 15 | 443 | 138 | 281 | 72 |
| Yoshida et al. [19]. | 45 | 23 | 51 | 23 | 22 | 4 | 141 | 69 | 95 | 31 |
| Gutie‘rrez et al. [20]. | 6 | 14 | 30 | 44 | 32 | 34 | 42 | 72 | 94 | 112 |
| Liu et al. [21]. | 10 | 105 | 8 | 78 | 12 | 15 | 28 | 288 | 32 | 108 |
| Hu et al. [22]. | 29 | 39 | 15 | 27 | 12 | 15 | 73 | 105 | 39 | 57 |
| Hanyu et al. [23]. | 21 | 17 | 18 | 27 | 6 | 13 | 60 | 61 | 30 | 53 |
| Frost et al. [24]. | 23 | 8 | 25 | 30 | 31 | 31 | 71 | 46 | 87 | 92 |
| Kimura et al. [25]. | 48 | 38 | 47 | 38 | 19 | 18 | 143 | 114 | 85 | 74 |
| Rabensteiner et al. [26]. | 11 | 23 | 46 | 44 | 37 | 14 | 68 | 90 | 120 | 72 |
| Solini et al. [27]. | 4 | 25 | 16 | 71 | 1 | 85 | 24 | 121 | 18 | 241 |
| Liao et al. [28]. | 33 | 35 | 21 | 26 | 14 | 15 | 87 | 96 | 49 | 56 |
| Xiang et al. [29]. | 12 | 65 | 23 | 70 | 14 | 27 | 47 | 200 | 51 | 124 |
| Wang et al. [30]. | 9 | 75 | 8 | 61 | 6 | 36 | 26 | 211 | 20 | 133 |
| Liu et al. [31]. | 33 | 63 | 38 | 71 | 29 | 30 | 104 | 197 | 96 | 131 |
| Van Ittersum et al. [32]. | 29 | 45 | 47 | 72 | 25 | 34 | 105 | 162 | 97 | 140 |
| Matsumoto et al. [33]. | 41 | 75 | 53 | 87 | 26 | 28 | 135 | 237 | 105 | 143 |
| Kankova et al. [34]. | 14 | 75 | 39 | 186 | 21 | 87 | 67 | 336 | 81 | 360 |
| Liao et al. [35]. | 11 | 46 | 18 | 72 | 13 | 60 | 40 | 164 | 44 | 192 |
| Yang et al. [36]. | 22 | 60 | 14 | 66 | 24 | 11 | 58 | 186 | 62 | 88 |
| Araz et al. [37]. | 20 | 42 | 62 | 124 | 38 | 91 | 102 | 208 | 138 | 306 |
| Viswanathan et al. [38]. | 17 | 10 | 45 | 5 | 24 | 8 | 79 | 25 | 93 | 21 |
| Petrovic et al. [39]. | 28 | 23 | 63 | 32 | 33 | 25 | 119 | 78 | 129 | 82 |
| Ha et al. [40]. | 48 | 20 | 85 | 34 | 47 | 5 | 181 | 74 | 179 | 44 |
| Crook et al. [41]. | 5 | 1 | 27 | 2 | 14 | 7 | 37 | 4 | 55 | 16 |
| Agardh et al. [42]. | 4 | 5 | 11 | 11 | 9 | 8 | 19 | 21 | 29 | 27 |
| Xu et al. [43]. | 11 | 66 | 31 | 53 | 16 | 23 | 53 | 185 | 63 | 99 |
| Thomas et al. [55]. | 157 | 231 | 129 | 212 | 40 | 58 | 443 | 674 | 209 | 328 |
| Wu et al. [44]. | 11 | 60 | 45 | 134 | 34 | 100 | 67 | 254 | 113 | 334 |
| Liao et al. [45]. | 19 | 9 | 16 | 7 | 9 | 5 | 54 | 25 | 34 | 17 |
| Degirmenci et al. [46]. | 6 | 19 | 34 | 39 | 17 | 25 | 46 | 77 | 68 | 89 |
| Chen et al. [47]. | 3 | 39 | 5 | 155 | 19 | 125 | 11 | 233 | 43 | 405 |
| Lee et al. [48]. | 47 | 67 | 69 | 68 | 14 | 39 | 163 | 202 | 97 | 146 |
| Liang et al. [49]. | 26 | 73 | 36 | 63 | 20 | 17 | 88 | 209 | 76 | 97 |
| Nikzamir et al. [50]. | 47 | 56 | 73 | 110 | 58 | 40 | 167 | 222 | 189 | 190 |
| Li et al. [51]. | 52 | 64 | 120 | 172 | 35 | 66 | 224 | 300 | 190 | 304 |
| Narne et al. [54]. | 46 | 64 | 76 | 66 | 27 | 32 | 168 | 194 | 130 | 130 |
| Total | 1278 | 1854 | 1947 | 2668 | 1027 | 1394 | 4503 | 63,762 | 4001 | 5456 |
| Genetic Model | Group | Sensitivity # | Studies | OR | 95% CI | p * | I2 (%) |
|---|---|---|---|---|---|---|---|
| ID vs. II | All studies | All | 40 | 1.14 | 1.00–1.30 | 0.02 | 33.8 |
| Sensitivity | 32 | 1.08 | 0.97–1.21 | 0.13 | 22.60 | ||
| Non-Asian | All | 15 | 1.04 | 0.86–1.25 | 0.09 | 35.30 | |
| Sensitivity | 15 | 1.04 | 0.86–1.25 | 0.09 | 35.30 | ||
| Asian | All | 25 | 1.14 | 1.01–1.29 | 0.05 | 34.50 | |
| Sensitivity | 17 | 1.11 | 0.96–1.29 | 0.32 | 11.50 | ||
| TIDM | All | 8 | 1.00 | 0.64–1.56 | 0.05 | 50.30 | |
| Sensitivity | 8 | 1.00 | 0.64–1.56 | 0.05 | 50.30 | ||
| T2DM | All | 33 | 1.13 | 1.00–1.24 | 0.05 | 31.20 | |
| Sensitivity | 26 | 1.07 | 1.00–1.21 | 0.30 | 11.40 | ||
| Non-Asian with T1DM | All | 7 | 0.98 | 0.84–1.14 | 0.04 | 55.40 | |
| Sensitivity | 7 | 0.98 | 0.84–1.14 | 0.04 | 55.40 | ||
| Non-Asian with T2DM | All | 9 | 1.03 | 0.96–1.10 | 0.49 | 0.00 | |
| Sensitivity | 9 | 1.03 | 0.96–1.10 | 0.49 | 0.00 | ||
| Asian with T1DM | All | 1 | 1.13 | 0.87–1.46 | NA | NA | |
| Sensitivity | 1 | 1.13 | 0.87–1.46 | NA | NA | ||
| Asian with T2DM | All | 24 | 1.14 | 1.01–1.30 | 0.05 | 36.10 | |
| Sensitivity | 16 | 1.11 | 1.00–1.29 | 0.29 | 13.90 | ||
| DD vs. II | All studies | All | 40 | 1.38 | 1.11–1.71 | 0.00 | 62.3 |
| Sensitivity | 32 | 1.46 | 1.15–1.87 | 0.00 | 62.20 | ||
| Non-Asian | All | 15 | 1.14 | 0.81–1.60 | 0.01 | 55.50 | |
| Sensitivity | 15 | 1.14 | 0.81–1.60 | 0.01 | 55.50 | ||
| Asian | All | 25 | 1.54 | 1.16–2.04 | 0.00 | 65.30 | |
| Sensitivity | 17 | 1.80 | 1.30–2.51 | 0.00 | 63.20 | ||
| TIDM | All | 8 | 1.08 | 0.63–1.87 | 0.01 | 61.70 | |
| Sensitivity | 8 | 1.08 | 0.63–1.87 | 0.01 | 61.70 | ||
| T2DM | All | 33 | 1.39 | 1.10–1.74 | 0.00 | 61.80 | |
| Sensitivity | 26 | 1.58 | 1.20–2.07 | 0.00 | 66.20 | ||
| Non-Asian with T1DM | All | 7 | 1.09 | 0.92–1.30 | 0.09 | 44.90 | |
| Sensitivity | 7 | 1.09 | 0.92–1.30 | 0.09 | 44.90 | ||
| Non-Asian with T2DM | All | 9 | 1.06 | 0.96–1.18 | 0.26 | 20.20 | |
| Sensitivity | 9 | 1.06 | 0.96–1.18 | 0.26 | 20.20 | ||
| Asian with T1DM | All | 1 | 0.99 | 0.64–1.53 | NA | NA | |
| Sensitivity | 1 | 0.99 | 0.64–1.53 | NA | NA | ||
| Asian with T2DM | All | 24 | 1.54 | 1.14–2.08 | 0.00 | 66.70 | |
| Sensitivity | 16 | 1.83 | 1.27–2.63 | 0.00 | 65.80 | ||
| Allele contrast | All studies | All | 40 | 1.17 | 1.05–1.30 | 0 | 64.7 |
| Sensitivity | 32 | 1.19 | 1.05–1.35 | 0.00 | 65.40 | ||
| Non-Asian | All | 15 | 1.02 | 0.86–1.22 | 0.00 | 62.10 | |
| Sensitivity | 15 | 1.02 | 0.86–1.22 | 0.00 | 62.10 | ||
| Asian | All | 25 | 1.26 | 1.10–1.45 | 0.00 | 65.40 | |
| Sensitivity | 17 | 1.35 | 1.15–1.59 | 0.00 | 64.00 | ||
| TIDM | All | 8 | 1.03 | 0.78–1.34 | 0.01 | 61.00 | |
| Sensitivity | 8 | 1.03 | 0.78–1.34 | 0.01 | 61.00 | ||
| T2DM | All | 33 | 1.17 | 1.04–1.32 | 0.00 | 64.90 | |
| Sensitivity | 26 | 1.22 | 1.06–1.40 | 0.00 | 66.50 | ||
| Non-Asian with T1DM | All | 7 | 1.02 | 0.89–1.16 | 0.01 | 65.40 | |
| Sensitivity | 7 | 1.02 | 0.89–1.16 | 0.01 | 65.40 | ||
| Non-Asian with T2DM | All | 9 | 1.01 | 0.92–1.10 | 0.02 | 54.80 | |
| Sensitivity | 9 | 1.01 | 0.92–1.10 | 0.02 | 54.80 | ||
| Asian with T1DM | All | 1 | 0.96 | 0.76–1.23 | NA | NA | |
| Sensitivity | 1 | 0.96 | 0.76–1.23 | NA | NA | ||
| Asian with T2DM | All | 24 | 1.26 | 1.09–1.47 | 0.00 | 66.90 | |
| Sensitivity | 16 | 1.36 | 1.14–1.63 | 0.00 | 66.30 | ||
| Recessive model | All studies | All | 40 | 1.24 | 1.02–1.51 | 0 | 67.6 |
| Sensitivity | 32 | 1.33 | 1.07–1.66 | 0.00 | 69.20 | ||
| Non-Asian | All | 15 | 1.03 | 0.79–1.35 | 0.00 | 59.70 | |
| Sensitivity | 15 | 1.03 | 0.79–1.35 | 0.00 | 59.70 | ||
| Asian | All | 25 | 1.42 | 1.08–1.85 | 0.00 | 71.10 | |
| Sensitivity | 17 | 1.73 | 1.24–2.41 | 0.00 | 71.90 | ||
| TIDM | All | 8 | 1.09 | 0.86–1.39 | 0.09 | 43.20 | |
| Sensitivity | 8 | 1.09 | 0.86–1.39 | 0.09 | 43.20 | ||
| T2DM | All | 33 | 1.24 | 1.01–1.54 | 0.00 | 69.50 | |
| Sensitivity | 26 | 1.36 | 1.06–1.74 | 0.00 | 71.90 | ||
| Non-Asian with T1DM | All | 7 | 1.09 | 0.92–1.30 | 0.09 | 44.90 | |
| Sensitivity | 7 | 1.09 | 0.92–1.30 | 0.09 | 44.90 | ||
| Non-Asian with T2DM | All | 9 | 1.00 | 0.75–1.25 | 0.00 | 67.20 | |
| Sensitivity | 9 | 1.00 | 0.75–1.25 | 0.00 | 67.20 | ||
| Asian with T1DM | All | 1 | 0.76 | 0.42–1.42 | NA | NA | |
| Sensitivity | 1 | 0.76 | 0.42–1.42 | NA | NA | ||
| Asian with T2DM | All | 24 | 1.42 | 1.07–1.88 | 0.00 | 71.80 | |
| Sensitivity | 16 | 1.76 | 1.23–2.51 | 0.00 | 72.90 | ||
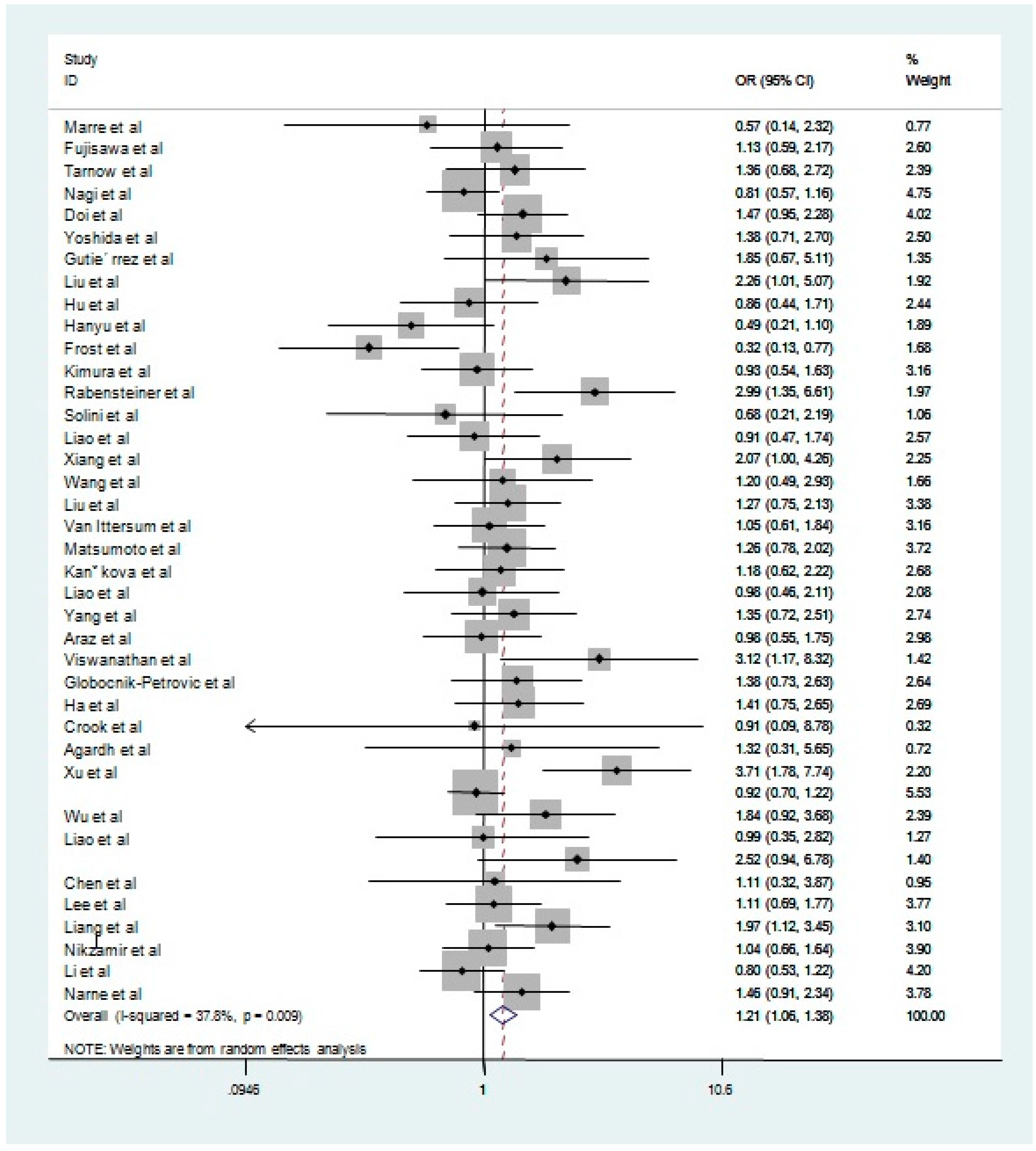
| Dominant model | All studies | All | 40 | 1.21 | 1.06–1.38 | 0.01 | 37.8 |
| Sensitivity | 32 | 1.17 | 1.06–1.31 | 0.05 | 30.50 | ||
| Non-Asian | All | 15 | 1.15 | 0.97–1.37 | 0.18 | 25.30 | |
| Sensitivity | 15 | 1.15 | 0.97–1.37 | 0.18 | 25.30 | ||
| Asian | All | 25 | 1.26 | 1.08–1.47 | 0.03 | 37.60 | |
| Sensitivity | 17 | 1.25 | 1.09–1.42 | 0.02 | 19.80 | ||
| TIDM | All | 8 | 1.03 | 0.66–1.61 | 0.02 | 57.30 | |
| Sensitivity | 8 | 1.03 | 0.66–1.61 | 0.02 | 57.30 | ||
| T2DM | All | 33 | 1.19 | 1.05–1.36 | 0.04 | 32.20 | |
| Sensitivity | 26 | 1.16 | 1.04–1.29 | 0.20 | 18.60 | ||
| Non-Asian with T1DM | All | 7 | 1.00 | 0.90–1.11 | 0.01 | 63.00 | |
| Sensitivity | 7 | 1.00 | 0.90–1.11 | 0.01 | 63.00 | ||
| Non-Asian with T2DM | All | 9 | 1.02 | 0.98–1.07 | 0.67 | 0.00 | |
| Sensitivity | 9 | 1.02 | 0.98–1.07 | 0.67 | 0.00 | ||
| Asian with T1DM | All | 1 | 1.05 | 0.89–1.25 | NA | NA | |
| Sensitivity | 1 | 1.05 | 0.89–1.25 | NA | NA | ||
| Asian with T2DM | All | 24 | 1.26 | 1.07–1.49 | 0.02 | 40.20 | |
| Sensitivity | 16 | 1.24 | 1.08–1.43 | 0.17 | 25.00 |
| Sub Group | Egger Test | Begg Test | ||
|---|---|---|---|---|
| Dominant | Recessive | Dominant | Recessive | |
| all study | 0.14 | 0.71 | 0.47 | 0.63 |
| T1DM | 0.96 | 0.86 | 1.00 | 1.00 |
| T2DM | 0.06 | 0.62 | 0.25 | 0.46 |
| Non-Asian | 0.08 | 0.12 | 0.11 | 0.43 |
| Asian | 0.09 | 0.12 | 0.34 | 0.18 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, S.; Shi, C.; Wang, F.; Wu, Z. Association between the Angiotensin-Converting Enzyme (ACE) Genetic Polymorphism and Diabetic Retinopathy—A Meta-Analysis Comprising 10,168 Subjects. Int. J. Environ. Res. Public Health 2016, 13, 1142. https://doi.org/10.3390/ijerph13111142
Luo S, Shi C, Wang F, Wu Z. Association between the Angiotensin-Converting Enzyme (ACE) Genetic Polymorphism and Diabetic Retinopathy—A Meta-Analysis Comprising 10,168 Subjects. International Journal of Environmental Research and Public Health. 2016; 13(11):1142. https://doi.org/10.3390/ijerph13111142
Chicago/Turabian StyleLuo, Shasha, Chao Shi, Furu Wang, and Zhifeng Wu. 2016. "Association between the Angiotensin-Converting Enzyme (ACE) Genetic Polymorphism and Diabetic Retinopathy—A Meta-Analysis Comprising 10,168 Subjects" International Journal of Environmental Research and Public Health 13, no. 11: 1142. https://doi.org/10.3390/ijerph13111142





