Health Effects Due to Radionuclides Content of Solid Minerals within Port of Richards Bay, South Africa
Abstract
:1. Introduction
2. Materials and Methods
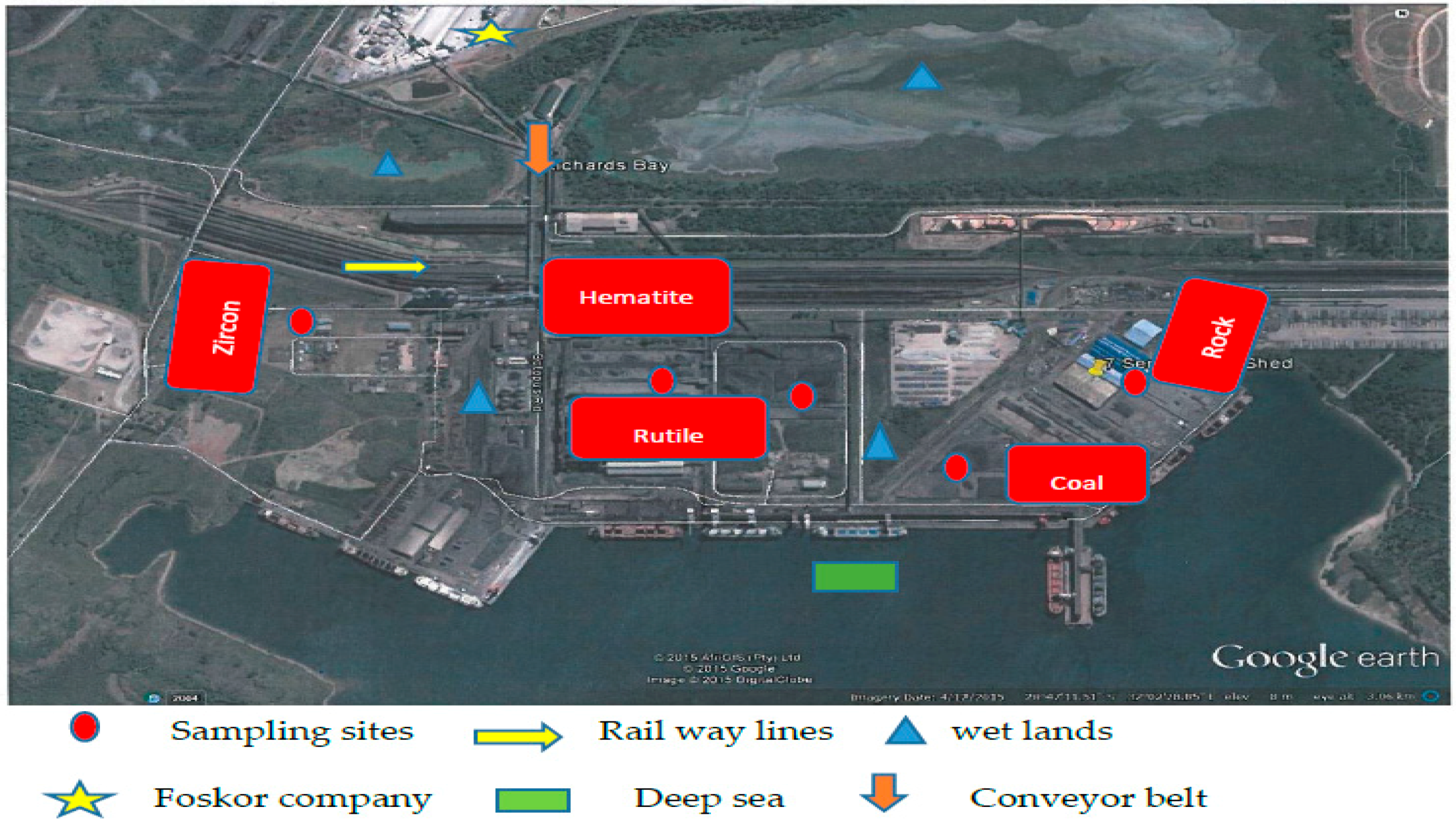
2.1. Review of Richards Bay
2.2. Sampling Techniques
2.3. Sample Preparation
2.4. Experimental Techniques
3. Radiological Health Hazards Assessment
3.1. Absorbed Dose Rate Determination
3.2. Radium Equivalent Dose Determination
3.3. Annual Effective Dose Determination
3.4. Effective Dose Rate to Different Organs (Dorgan)
3.5. Radiological Hazard Indexes
3.6. Excess Lifetime Cancer Risk (ELCR) Determination
4. Results
5. Discussion
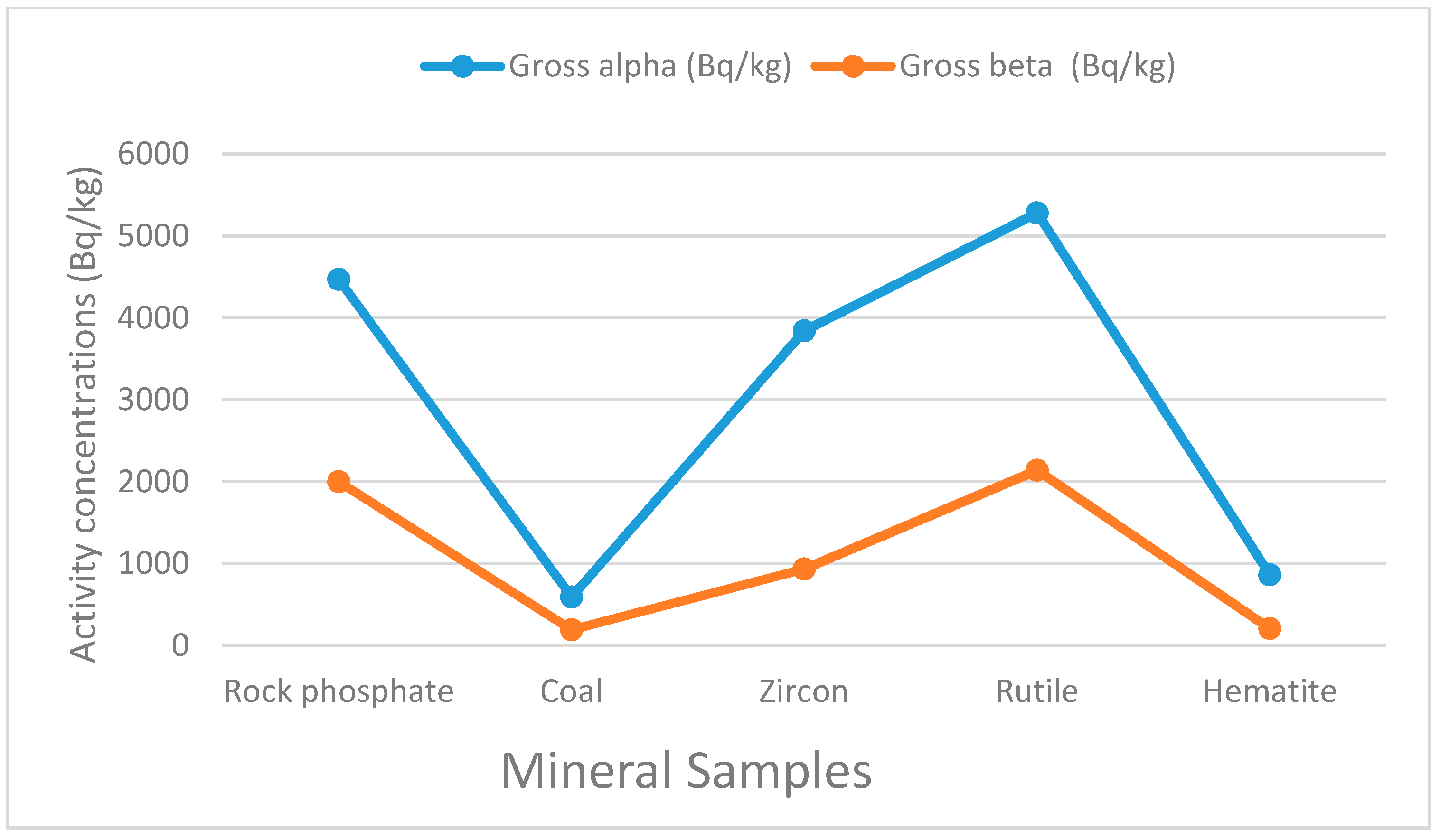
5.1. Gross Alpha and Beta Concentrations in Rock Phosphate, Coal, Zircon, Rutile and Hematite
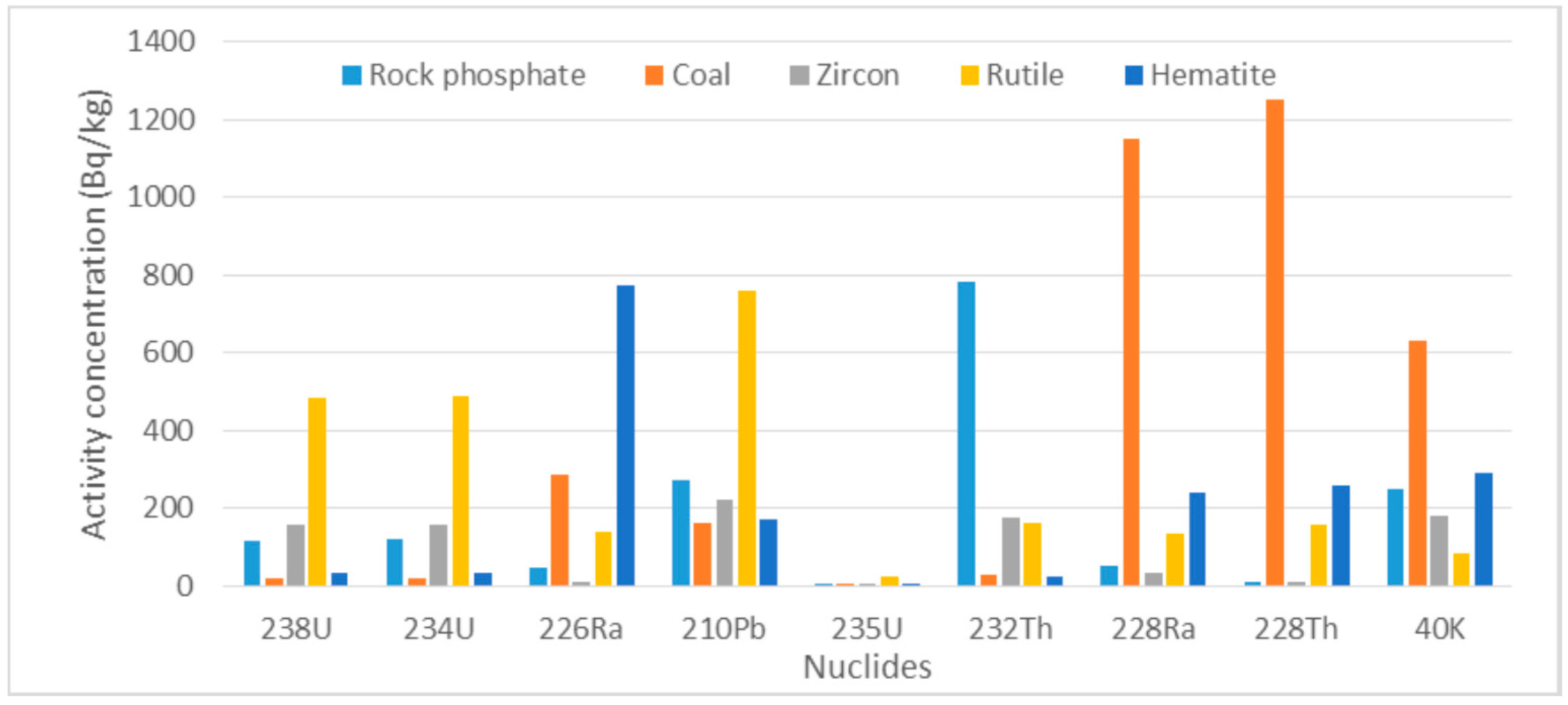
5.2. Average Activity Concentrations of Radionuclides in Mineral Ores in This Study Area
5.3. Comparison of Average Activity Concentrations of Radionuclides in Mineral Ore from This Study Area and Other Countries
5.4. Assessment of Radiological Health Effects
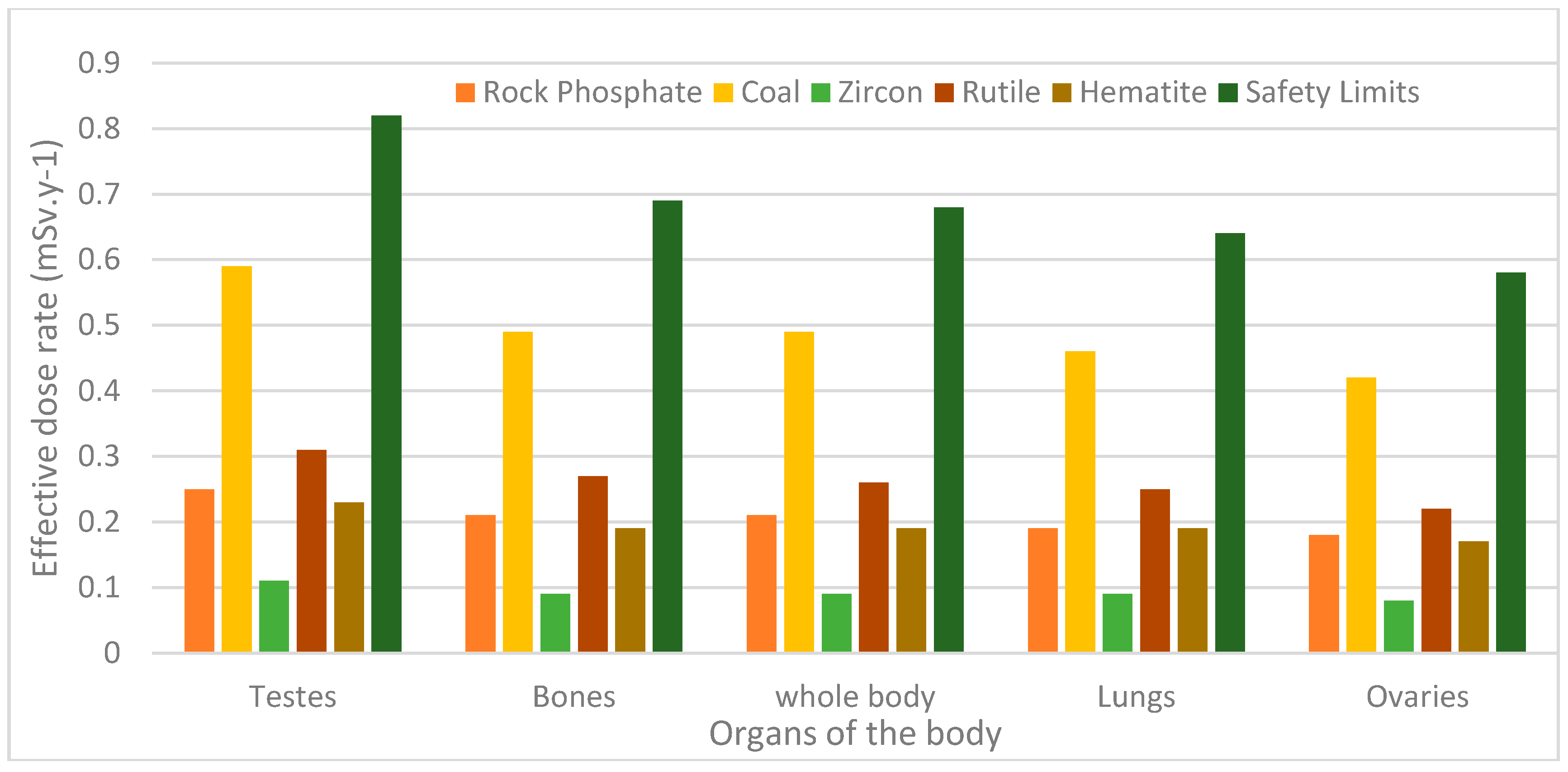
5.5. Effective Dose Rate to Various Parts of the Body
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and Effects of Ionizing Radiation. In UNSCEAR Report to the General Assembly with Scientific Annexes; United Nations Sales Publication: New York, NY, USA, 2000; Volume 1. [Google Scholar]
- Eisenbud, M.; Gesell, T. Environmental Radioactivity from Natural, Industrial and Military Sources, 4th ed.; Academic Press: London, UK, 1997. [Google Scholar]
- Klement, A.W. CRC Handbook of Environmental Radiation; CRC Press, Inc.: Boca Raton, FL, USA, 1982. [Google Scholar]
- Watson, S.J.; Jones, A.L.; Oatway, W.B.; Hunghes, J.S. Ionizing Radiation Exposure of UK Population: 2005 Review; Health Protection Agency, Center for Radiation, Chemical and Environmental Hazards, Radiation Protection Division: Chilton/Didcot/Oxfordshire, UK, 2005.
- National Council on Radiation Protection and Measurements. Environmental Radiation Measurements; NCRP Report No. 50 NCRP; National Council on Radiation Protection and Measurements: Bethesda, MD, USA, 1977. [Google Scholar]
- World Nuclear Association. Nuclear Radiation and Health Effects- World Nuclear Association. Available online: http://www.world-nuclear.org/information-library/safety-and-security/radiation-and-health/naturally-occurring-radioactive-materials-norm.aspx (accessed on 15 July 2016).
- International Atomic Energy Agency. IAEA-TECDOC-1472. In Proceedings of the International Conference on Naturally Occurring Radioactive Materials (NORM IV), Szczyrk, Poland, 17–21 May 2004; IAEA: Vienna, Austria, 2005. [Google Scholar]
- International Atomic Energy Agency (IAEA). Extent of Environmental Contamination by Naturally Occurring Radioactive Material (NORM) and Technological Options for Mitigation; Technical Reports Series No. 419, STI/DOC/010/419 (ISBN: 9201125038); International Atomic Energy Agency (IAEA): Vienna, Austria, 2003; p. 90. [Google Scholar]
- Austrialian Radiation Protection and Nuclear Safety Agency (ARPNSA). Radiation Exposure in the Transport of Heavy Mineral Sands; Report for the Austrialian Radiation Protection and Nuclear Safety Agency (ARPNSA); Calytrix Consulting Pty Ltd.: Perth, Australia, 2008. [Google Scholar]
- International Atomic Energy Agency. Naturally Occurring Radioactive Material (NORM VII). STI/PUB/1664, (ISBN: 978-92-0-104014–5), IAEA Technical Report 419. In Proceedings of the Seventh International Symposium, Beijing, China, 22–26 April 2013; p. 84.
- Transnet National Port Authority. The Port Development Frame Work Plans; Transnet National Port Authority: Johannesburg, South Africa, 2014; pp. 3–5. [Google Scholar]
- Williams, G.E.; Steenkamp, J.D. Heavy Minerals Processing at Richards Bay Minerals; South African Pyrometallurgy; Jones, R.T., Ed.; South African Institute of Mining and Metallurgy: Johannesburg, South Africa, 2006. [Google Scholar]
- Richards Bay Coal Terminal (RBCT). ISH Energy South African Coal Export Conference; Richards Bay Coal Terminal (RBCT): KwaZulu-Natal, South Africa, 2015. [Google Scholar]
- The Geographical Map of Richards Bay Precinct. Available online: https://www.google.co.za/maps/place/Transnet+Port+Terminals+Richards+Bay/@-28.7852243,32.0284497,3078m/data=!3m1!1e3!4m5!3m4!1s0x0:0xc4a3a0544935ebc6!8m2!3d-28.7852243!4d32.0284497 (accessed on 15 July 2016).
- International Atomic Energy Agency. Measurement of Radionuclides in Food and Environment; Technical Report Series No. 295; International Atomic Energy Agency: Vienna, Australia, 1989. [Google Scholar]
- Sing, S.; Rani, A.; Kumar Mahajan, R. 226Ra, 232Th and 40K analysis in soil samples from some areas of Punjab and Himachal Pradesh, India using gamma ray spectrometry. Radiat. Meas. 2005, 39, 431–439. [Google Scholar] [CrossRef]
- Radio-Analysis Quality Management System, Based on ISO/IEC Standard 17025 (SANAS)-Accreditation Schedule T0111. Available online: http://www.sanas.co.za/af-directory/calibration_list.php?s_lab_no=&s_lab_name=necsa&s_disciplines=&s_accreditation_status=Accredited&s_phys_city=&s_province (accessed on 16 July 2016).
- Radiation; Effects and Sources, United Nations Environmental Programme 2016. Available online: http://drustage.unep.org/newyork/radiation-effects-and-sources (accessed on 16 July 2016).
- Masok, F.B.; Masiteng, P.L.; Jwanbot, D.I. Natural radioactivity concentrations and effective dose rate from Jostin mining dumpsites in Ray-field, Nigeria. J. Environ. Earth Sci. 2015, 5, 51–55. [Google Scholar]
- Veiga, R.; Sanches, N.; Anjos, R.M.; Macario, K.; Bastos, J.; Iguatemy, M.; Aguiar, J.G.; Santos, A.M.A.; Mosquera, B.; Carvalho, C.; et al. Measurement of natural radioactivity in Brazilian beach sands. Radiat. Meas. 2006, 14, 189–196. [Google Scholar] [CrossRef]
- Darwish, D.A.E.; Abul-Nasr, K.T.M.; El-Khayatt, A.M. The assessment of natural radioactivity and its associated radiological hazards and dose parameters in granite samples from South Sinai, Egypt. J. Radiat. Res. Appl. Sci. 2015, 8, 17–25. [Google Scholar] [CrossRef]
- Kessaratikoon, P.; Awaekechi, S. Natural radioactivity measurement in soil samples collected from municipal areaof Hat Yai District in Songkhla Province. King Mongkut’s Inst. of Technol. Ladkrabang Sci. J. 2008, 8, 52–58. [Google Scholar]
- O’Brien, K.; Sanna, R. The distribution of absorbed dose rates in humans from exposure to environmental gamma rays. Health Phys. 1976, 30, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Nada, A. Evaluation of natural radionuclides at Um-Greifat area, eastern desert of Egypt. Appl. Radiat. Isot. 2003, 58, 275–280. [Google Scholar] [CrossRef]
- Arafat, W. Specific activity and hazards of granite samples collected from the Eastern Desert of Egypt. J. Environ. Radioact. 2004, 75, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Statistic South Africa. Midyear Population Estimates; Statistic South Africa: Gauteng, South Africa, 2015.
- International Commission for Radiological Protection (ICPR). Recommendations of International Commissions on Radiation Protection; Annals of ICRP 2007; ICPR Publication: London, UK, 2007; p. 103. [Google Scholar]
| Organ | C-Factor |
|---|---|
| Testes | 8.2 × 10−1 |
| Bone marrow | 6.9 × 10−1 |
| Whole body | 0.68 × 10−1 |
| Lungs | 6.4 × 10−1 |
| Ovaries | 5.8 × 10−1 |
| NORM | Mean Activity Concentrations of Nuclides in Minerals (Bq·kg−1) | |||||
|---|---|---|---|---|---|---|
| Origin | Nuclides | Rock Phosphate | Coal | Zircon | Rutile | Hematite |
| 238U Series | 238U | 118.00 ± 7.00 | 18.40 ± 1.20 | 158.00 ± 9.00 | 485.00 ± 84.00 | 31.50 ± 1.90 |
| 234U | 119.00 ± 7.00 | 18.50 ± 1.20 | 159.00 ± 9.00 | 489.00 ± 85.00 | 31.70 ± 1.90 | |
| 226Ra | 44.50 ± 8.50 | 285.00 ± 31.00 | 11.00 ± 4.00 | 141.00 ± 10.00 | 772.00 ± 27.00 | |
| 210Pb | 270.00 ± 1.00 | 160.00 ± 0.01 | 220.00 ± 0.01 | 759.00 ± 106.00 | 170.00 ± 0.01 | |
| 235U Series | 235U | 5.45 ± 0.31 | 0.85 ± 0.06 | 7.28 ± 0.41 | 22.30 ± 3.90 | 1.45 ± 0.09 |
| 232Th series | 232Th | 783.00 ± 24.00 | 27.80 ± 1.50 | 174.00 ± 5.00 | 163.00 ± 6.00 | 23.90 ± 2.80 |
| 228Ra | 51.00 ± 1.00 | 1150 ± 80.00 | 32.00 ± 0.01 | 133.00 ± 16.00 | 239.00 ± 27.00 | |
| 228Th | 11.00 ± 4.00 | 1250.00 ± 120 | 10.00 ± 4.60 | 155.00 ± 12.00 | 258.00 ± 42.00 | |
| Primordial | 40K | 240.00 ± 1.00 | 630.00 ± 0.01 | 180 ± 0.01 | 83.00 ± 39.00 | 290.00 ± 0.01 |
| Gross alpha | 4470.00 ± 940.00 | 593.00 ± 179.00 | 3840.00 ± 340 | 5280.00 ± 430.00 | 860.00 ± 192.00 | |
| Gross beta | 2000.00 ± 120.00 | 193.00 ± 20.00 | 933.00 ± 36.00 | 2140 .00 ± 50.00 | 207.00 ± 20.00 | |
| Minerals/NORMs | Mean Activity of Series (Bq·kg−1) | |||
|---|---|---|---|---|
| 226Ra | 235U | 232Th | 40K | |
| Rock phosphate | 137.63 ± 5.88 | 5.45 ± 0.31 | 281.67 ± 9.67 | 250.00 ± 0.01 |
| Coal | 120.48 ± 8.35 | 0.85 ± 0.06 | 809.27 ± 43.17 | 630.00 ± 0.01 |
| Zircon | 137.00 ± 5.50 | 7.28 ± 0.41 | 72.00 ± 3.20 | 180.00 ± 0.01 |
| Rutile | 468.50 ± 71.25 | 22.30 ± 3.90 | 150.33 ± 11.33 | 83.00 ± 39.00 |
| Hematite | 251.30 ± 7.70 | 1.45 ± 0.09 | 173.63 ± 23.93 | 290.00 ± 0.01 |
| Country | 238U (Bq·kg−1) | 226Ra (Bq·kg−1) | 232Th (Bq·kg−1) |
|---|---|---|---|
| South Africa (++) | 118 | 44.5 | 783 |
| USA (Western) (+,*) | 259–3700 | 1540 | 3.7–22 |
| USA (Florida) (+,*) | 1500–1900 | 1800 | 16–59 |
| Brazil (+,*) | 114–880 | 330–700 | 204–753 |
| Chile (+,*) | 40 | 40 | 30 |
| Algeria (+,*) | 1295 | 1150 | 56 |
| Morocco (+,*) | 1500–1700 | 1500–1700 | 10–200 |
| Senegal (+,*) | 1332 | 1370 | 67 |
| Tunisia (+,*) | 590 | 520 | 92 |
| Egypt (+,*) | 1520 | 1370 | 26 |
| Jordan (+,*) | 1300–1850 | 28–90 | NA |
| Australia (+,*) | 15–900 | 28–90 | 5–47 |
| Country | Activity Concentrations of Nuclides (Bq·kg−1) | |||||
|---|---|---|---|---|---|---|
| 238U | 226Ra | 210Pb | 232Th | 228Ra | 40K | |
| South Africa × | 18.4 | 285 | 160 | 27.80 | 1150 | 630 |
| USA ** | 6–73 | 8.9–59 | 12–78 | 4–21 | N+ | N+ |
| UK ** | 7–19 | 8–22 | N+ | 7–19 | N+ | 55–314 |
| Hungary ** | 20–480 | NA | N+ | N+ | 12–97 | 30–384 |
| China ** | Range 10–25 | Av. 25 | ||||
| Greece ** | 111–390 | 44–206 | 59–205 | N+ | 9–41 | N+ |
| Romania ** | Av. 80.00 | Av. 126 | Av. 210 | Av. 62 | N+ | N+ |
| Samples | ADrate (nGy·h−1) | AEDR (mSv·y−1) | ELCR (mSv·y−1) | Radiological Hazards Indexes (Bq·kg−1) | ||
|---|---|---|---|---|---|---|
| Raeq | Hex | Hin | ||||
| Rock phosphate | 248.93 | 298.12 | 0.95 | 560.42 | 1.51 | 1.88 |
| Coal | 584.48 | 711.77 | 2.24 | 1328.12 | 3.58 | 3.91 |
| Zircon | 115.51 | 134.05 | 0.44 | 254.36 | 0.69 | 1.06 |
| Rutile | 3131.26 | 358.09 | 1.19 | 690.11 | 1.86 | 3.13 |
| Hematite | 236.12 | 275.59 | 0.90 | 522.79 | 1.41 | 2.09 |
| Samples/Organs | Effective Dose Rate (mSv·y−1) | ||||
|---|---|---|---|---|---|
| Testes | Bones | Whole | Lungs | Ovaries | |
| Rock phosphate | 0.25 | 0.21 | 0.21 | 0.19 | 0.18 |
| Coal | 0.59 | 0.49 | 0.49 | 0.46 | 0.42 |
| Zircon | 0.11 | 0.09 | 0.09 | 0.09 | 0.08 |
| Rutile | 0.31 | 0.27 | 0.26 | 0.25 | 0.22 |
| Hematite | 0.23 | 0.19 | 0.19 | 0.19 | 0.17 |
| Permissible limit [21] | 0.82 | 0.69 | 0.68 | 0.64 | 0.58 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masok, F.B.; Masiteng, P.L.; Mavunda, R.D.; Maleka, P.P. Health Effects Due to Radionuclides Content of Solid Minerals within Port of Richards Bay, South Africa. Int. J. Environ. Res. Public Health 2016, 13, 1180. https://doi.org/10.3390/ijerph13121180
Masok FB, Masiteng PL, Mavunda RD, Maleka PP. Health Effects Due to Radionuclides Content of Solid Minerals within Port of Richards Bay, South Africa. International Journal of Environmental Research and Public Health. 2016; 13(12):1180. https://doi.org/10.3390/ijerph13121180
Chicago/Turabian StyleMasok, Felix B., Paulus L. Masiteng, Risimati D. Mavunda, and Peane P. Maleka. 2016. "Health Effects Due to Radionuclides Content of Solid Minerals within Port of Richards Bay, South Africa" International Journal of Environmental Research and Public Health 13, no. 12: 1180. https://doi.org/10.3390/ijerph13121180





