Effects of Fetal Exposure to Asian Sand Dust on Development and Reproduction in Male Offspring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. ASD Particles
2.3. Particle Administration
2.4. Offspring
2.5. Testicle and Epididymis Removal
2.6. Sperm Characteristics
2.7. Testicular Daily Sperm Production (DSP)
2.8. Serum Testosterone Measurement
2.9. Histological Analysis
2.10. Statistical Analysis
3. Results
3.1. Effects of ASD Administration on Maternal and Fetal Growth
3.2. Effects of Fetal ASD Exposure on Body, Testis, and Epididymis Weights
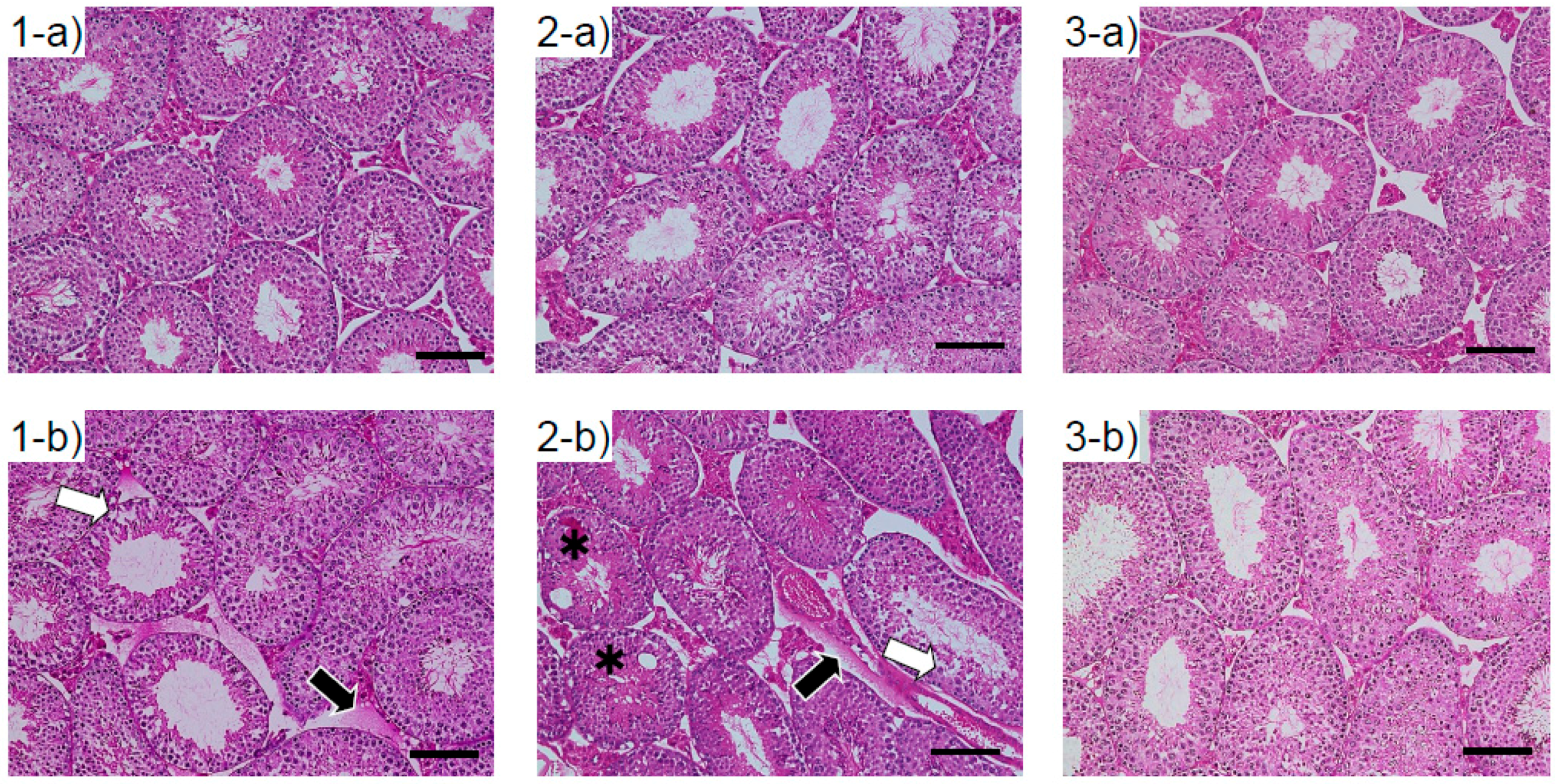
3.3. Histological Changes in the Testes of Mice Exposed to ASD During the Fetal Period
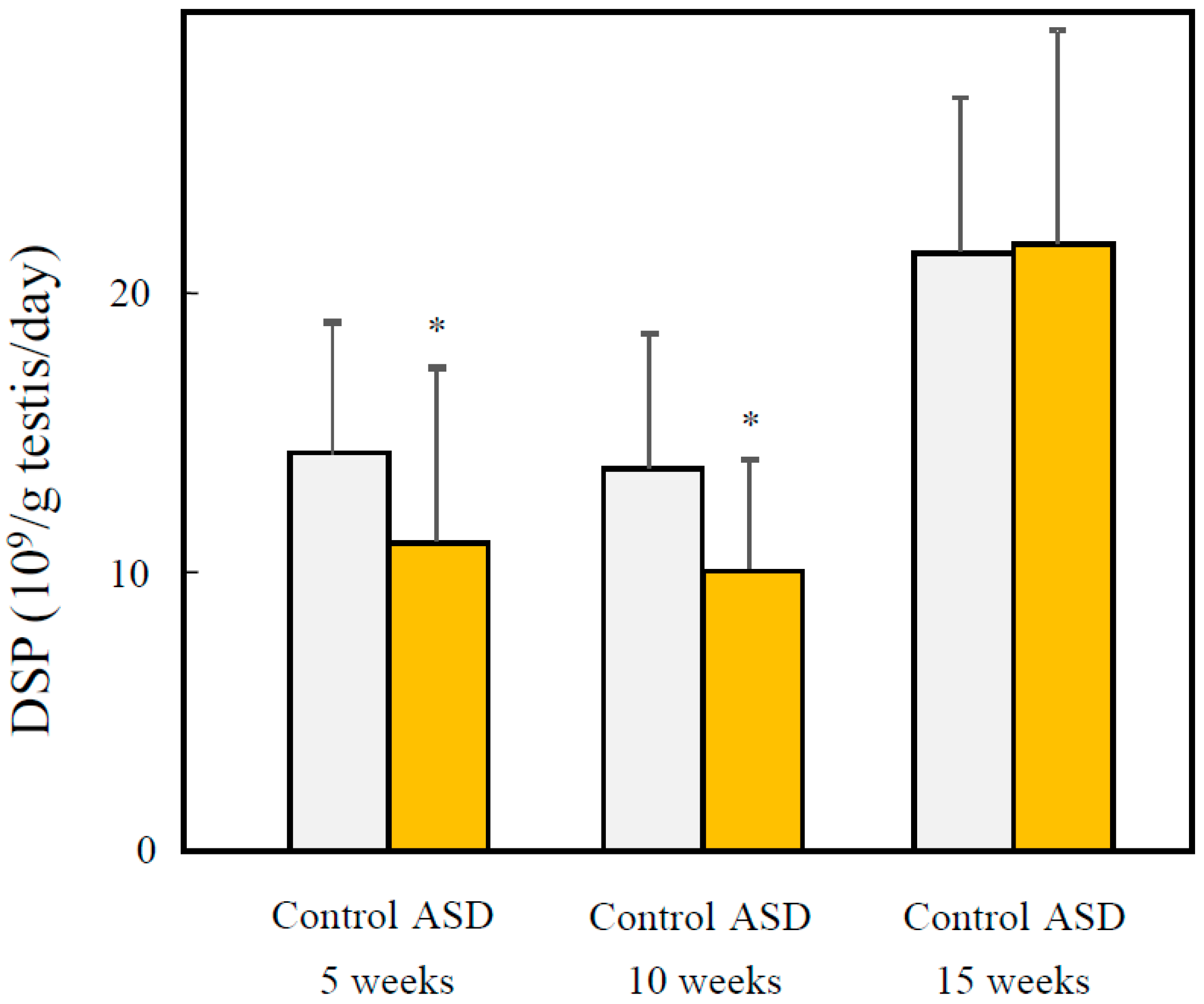
3.4. Effects of Fetal ASD Exposure on Sperm Production
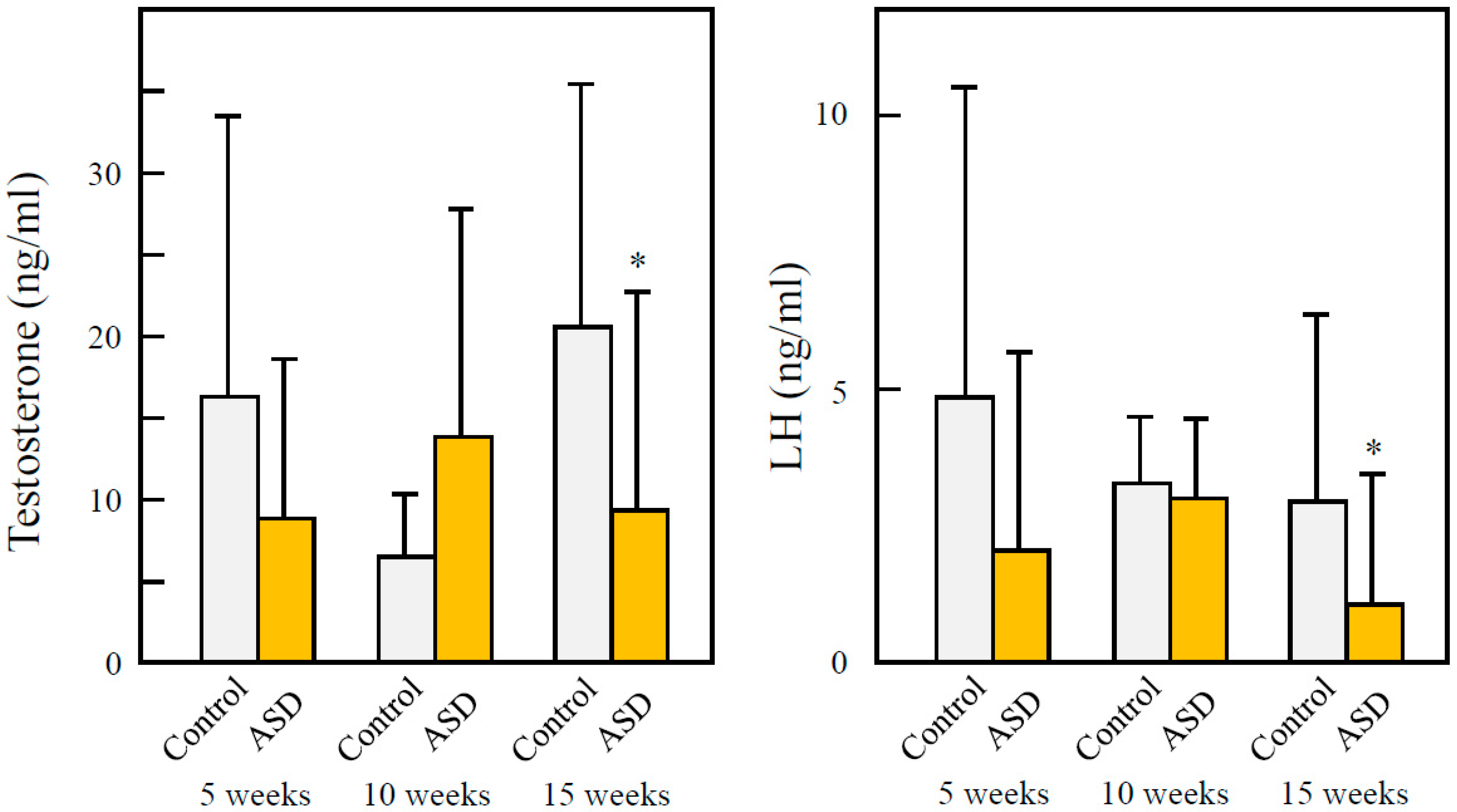
3.5. Effects of ASD on Serum Testosterone and LH
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ASD | Asian sand dust |
| DE | Diesel exhaust |
| DEP | Diesel exhaust particles |
| DSP | Daily sperm production |
| HE | hematoxylin and eosin |
| ICP-AES | inductively-coupled plasma atomic emission spectroscopy |
| LH | luteinizing hormone |
| LIDAR | Light Detection and Ranging |
| p.c. | post-coitum |
| SSR | Secondary sex ratio |
References
- Duce, R.A.; Unni, C.K.; Ray, B.J.; Prospero, J.M.; Merrill, J.T. Long-range atmospheric transport of soil dust from Asia to the tropical north pacific: Temporal variability. Science 1980, 209, 1522–1524. [Google Scholar] [CrossRef] [PubMed]
- Husar, R.B.; Tratt, D.M.; Schichtel, B.A.; Falke, S.R.; Li, F.; Jaffe, D.; Gass, S.; Gill, T.; Laulainen, N.S.; Lu, F.; et al. Asian dust events of April 1998. J. Geophys. Res. 2001, 106, 18316–18330. [Google Scholar] [CrossRef]
- Kim, B.G.; Han, J.S.; Park, S.U. Transport SO2 and aerosol over the Yellow Sea. Atmos. Environ. 2001, 35, 727–737. [Google Scholar] [CrossRef]
- Uno, I.; Eguchi, K.; Yumimoto, K.; Takemura, T.; Shimizu, A.; Uematsu, M.; Liu, Z.; Wang, Z.; Hara, Y.; Sugimoto, N. Asian dust transported one full circuit around the globe. Nat. Geosci. 2009, 2, 557–560. [Google Scholar] [CrossRef]
- Kanatani, K.T.; Ito, I.; Al-Delaimy, W.K.; Adachi, Y.; Mathews, W.C.; Ramsdell, J.W. Desert dust exposure is associated with increased risk of asthma hospitalization in children. Am. J. Respir. Crit. Care Med. 2010, 182, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Yamasaki, A.; Burioka, N.; Kurai, J.; Yoneda, K.; Yoshida, A.; Igishi, T.; Fukuoka, Y.; Nakamoto, M.; Takeuchi, H.; et al. Correlation between Asian dust storms and worsening asthma in western Japan. Allergol. Int. 2011, 60, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Lee, I.M.; Tsai, S.S.; Yang, C.Y. Correlation of Asian dust storm events with daily clinic visits for allergic rhinitis in Taipei, Taiwan. J. Toxicol. Environ. Health A 2006, 69, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Sato, T. The study on the change of symptoms in allergic rhinitis during Asian sand dust phenomenon. Jibi Ryensyou 2009, 102, 831–839. (In Japanese) [Google Scholar] [CrossRef]
- Meng, Z.; Lu, B. Dust events as a risk factor for daily hospitalization for respiratory and cardiovascular diseases in Minqin. China Atmos. Environ. 2007, 41, 7048–7058. [Google Scholar] [CrossRef]
- Kwon, H.J.; Cho, S.H.; Chun, Y.; Lagarde, F.; Pershagen, G. Effects of the Asian dust events on daily mortality in Seoul, Korea. Environ. Res. 2002, 90, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Yang, C.Y. Effects of Asian dust storm events on daily hospital admissions for cardiovascular disease in Taipei, Taiwan. J. Toxicol. Environ. Health A 2005, 68, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Sheen, P.C.; Chen, E.R.; Liu, Y.K.; Wu, T.N.; Yang, C.Y. Effects of Asian dust storm events on daily mortality in Taipei, Taiwan. Environ. Res. 2004, 95, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Ichinose, T.; He, M.; Song, Y.; Yoshida, Y.; Yoshida, S.; Nishikawa, M.; Takano, H.; Sun, G.; Shibamoto, T. Enhancement of OVA-induced murine lung eosinophilia by co-exposure to contamination levels of LPS in Asian sand dust and heated dust. Allergy Asthma Clin. Immunol. 2014. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Ichinose, T.; Song, Y.; Yoshida, Y.; Arashidani, K.; Yoshida, S.; Liu, B.; Nishikawa, M.; Takano, H.; Sun, G. Effects of two Asian sand dusts transported from the dust source regions of Inner Mongolia and northeast China on murine lung eosinophilia. Toxicol. Appl. Pharmacol. 2013, 272, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Hiyoshi, K.; Ichinose, T.; Nisikawa, M.; Takano, H.; Sugawara, I.; Takeda, K. Aggravating effect of natural sand dust on male reproductive function in mice. Reprod. Med. Biol. 2009, 8, 151–156. [Google Scholar] [CrossRef]
- Yoshida, S.; Sagai, M.; Oshio, S.; Umeda, T.; Ihara, T.; Sugamata, M.; Sugawara, I.; Takeda, K. Exposure to diesel exhaust affects the male reproductive system of mice. Int. J. Androl. 1999, 22, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Hiyoshi, K.; Ichinose, T.; Takano, H.; Oshio, S.; Sugawara, I.; Takeda, K.; Shibamoto, T. Effect of nanoparticles on the male reproductive system of mice. Int. J. Androl. 2009, 32, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Ono, N.; Oshio, S.; Niwata, Y.; Yoshida, S.; Tsukue, N.; Sugawara, I.; Takano, H.; Takeda, K. Prenatal exposure to diesel exhaust impairs mouse spermatogenesis. Inhal. Toxicol. 2007, 19, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Hiyoshi, K.; Oshio, S.; Takano, H.; Takeda, K.; Ichinose, T. Effects of fetal exposure to carbon nanoparticles on reproductive function in male offspring. Fertil. Steril. 2010, 93, 1695–1699. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.L.; Gottlieb, M.B.; Stampnitzky, J.R. Reduced ratio of male to female births in several industrial countries: A sentinel health indicator? JAMA 1998, 279, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Miraglia, S.G.; Veras, M.M.; Amato-Lourenço, L.F.; Rodrigues-Silva, F.; Saldiva, P.H. Follow-up of the air pollution and the human male-to-female ratio analysis in Sao Paulo, Brazil: A times series study. BMJ Open 2013, 3, e002552. [Google Scholar] [CrossRef] [PubMed]
- Joyce, K.L.; Porcelli, J.; Cooke, P.S. Neonatal goitrogen treatment increases adult testis size and sperm production in the mouse. J. Androl. 1993, 14, 448–455. [Google Scholar] [PubMed]
- Allan, B.B.; Brant, R.; Seidel, J.E.; Jarrell, J.F. Declining sex ratios in Canada. CMAJ 1997, 156, 37–41. [Google Scholar] [PubMed]
- Davis, D.L.; Webster, P.; Stainthorpe, H.; Chilton, J.; Jones, L.; Doi, R. Declines in sex ratio at birth and fetal deaths in Japan, and in U.S. whites but not African Americans. Environ. Health Perspect. 2007, 115, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N. Decreased number of sperms and Sertoli cells in mature rats exposed to diesel exhaust as fetuses. Toxicol. Lett. 2005, 155, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Lui, W.Y.; Mruk, D.; Lee, W.M.; Cheng, C.Y. Sertoli cell tight junction dynamics: Their regulation during spermatogenesis. Biol. Reprod. 2003, 68, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
| Variable | Control | ASD |
|---|---|---|
| Fertility (%) | 95% | 80% |
| Gestation length (day) | 18.6 ± 0.2 | 18.8 ± 0.3 |
| Litter size | 14.6 ± 1.9 | 13.3 ± 3.6 |
| Sex ratio at birth | 0.542 | 0.429 * |
| Weeks | Pups Number | Body Weight (g) | Testis (mg) | Epididymis (mg) | ||||
|---|---|---|---|---|---|---|---|---|
| Control | ASD | Control | ASD | Control | ASD | Control | ASD | |
| 5 | 16 | 12 | 33.9 ± 2.8 | 33.0 ± 3.6 | 118.2 ± 9.3 | 102.1 ± 25.0 ** | 31.3 ± 4.5 | 27.8 ± 4.0 * |
| 10 | 16 | 13 | 43.7 ± 3.2 | 44.8 ± 2.4 | 145.7 ± 23.8 | 147.9 ± 11.4 | 54.0 ± 5.3 | 54.8 ± 4.8 |
| 15 | 16 | 13 | 46.4 ± 3.4 | 46.9 ± 4.6 | 159.9 ± 27.7 | 147.2 ± 36.3 | 64.5 ± 7.2 | 57.9 ± 8.1 * |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, S.; Ichinose, T.; Arashidani, K.; He, M.; Takano, H.; Shibamoto, T. Effects of Fetal Exposure to Asian Sand Dust on Development and Reproduction in Male Offspring. Int. J. Environ. Res. Public Health 2016, 13, 1173. https://doi.org/10.3390/ijerph13111173
Yoshida S, Ichinose T, Arashidani K, He M, Takano H, Shibamoto T. Effects of Fetal Exposure to Asian Sand Dust on Development and Reproduction in Male Offspring. International Journal of Environmental Research and Public Health. 2016; 13(11):1173. https://doi.org/10.3390/ijerph13111173
Chicago/Turabian StyleYoshida, Seiichi, Takamichi Ichinose, Keiichi Arashidani, Miao He, Hirohisa Takano, and Takayuki Shibamoto. 2016. "Effects of Fetal Exposure to Asian Sand Dust on Development and Reproduction in Male Offspring" International Journal of Environmental Research and Public Health 13, no. 11: 1173. https://doi.org/10.3390/ijerph13111173





