Subchronic Toxicities of HZ1006, a Hydroxamate-Based Histone Deacetylase Inhibitor, in Beagle Dogs and Sprague-Dawley Rats
Abstract
:1. Introduction
2. Materials and Methods
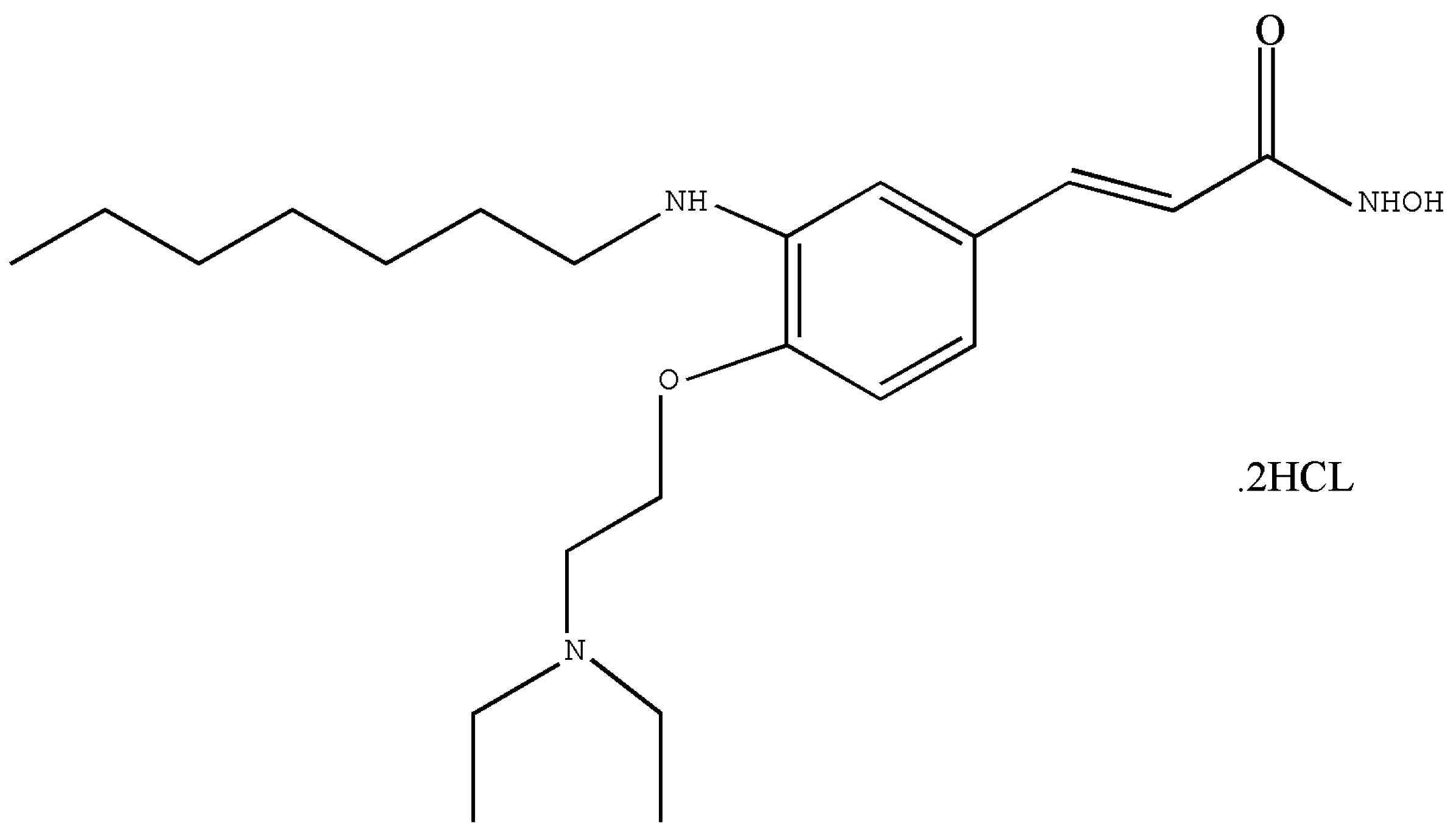
2.1. Test Article
2.2. Animals
2.3. Doses and Treatment Plans
2.3.1. Subchronic Toxicity Test in Beagle Dogs
2.3.2. Subchronic Toxicity Test in SD Rats
2.4. Clinical Symptoms Observed on Dogs and Rats in Repeated Dose Toxicity Studies
2.5. Clinical Examination Parameters
2.5.1. Electrocardiogram (ECG)
2.5.2. Hematology
2.5.3. Serum Biochemistry
2.5.4. Urinalysis
2.5.5. Bone Marrow Examination
2.5.6. Histopathology
2.6. Toxicokinetics
2.7. Statistical Analysis
3. Results
3.1. Subchronic Toxicology Study in Dogs
3.1.1. Clinical Observation
3.1.2. Hematology
3.1.3. Clinical Chemistry
3.1.4. Urinalysis
3.1.5. ECG Parameters
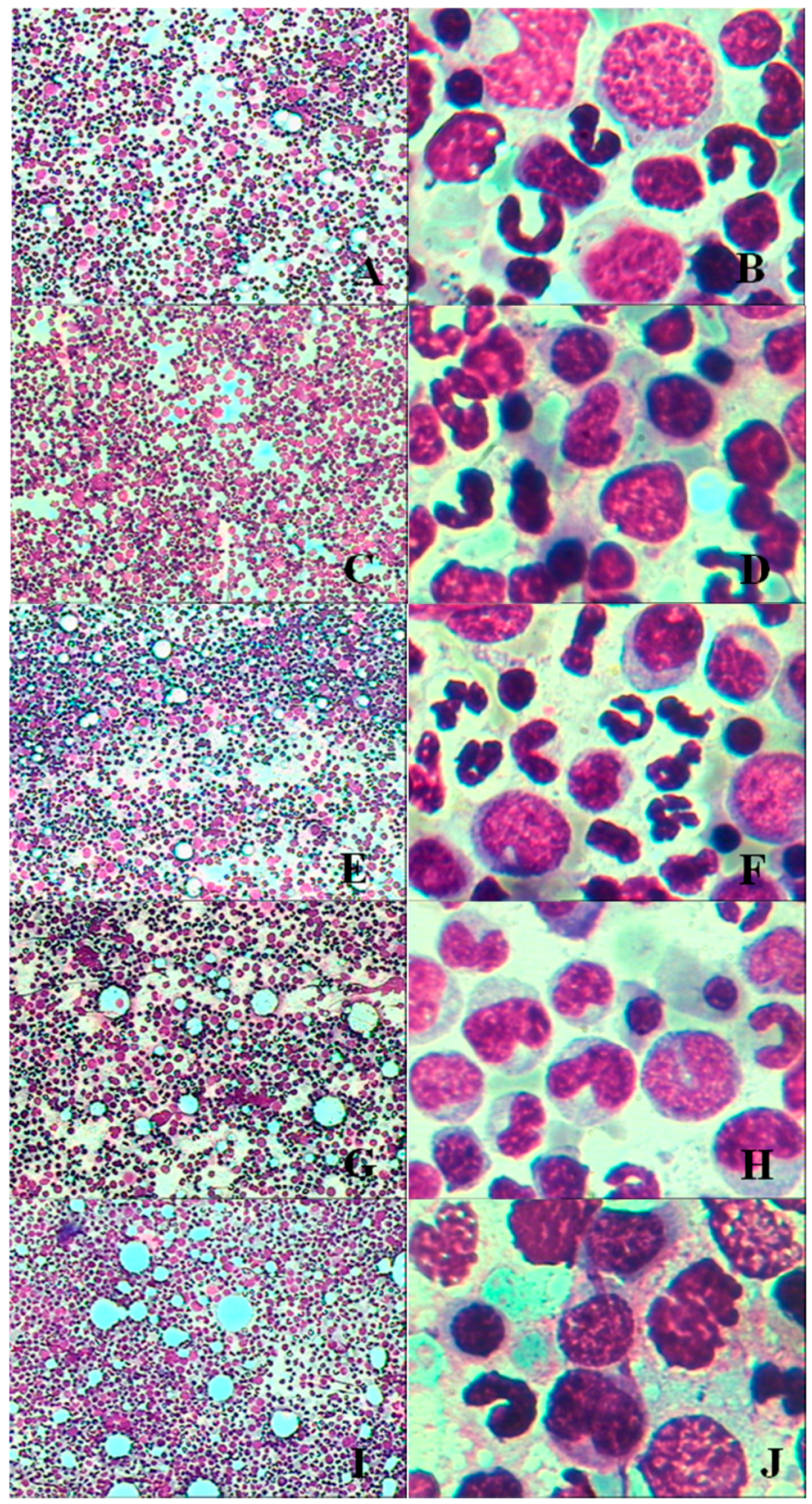
3.1.6. Bone Marrow Examination
3.1.7. Necropsy
Gross Pathology and Organ Weights
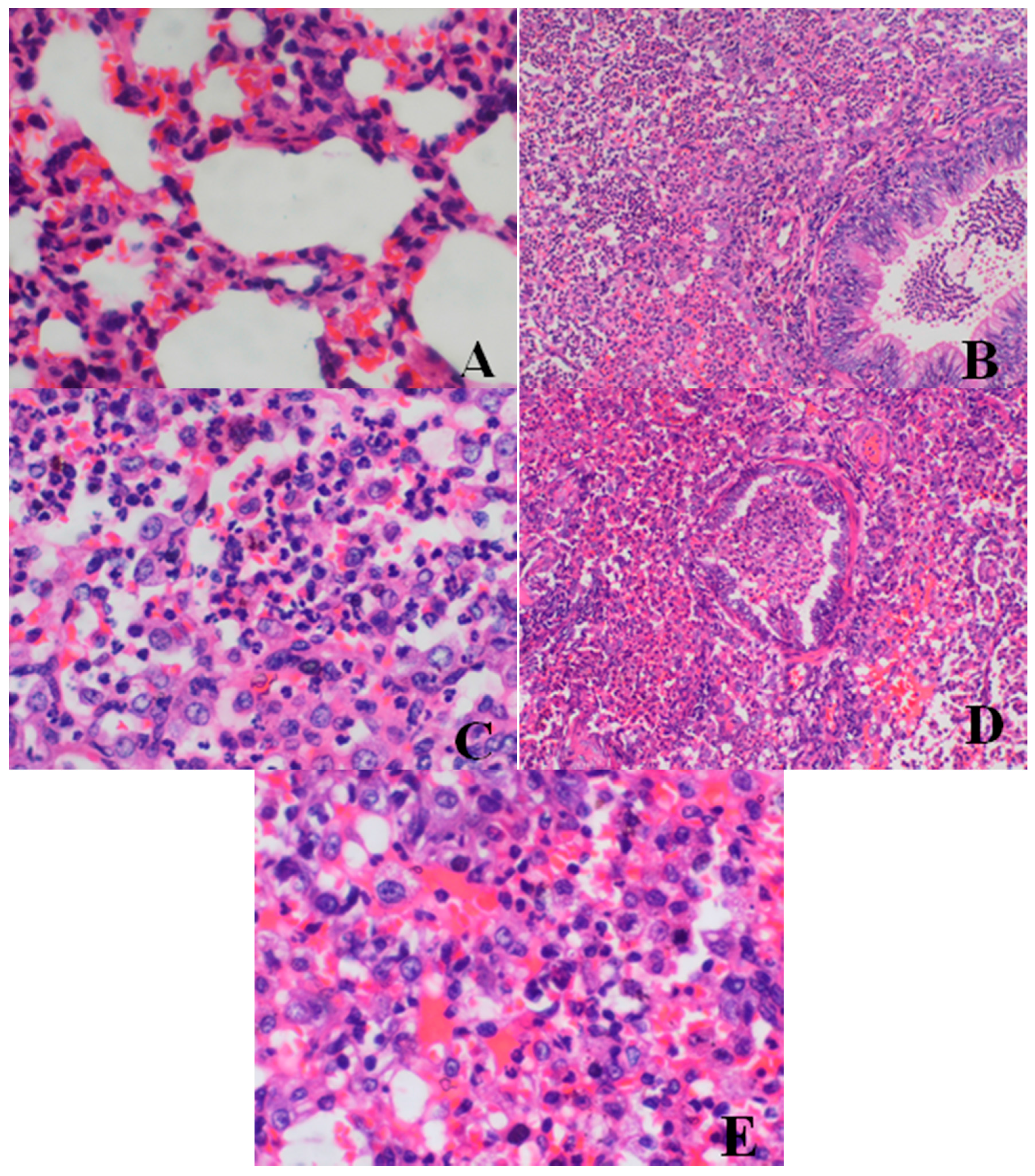
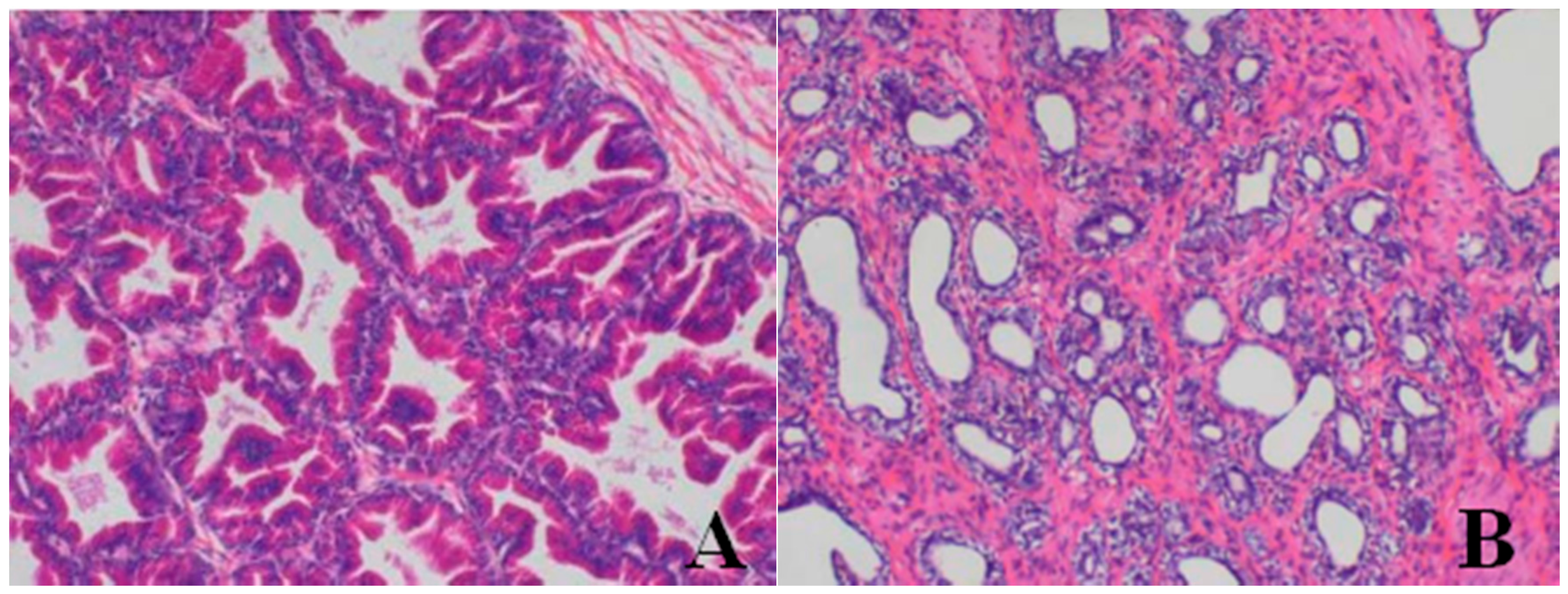
Histopathological Findings
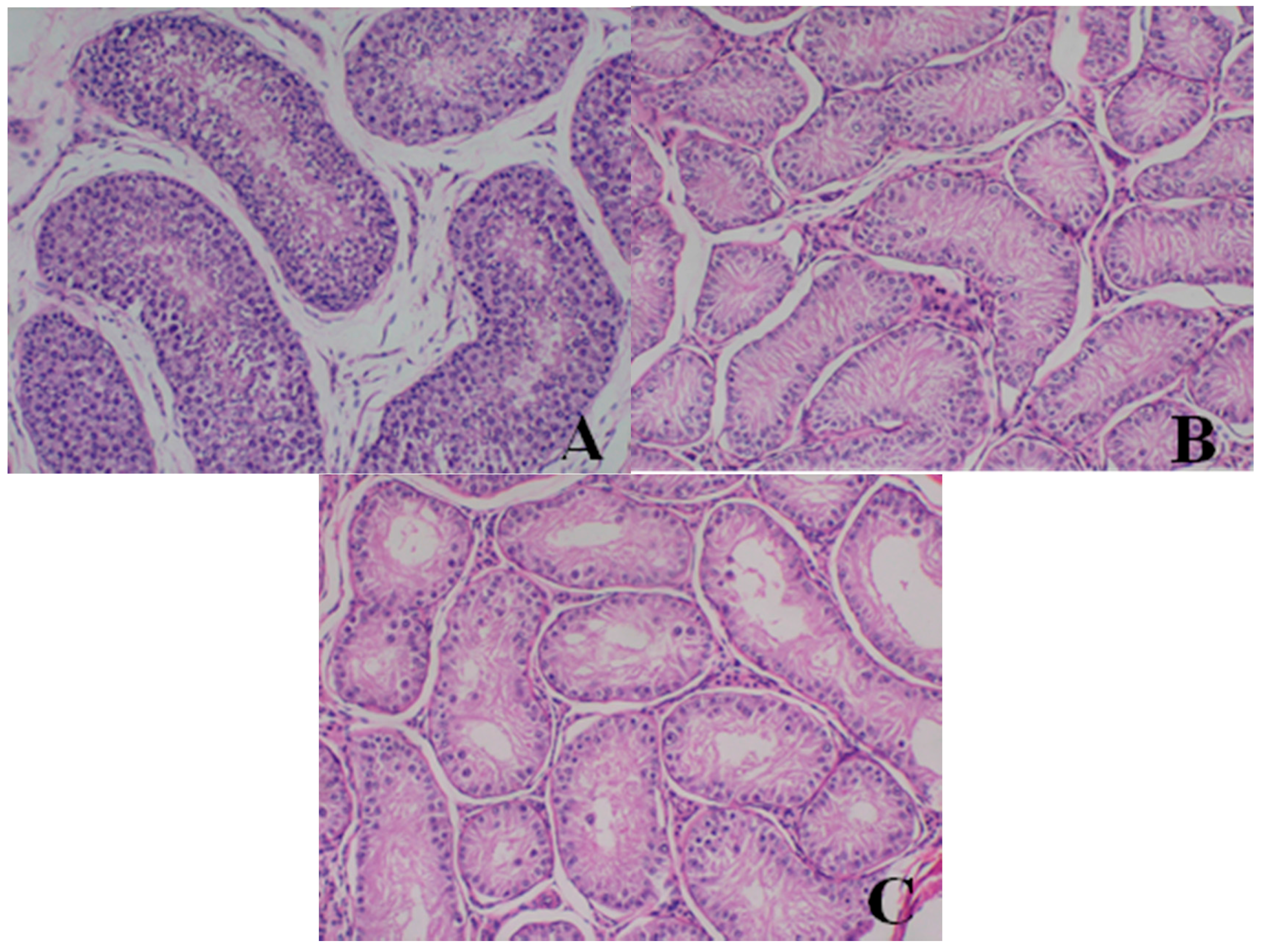
Male reproductive organs (testis, epididymis and prostate)
- Testis: The numbers of seminiferous tubules decreased in dogs belonging to the 80- and 40-mg/kg dose groups, while the tubule lumen decreased and had reduced numbers and types of spermatogenic cells when compared with the control group. Only spermatogonia and some primary and secondary spermatocytes were observed, and there were no mature sperm compared with the control group.
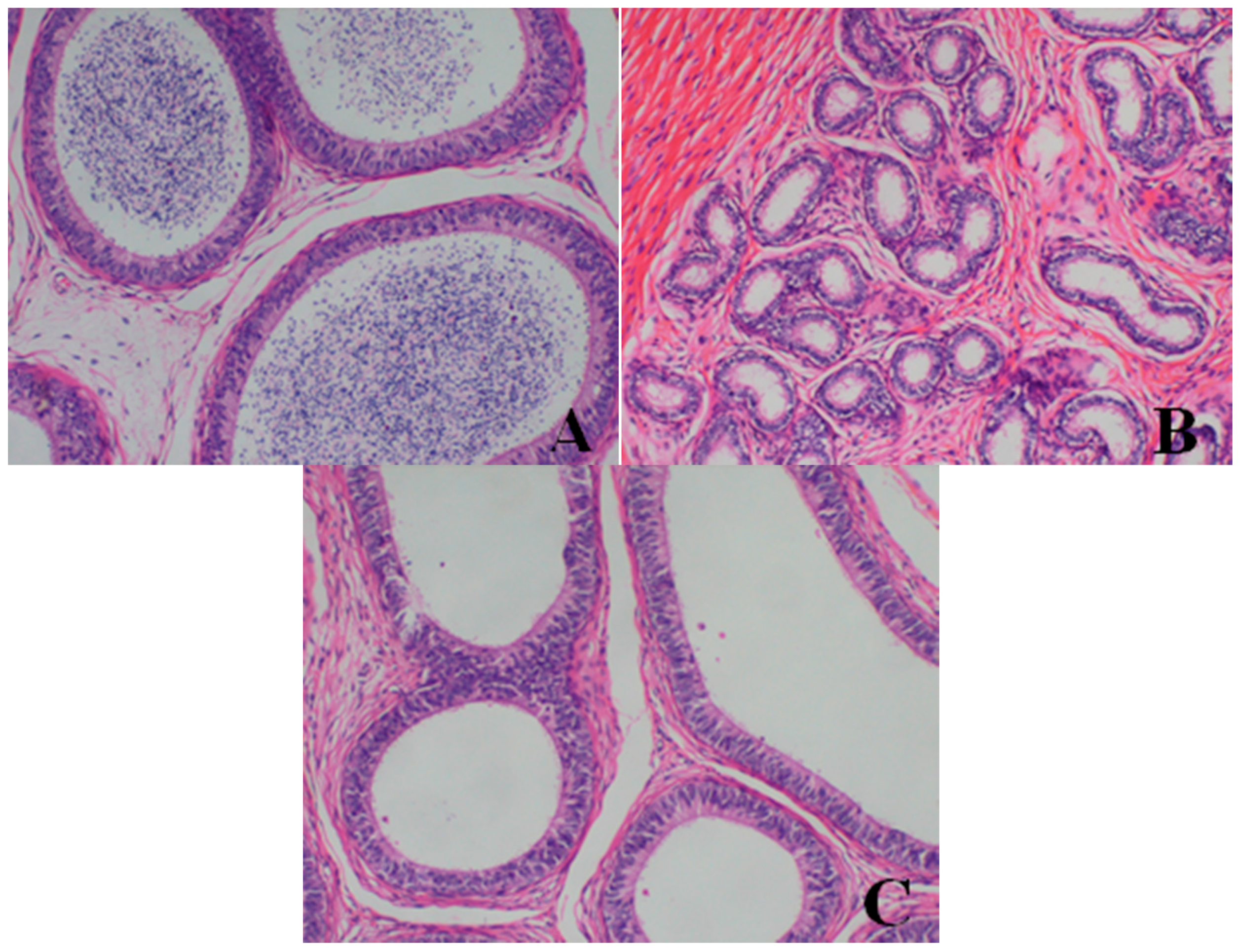
- Epididymis: When compared with the control group, there was a notable lack of mature sperm cells and secretions in the epididymal lumen, as well as a decrease in epididymis lumen size, an irregular shape of the epididymal lumen and an increase in the connective tissue in the interstitium observed in dogs in the 80- and 40-mg/kg dose groups.
- Prostate: When compared with the control group, a reduction in the numbers and size, a thinner epithelium, narrower glandular cavity and increase in the connective tissue in the interstitium were observed in the prostatic acini of dogs in the 80- and 40-mg/kg dose groups.
3.1.8. Toxicokinetics
3.2. Subchronic Toxicology Study on Rats
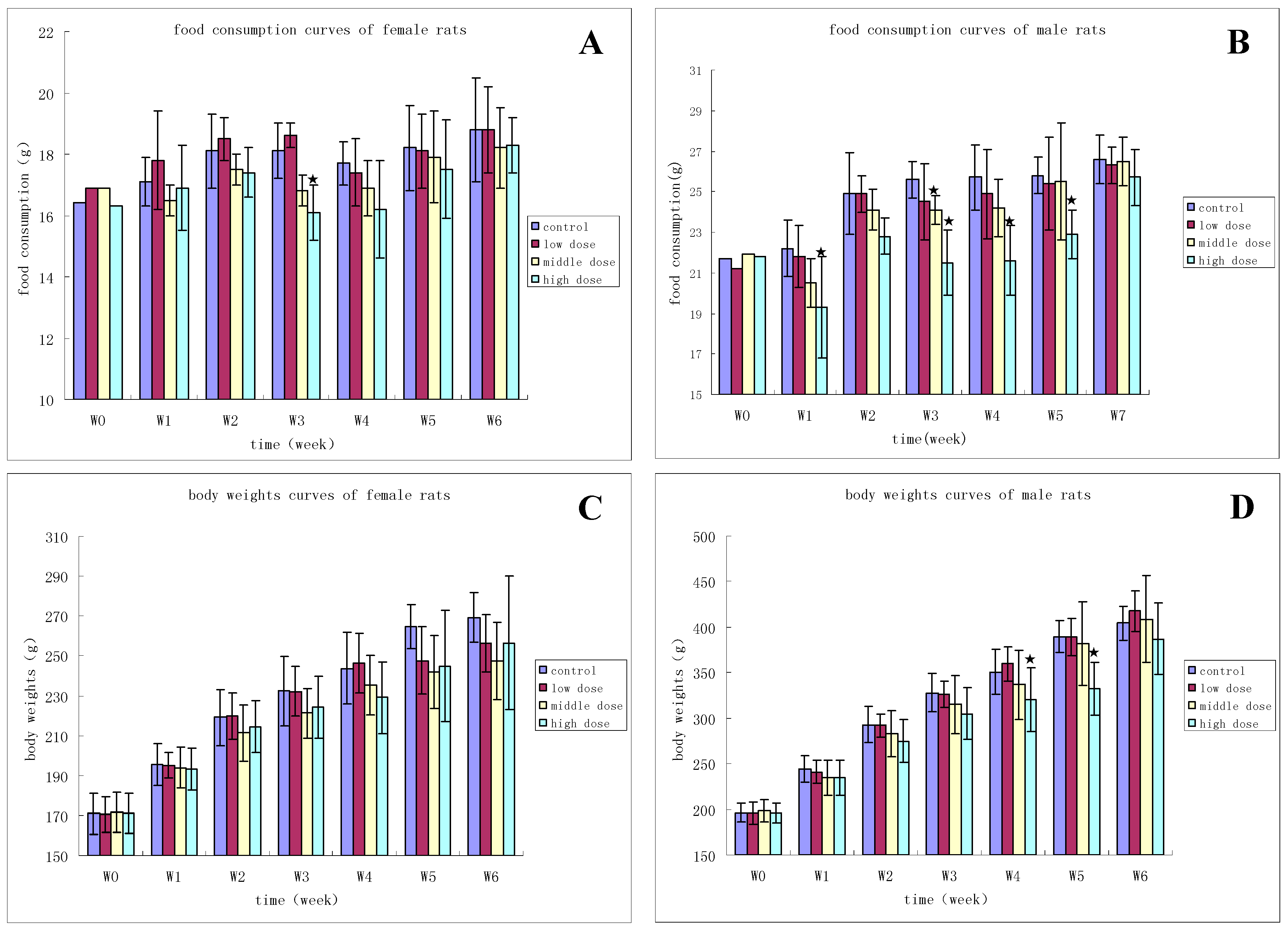
3.2.1. Clinical Observation
3.2.2. Hematology
3.2.3. Clinical Chemistry
3.2.4. Urinalysis
3.2.5. Bone Marrow Examination
3.2.6. Necropsy
Gross Pathology and Organ Weights
Histopathological Findings
3.2.7. Toxicokinetics
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kristeleit, R.; Fong, P.; Aherne, G.W.; de Bono, J. Histone deacetylase inhibitors: Emerging anticancer therapeutic agents? Clin. Lung Cancer 2005, 7, S19–S30. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.H.; Binkley, P.; Chan, K.; Xiao, J.; Arbogast, D.; Collamore, M.; Farra, Y.; Young, D.; Grever, M. Cardiotoxicity of histone deacetylase inhibitor depsipeptide in patients with metastatic neuroendocrine tumors. Clin. Cancer Res. 2006, 12, 3997–4003. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.L.; Rizvi, N.; Kauh, J.; Dahut, W.; Figuera, M.; Kang, M.H.; Figg, W.D.; Wainer, I.; Chaissang, C.; Li, M.Z.; et al. A phase I trial of depsipeptide (FR901228) in patients with advanced cancer. J. Exp. Ther. Oncol. 2002, 2, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Razak, A.R.; Hotte, S.J.; Siu, L.L.; Chen, E.X.; Hirte, H.W.; Powers, J.; Walsh, W.; Stayner, L.A.; Laughlin, A.; Novotny-Diermayr, V.; et al. Phase I clinical, pharmacokinetic and pharmacodynamic study of SB939, an oral histone deacetylase (HDAC) inhibitor, in patients with advanced solid tumours. Br. J. Cancer 2011, 104, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Xia, Z.; Zhang, X.; Zong, Y.; Zhu, L.; Yuan, B.; Lu, G. Evaluation of subchronic toxicity of SIM010603, a potent inhibitor of receptor tyrosine kinase, after 28-day repeated oral administration in SD rats and beagle dogs. Food Chem. Toxicol. 2012, 20, 1256–1270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yuan, B.; Mao, Y.; Dai, X.; Zhang, X.; Lu, G. Acute and subchronic toxicities of QX100626, a 5-HT4 receptor agonist, in rodents and Beagle dogs. Regul. Toxicol. Pharmacol. 2014, 70, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.S.; Galloway, S.; Lagrutta, A.; Armstrong, M.; Miller, T.; Richon, V.M.; Andrews, P.A. Nonclinical safety assessment of the histone deacetylase inhibitor Vorinostat. Int. J. Toxicol. 2010, 29, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Duvic, M.; Talpur, R.; Ni, X.; Zhang, C.; Hazarika, P.; Kelly, C.; Chiao, J.H.; Reilly, J.F.; Ricker, J.L.; Richon, V.M.; et al. Phase 2 trial of oral Vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood 2007, 109, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Welsbie, D.S.; Xu, J.; Chen, Y.; Borsu, L.; Scher, H.I.; Rosen, N.; Sawyers, C.L. Histone deacetylases are required for androgen receptor function in hormone-sensitive and castrate-resistant prostate cancer. Cancer Res. 2009, 69, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Solit, D.B.; Scher, H.I.; Rosen, N. HSP90 as a therapeutic target in prostate cancer. Semin. Oncol. 2003, 30, 709–716. [Google Scholar] [CrossRef]
- Fiskus, W.; Ren, Y.; Mohapatra, A.; Bali, P.; Mandawat, A.; Rao, R.; Herger, B.; Yang, Y.; Atadja, P.; Wu, J.; et al. Hydroxamic acid analogue histone deacetylase inhibitors attenuate estrogen receptor-a levels and transcriptional activity: A result of hyperacetylation and inhibition of chaperone function of heat shock protein 90. Clin. Cancer Res. 2007, 13, 4882–4890. [Google Scholar] [CrossRef] [PubMed]
- Molife, L.R.; Attard, G.; Fong, P.C.; Karavasilis, V.; Reid, A.H.; Patterson, S.; Riggs, C.E., Jr.; Higano, C.; Stadler, W.M.; McCulloch, W.; et al. Phase II, two-stage, single-arm trial of the histone deacetylase inhibitor (HDACi) romidepsin in metastatic castration-resistant prostate cancer (CRPC). Ann. Oncol. 2010, 21, 109–113. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.S.; Kristeleit, R.; Tolcher, A.; Fong, P.; Pacey, S.; Karavasilis, V.; Mita, M.; Shaw, H.; Workman, P.; Kaye, S.; et al. Phase I, pharmacokinetic and pharmacodynamic study of LAQ824, a hydroxamate histone deacetylase inhibitor with an HSP90 inhibitory profile, in patients with advanced solid tumors. Clin. Cancer Res. 2008, 14, 6663–6673. [Google Scholar] [CrossRef] [PubMed]

| Animal Number | Frequencies of Vomiting (Days) | Frequencies of Diarrhea (Days) | Animal Number | Frequencies of Vomiting (Days) | Frequencies of Diarrhea (Days) |
|---|---|---|---|---|---|
| Control | |||||
| ♂9 | 0 | 0 | ♀14 | 0 | 0 |
| ♂21 | 0 | 0 | ♀26 | 0 | 0 |
| ♂29 | 0 | 0 | ♀30 | 0 | 0 |
| ♂33 | 0 | 0 | ♀36 | 0 | 0 |
| 5 mg/kg | |||||
| ♂7 | 0 | 0 | ♀4 | 0 | 0 |
| ♂31 | 0 | 0 | ♀34 | 0 | 0 |
| ♂25 | 0 | 0 | ♀38 | 0 | 0 |
| ♂35 | 0 | 0 | ♀42 | 0 | 0 |
| 20 mg/kg | |||||
| ♂15 | 2 | 0 | ♀12 | 1 | 0 |
| ♂19 | 0 | 0 | ♀16 | 6 | 0 |
| ♂27 | 2 | 0 | ♀22 | 1 | 0 |
| ♂37 | 1 | 0 | ♀32 | 4 | 0 |
| 40 mg/kg | |||||
| ♂1 | 6 | 1 | ♀10 | 1 | 2 |
| ♂11 | 9 | 8 | ♀20 | 6 | 0 |
| ♂23 | 9 | 1 | ♀24 | 3 | 2 |
| ♂39 | 5 | 3 | ♀28 | 4 | 3 |
| 80 mg/kg | |||||
| ♂3 | 9 | 3 | ♀2 | 5 | 8 |
| ♂5 | 2 | 11 | ♀6 | 4 | 0 |
| ♂13 | 12 | 5 | ♀8 | 3 | 2 |
| ♂17 | 4 | 2 | ♀18 | 6 | 9 |
| Items | Days | Control | 5 mg/kg | 20 mg/kg | 40 mg/kg | 80 mg/kg | ♀6 a | ♀28 b |
|---|---|---|---|---|---|---|---|---|
| RBC (×1012/L) | d0 | 6.29 ± 0.68 | 6.31 ± 0.45 | 6.20 ± 0.58 | 6.01 ± 0.31 | 5.92 ± 0.42 | ||
| d14 | 6.51 ± 0.76 | 6.23 ± 0.46 | 5.57 ± 0.30 ##,* | 5.6 ± 0.36 ##,* | 5.09 ± 0.42 ##,* | |||
| d28 | 6.50 ± 0.54 | 6.22 ± 0.43 | 5.57 ± 0.16 ##,* | 5.00 ± 0.47 ##,* | 4.17 ± 0.33 ##,** | |||
| d42 | 6.27 ± 0.36 | 6.21 ± 0.36 | 5.96 ± 0.13 | 5.96 ± 0.06 | 5.71 ± 0.50 | |||
| HCT (%) | d0 | 42.4 ± 4.4 | 43.0 ± 2.6 | 42.2 ± 3.9 | 41.7 ± 2.0 | 40.3 ± 3.0 | ||
| d14 | 43.2 ± 4.3 | 41.8 ± 2.7 | 36.9 ± 2.2 ##,* | 35.5 ± 2.6 ##,** | 33.4 ± 2.6 ##,* | |||
| d28 | 42.7 ± 3.0 | 41.6 ± 2.4 | 36.6 ± 1.0 ##,** | 32.8 ± 2.8 ##,** | 26.9 ± 1.7 ##,** | |||
| d42 | 41.8 ± 2.3 | 41.0 ± 1.8 | 39.4 ± 1.7 | 39.7 ± 0.1 | 37.5 ± 3.1 | |||
| HGB (g/L) | d0 | 142 ± 14 | 139 ± 8 | 142 ± 13 | 139 ± 6 | 134 ± 10 | ||
| d14 | 139 ± 14 | 138 ± 9 | 118 ± 7 ##,** | 114 ± 8 ##,** | 107 ± 8 ##,** | |||
| d28 | 137 ± 9 | 135 ± 8 | 118 ± 3 ##,** | 105 ± 9 ##,** | 86 ± 5 ##,** | |||
| d42 | 136 ± 7 | 134 ± 7 | 128 ± 8 | 124 ± 1 | 126 ± 4 | |||
| RET (%) | d0 | 0.87 ± 0.34 | 0.77 ± 0.38 | 0.96 ± 0.24 | 0.86 ± 0.17 | 0.92 ± 0.27 | 1.37 | 0.75 |
| d14 | 0.63 ± 0.29 | 0.62 ± 0.29 | 0.53 ± 0.23 * | 0.13 ± 0.11 ##,** | 0.08 ± 0.12 ##,** | 0.14 | 0.04 | |
| d28 | 0.48 ± 0.21 | 0.49 ± 0.23 | 0.54 ± 0.19 ** | 0.51 ± 0.22* | 0.21 ± 0.16 #,** | |||
| d42 | 0.65 ± 0.25 | 0.61 ± 0.22 | 0.83 ± 0.23 | 0.89 ± 0.65 | 0.88 ± 0.27 | |||
| MON (%) | d0 | 4.8 ± 1.0 | 4.4 ± 1.6 | 4.7 ± 1.3 | 6.0 ± 1.8 | 5.0 ± 0.8 | ||
| d14 | 4.5 ± 1.3 | 4.2 ± 1.0 | 8.0 ± 2.3 ** | 9.2 ± 5.3 | 19.7 ± 19.7 # | |||
| d28 | 4.7 ± 1.0 | 4.4 ± 1.4 | 9.0 ± 1.4 #,** | 11.6 ± 4.5 ## | 12.5 ± 2.9 ##,** | |||
| d42 | 4.7 ± 1.0 | 4.0 ± 1.2 | 5.8 ± 1.0 | 6.4 ± 3.5 | 5.0 ± 1.9 | |||
| APTT (s) | d0 | 12.7 ± 0.8 | 13.6 ± 0.6 | 12.4 ± 0.3 | 12.8 ± 0.5 | 13.4 ± 0.6 | 13.9 | 12.4 |
| d14 | 13.0 ± 0.9 | 13.2 ± 0.6 | 12.5 ± 0.5 | 16.8 ± 0.7 ##,** | 16.2 ± 3.0 ## | 48.7 | 18.5 | |
| d28 | 12.1 ± 0.9 | 13.1 ± 0.5 | 12.0 ± 0.7 | 12.8 ± 0.8 | 12.5 ± 0.5 ** | |||
| d42 | 13.8 ± 1.0 | 13.4 ± 0.4 | 12.8 ± 0.3 | 12.9 ± 0.8 | 12.7 ± 0.9 | |||
| FIB (g/L) | d0 | 3.86 ± 0.86 | 2.25 ± 0.37 | 4.07 ± 0.94 | 4.84 ± 0.89 | 3.80 ± 0.84 | ||
| d14 | 3.93 ± 1.26 | 2.53 ± 0.46 | 4.30 ± 1.17 | 7.19 ± 1.58 ## | 7.06 ± 2.14 ##,** | |||
| d28 | 3.18 ± 0.58 | 2.56 ± 0.40 | 4.48 ± 1.68 | 4.93 ± 1.22 | 6.69 ± 0.62 ##,** | |||
| d42 | 2.96 ± 0.70 | 2.89 ± 0.60 | 4.26 ± 0.49 | 4.22 ± 1.25 | 3.72 ± 2.00 |
| Items | Days | Control | 5 mg/kg | 20 mg/kg | 40 mg/kg | 80 mg/kg | ♀6 a | ♀28 b |
|---|---|---|---|---|---|---|---|---|
| ALT (nmol/s/L) | d0 | 590 ± 167 | 559 ± 120 | 535 ± 92 | 526 ± 179 | 591 ± 143 | 487 | 428 |
| d14 | 563 ± 196 | 521 ± 116 | 425 ± 105 ** | 433 ± 304 | 312 ± 38 * | 466 | 852 | |
| d28 | 611 ± 155 | 593 ± 105 | 486 ± 90 | 347 ± 78 * | 307 ± 51 ** | |||
| d42 | 562 ± 165 | 558 ± 90 | 523 ± 148 | 514 ± 112 | 549 ± 181 | |||
| AST (nmol/s/L) | d0 | 436 ± 85 | 463 ± 106 | 456 ± 92 | 462 ± 57 | 493 ± 103 | 456 | 398 |
| d14 | 472 ± 72 | 442 ± 105 | 384 ± 56 * | 313 ± 23 ** | 386 ± 104 | 1907 | 4924 | |
| d28 | 419 ± 68 | 424 ± 109 | 408 ± 57 | 378 ± 71 * | 310 ± 59 ** | |||
| d42 | 470 ± 68 | 451 ± 79 | 412 ± 31 | 468 ± 80 | 460 ± 83 | |||
| LDH (μmol/s/L) | d0 | 3.02 ± 1.38 | 3.08 ± 1.36 | 2.85 ± 0.86 | 3.39 ± 1.55 | 2.71 ± 0.97 | 2.78 | 2.21 |
| d14 | 2.28 ± 0.37 | 2.27 ± 1.24 | 1.23 ± 0.45 ** | 1.29 ± 0.68 | 1.76 ± 1.85 | 2.91 | 2.06 | |
| d28 | 2.59 ± 0.45 | 2.71 ± 1.12 | 1.54 ± 0.74 * | 2.76 ± 2.49 | 0.97 ± 0.30 ** | |||
| d42 | 2.25 ± 0.27 | 2.21 ± 1.32 | 2.28 ± 0.93 | 3.50 ± 2.49 | 3.20 ± 1.62 ** | |||
| CPK (μmol/s/L) | d0 | 2.98 ± 0.63 | 2.94 ± 1.40 | 2.98 ± 0.64 | 3.27 ± 0.76 | 3.24 ± 0.77 | 3.06 | 2.47 |
| d14 | 2.78 ± 0.27 | 2.63 ± 1.36 | 2.43 ± 0.48 | 2.36 ± 0.19 * | 3.29 ± 1.86 | 57.57 | 6.39 | |
| d28 | 2.58 ± 0.29 | 2.43 ± 0.19 | 2.38 ± 0.53 ** | 3.00 ± 0.94 | 1.73 ± 0.24 ** | |||
| d42 | 2.56 ± 0.20 | 2.97 ± 0.18 | 2.17 ± 0.30 | 3.00 ± 1.32 | 2.86 ± 0.53 | |||
| ALP (μmol/s/L) | d0 | 1.55 ± 0.43 | 1.47 ± 0.30 | 1.48 ± 0.48 | 1.62 ± 0.40 | 1.62 ± 0.49 | 1.50 | 1.33 |
| d14 | 1.49 ± 0.28 | 1.45 ± 0.35 | 0.85 ± 0.25 ** | 1.03 ± 0.32 ** | 1.27 ± 1.15 | 3.86 | 3.16 | |
| d28 | 1.52 ± 0.48 | 1.51 ± 0.29 | 0.98 ± 0.27 ** | 1.30 ± 0.24 | 1.01 ± 0.27 ** | |||
| d42 | 1.27 ± 0.20 | 1.45 ± 0.37 | 1.00 ± 0.24 | 1.34 ± 0.34 | 0.99 ± 0.14 | |||
| TBIL (μmol/L) | d0 | 2.50 ± 0.30 | 2.34 ± 0.31 | 2.55 ± 0.28 | 2.24 ± 0.24 | 2.64 ± 0.41 | 2.95 | 2.18 |
| d14 | 2.17 ± 0.46 | 2.01 ± 0.41 | 1.85 ± 0.16 | 1.81 ± 0.24 | 2.74 ± 2.93 | 11.53 | 9.65 | |
| d28 | 2.17 ± 0.36 | 2.08 ± 0.52 | 2.29 ± 0.31 | 2.04 ± 0.43 | 2.19 ± 0.20 | |||
| d42 | 1.99 ± 0.39 | 2.01 ± 0.33 | 1.88 ± 0.19 | 1.88 ± 0.08 | 1.90 ± 0.23 | |||
| BU (mmol/L) | d0 | 4.29 ± 1.07 | 4.03 ± 0.52 | 4.35 ± 0.98 | 3.95 ± 0.48 | 4.38 ± 0.72 | 4.04 | 4.08 |
| d14 | 4.00 ± 0.75 | 4.10 ± 0.90 | 4.52 ± 1.05 | 4.52 ± 1.05 | 4.66 ± 0.93 | 15.20 | 4.89 | |
| d28 | 3.63 ± 0.31 | 3.82 ± 0.51 | 5.37 ± 1.78 | 4.66 ± 0.96 | 5.25 ± 1.21 | |||
| d42 | 4.74 ± 0.40 | 4.83 ± 0.20 | 4.05 ± 0.37 | 4.87 ± 1.56 | 4.93 ± 0.50 | |||
| TCH (mmol/L) | d0 | 4.45 ± 0.70 | 4.28 ± 0.36 | 4.31 ± 0.36 | 5.38 ± 1.95 | 4.03 ± 1.04 | 3.27 | 5.85 |
| d14 | 4.08 ± 0.78 | 3.98 ± 0.64 | 3.16 ± 0.34 | 4.47 ± 0.71 | 4.62 ± 2.51 | 11.04 | 10.73 | |
| d28 | 4.48 ± 0.80 | 4.24 ± 0.56 | 3.95 ± 0.55 | 4.66 ± 1.85 | 4.16 ± 1.39 | |||
| d42 | 4.12 ± 1.21 | 3.99 ± 0.41 | 3.81 ± 0.18 | 4.55 ± 1.10 | 3.52 ± 0.84 | 0.31 | 0.39 | |
| TG (mmol/L) | d0 | 0.43 ± 0.12 | 0.43 ± 0.11 | 0.42 ± 0.09 | 0.41 ± 0.10 | 0.38 ± 0.15 | 1.60 | 1.40 |
| d14 | 0.38 ± 0.15 | 0.39 ± 0.08 | 0.29 ± 0.08 | 0.41 ± 0.12 | 0.56 ± 0.28 | |||
| d28 | 0.33 ± 0.08 | 0.35 ± 0.11 | 0.34 ± 0.05 | 0.43 ± 0.18 | 0.45 ± 0.11 | |||
| d42 | 0.29 ± 0.05 | 0.31 ± 0.09 | 0.33 ± 0.12 | 0.45 ± 0.04 | 0.40 ± 0.14 | |||
| CREA (μmol/L) | d0 | 50.9 ± 7.9 | 51.5 ± 9.3 | 51.6 ± 7.5 | 49.2 ± 6.2 | 53.4 ± 5.2 | 52.8 | 47.9 |
| d14 | 50.3 ± 5.5 | 51.0 ± 9.9 | 58.6 ± 10.6 | 52.5 ± 6.1 | 58.2 ± 4.8 | 82.4 | 37.8 | |
| d28 | 50.9 ± 5.2 | 51.4 ± 10.9 | 52.1 ± 10.3 | 52.6 ± 9.8 | 54.3 ± 8.9 | |||
| d42 | 56.4 ± 5.7 | 55.6 ± 4.5 | 49.6 ± 0.6 | 58.8 ± 2.0 | 65.9 ± 11.3 |
| Organ | Days | Control | 5 mg/kg | 20 mg/kg | 40 mg/kg | 80 mg/kg | ♂23 | ♂5 |
|---|---|---|---|---|---|---|---|---|
| Absolute weight | ||||||||
| Lung (g) | d28 | 69.9 ± 3.0 | 72.7 ± 8.2 | 71.4 ± 12.6 | 92.4 ± 15.4 | 95.8 ± 35.3 | 109.8 | 135.9 |
| d42 | 74.7 ± 7.1 | ± | 74.0 ± 8.8 | 70.4 ± 5.0 | 82.9 ± 6.8 | |||
| Testis (g) | d28 | 13.625 ± 0.714 | 13.550 ± 0.494 | 13.953 ± 0.081 | 6.631 ± 2.855 ** | 5.660 ± 1.608 ** | ||
| d42 | 12.820 ± 1.556 | 14.050 ± 0.212 | 12.179 ± 0.518 | 9.400 ± 0.063 ** | 6.017 ± 2.937 ** | |||
| Relative weight | ||||||||
| Lung | d28 | 6.93 ± 0.52 | 6.90 ± 0.90 | 6.96 ± 1.02 | 9.87 ± 2.14 | 9.87 ± 3.27 | 12.34 | 13.39 |
| d42 | 7.46 ± 0.43 | ± | 7.37 ± 0.67 | 7.10 ± 1.13 | 8.49 ± 0.95 | |||
| Testis | d28 | 1.44 ± 0.02 | 1.23 ± 0.03 | 1.31 ± 0.26 | 0.69 ± 0.24 ** | 0.61 ± 0.23 ** | ||
| d42 | 1.29 ± 0.08 | 1.45 ± 0.04 | 1.21 ± 0.01 | 0.78 ± 0.21 ** | 0.63 ± 0.31 ** | |||
| Organ | Findings | Dose (mg/kg/day) | ||||
|---|---|---|---|---|---|---|
| 0 | 5 | 20 | 40 | 80 | ||
| Lung | large confluent bronchopneumonia | 0/4 | 0/4 | 0/4 | 2/4 | 2/4 |
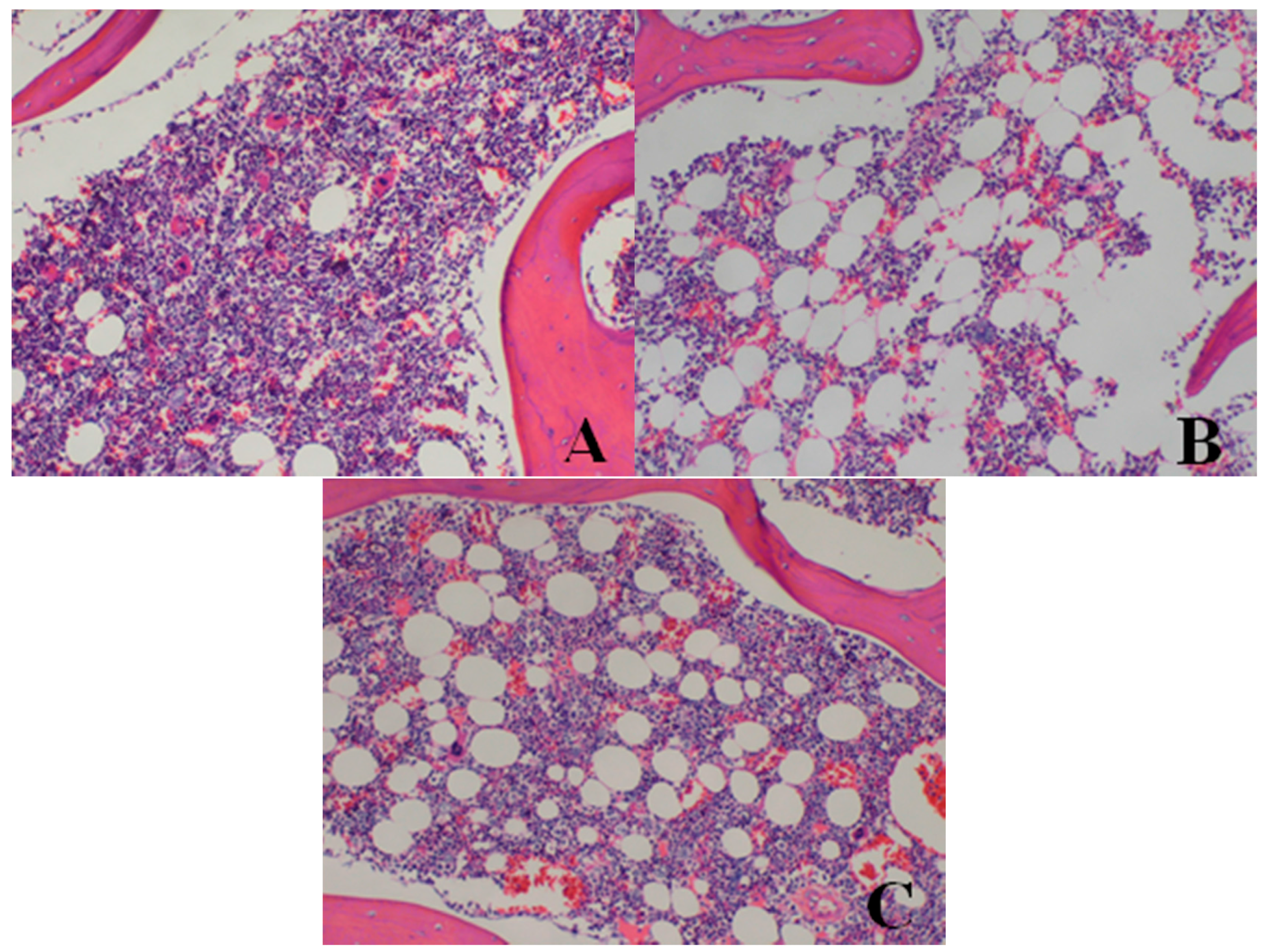
| Bone marrow of sternum | decrease in hematopoietic cell numbers | 0/4 | 0/4 | 0/4 | 2/4 | 3/4 |
| Testis | Reduction in numbers and types of spermatogenic cells | 0/2 | 0/2 | 0/2 | 2/2 | 2/2 |
| Epididymis | lack of mature sperm and secretions in the epididymal lumen as well as decrease of epididymal lumen size | 0/2 | 0/2 | 0/2 | 2/2 | 2/2 |
| Prostate | reduction in numbers and size, thinner epithelium, narrowing of the glandular cavity of prostatic acini | 0/2 | 0/2 | 0/2 | 2/2 | 2/2 |
| Dose (mg/kg/day) | Duration of Administration | AUC0–∞ (ng·h·mL−1) | Cmax (ng·mL−1) | Tmax (h) | t1/2 (h) |
|---|---|---|---|---|---|
| 5 (n = 6) | single | 193.1 ± 64.6 | 35.6 ± 15.1 | 3.67 ± 1.52 | 4.40 ± 0.61 |
| 14 days | 198.9 ± 69.6 | 30.1 ± 14.2 | 4.20 ± 2.01 | 4.75 ± 1.23 | |
| 28 days | 616.1 ± 242.4 | 65.7 ± 42.0 | 4.33 ± 2.94 | 7.34 ± 1.02 | |
| 20 (n = 6) | single | 1191.2 ± 319.9 | 139.1 ± 73.4 | 3.50 ± 2.51 | 5.54 ± 1.53 |
| 14 days | 620.3 ± 248.7 | 68.1 ± 22.8 | 4.00 ± 2.19 | 5.21 ± 0.53 | |
| 28 days | 388.9 ± 103.8 | 55.2 ± 21.8 | 3.17 ± 1.33 | 5.35 ± 0.73 | |
| 40 (n = 6) | single | 2500.4 ± 1490.8 | 456.0 ± 375.5 | 2.70 ± 2.70 | 6.93 ± 3.03 |
| 14 days | 1021.5 ± 976.6 | 199.0 ± 174.6 | 1.90 ± 1.34 | 7.34 ± 3.82 | |
| 28 days | 927.9 ± 297.5 | 162.2 ± 101.6 | 2.20 ± 1.10 | 5.08 ± 1.04 | |
| 80 (n = 6) | single | 3874.9 ± 2632.8 | 365.4 ± 256.7 | 3.50 ± 2.51 | 6.74 ± 2.20 |
| 14 days | 1547.4 ± 758.0 | 173.5 ± 123.0 | 4.43 ± 3.53 | 7.23 ± 3.45 | |
| 28 days | 1820.9 ± 570.0 | 180.2 ± 83.4 | 4.60 ± 3.29 | 7.56 ± 3.27 |
| Items | Days | Control | 20 mg/kg | 60 mg/kg | 120 mg/kg |
|---|---|---|---|---|---|
| Female | |||||
| WBC | d28 | 3.32 ± 0.54 | 3.17 ± 1.23 | 2.82 ± 0.60 | 3.95 ± 0.77 |
| (×109·L−1) | d42 | 3.78 ± 0.92 | 2.68 ± 0.63 * | 2.53 ± 0.60 * | 3.00 ± 0.43 |
| FIB | d28 | 1.78 ± 0.11 | 1.87 ± 0.09 | 1.89 ± 0.06 | 2.01 ± 0.40 |
| (g/L) | d42 | 1.89 ± 0.19 | 1.62 ± 0.17 * | 1.62 ± 0.18 * | 1.85 ± 0.12 |
| APTT | d28 | 24.3 ± 1.7 | 24.3 ± 3.5 | 23.0 ± 2.4 | 22.6 ± 2.4 |
| (s) | d42 | 26.2 ± 2.0 | 22.2 ± 3.0 ** | 25.2 ± 1.3 | 25.2 ± 1.6 |
| Neu | d28 | 8.40 ± 0.80 | 8.70 ± 1.72 | 12.08 ± 4.77 | 19.28 ± 8.26 |
| (%) | d42 | 12.42 ± 1.73 | 11.58 ± 4.04 | 12.06 ± 6.70 | 10.76 ± 1.81 |
| Lym | d28 | 88.40 ± 0.77 | 88.38 ± 1.68 | 84.70 ± 5.45 | 77.22 ± 8.78 |
| (%) | d42 | 84.30 ± 1.57 | 85.40 ± 3.85 | 85.36 ± 6.93 | 85.88 ± 1.85 |
| Males | |||||
| RET | d28 | 1.61 ± 0.21 | 1.88 ± 0.32 | 1.43 ± 0.22 | 2.04 ± 0.31 * |
| (%) | d42 | 2.03 ± 1.19 | 1.92 ± 0.31 | 1.82 ± 0.43 | 2.16 ± 0.22 |
| Organ | Days | Control | 20 mg/kg | 60 mg/kg | 120 mg/kg |
|---|---|---|---|---|---|
| Absolute weight (female) | |||||
| Adrenal gland (g) | d28 | 0.068 ± 0.007 | 0.081 ± 0.005 ** | 0.068 ± 0.003 | 0.066 ± 0.008 |
| d42 | 0.077 ± 0.009 | 0.072 ± 0.008 | 0.071 ± 0.012 | 0.069 ± 0.011 | |
| Relative weight (female) | |||||
| Brain (g/100 g) | d28 | 0.799 ± 0.045 | 0.728 ± 0.047 * | 0.799 ± 0.062 | 0.834 ± 0.042 |
| d42 | 0.739 ± 0.045 | 0.768 ± 0.020 | 0.768 ± 0.068 | 0.745 ± 0.089 | |
| Absolute weight (male) | |||||
| Testis (g) | d28 | 2.774 ± 0.209 | 2.707 ± 0.090 | 2.624 ± 0.170 | 2.666 ± 0.115 |
| d42 | 2.546 ± 0.082 | 2.798 ± 0.112 ** | 2.787 ± 0.097 ** | 2.723 ± 0.161 * | |
| Relative weight (male) | |||||
| Kidney (g/100 g) | d28 | 0.759 ± 0.051 | 0.707 ± 0.024 * | 0.705 ± 0.039 * | 0.673 ± 0.023 ** |
| d42 | 0.705 ± 0.028 | 0.693 ± 0.033 | 0.687 ± 0.020 | 0.624 ± 0.053 ** | |
| Dose (mg/kg/day) | Duration of Administration | AUC0–∞ (ng·h·mL−1) | Cmax (ng·mL−1) | Tmax (h) | t1/2 (h) |
|---|---|---|---|---|---|
| 20 (n = 6) | single | 8.0 ± 9.1 | 7.5 ± 7.2 | 0.86 ± 1.54 | 0.60 ± 0.37 |
| 28 days | 32.7 ± 14.9 | 12.9 ± 9.5 | 0.34 ± 0.18 | 7.32 ± 2.93 | |
| 60 (n = 6) | single | 39.1 ± 15.0 | 13.3 ± 5.5 | 0.50 ± 0.00 | 6.83 ± 2.69 |
| 28 days | 114.1 ± 30.9 | 19.7 ± 3.3 | 0.28 ± 0.17 | 7.57 ± 2.31 | |
| 120 (n = 6) | single | 69.9 ± 22.9 | 20.4 ± 3.8 | 0.45 ± 0.13 | 6.51 ± 1.62 |
| 28 days | 446.8 ± 282.2 | 57.5 ± 26.1 | 1.92 ± 3.07 | 7.03 ± 1.90 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, X.; Yuan, B.; Ren, L.; Zhang, T.; Lu, G. Subchronic Toxicities of HZ1006, a Hydroxamate-Based Histone Deacetylase Inhibitor, in Beagle Dogs and Sprague-Dawley Rats. Int. J. Environ. Res. Public Health 2016, 13, 1190. https://doi.org/10.3390/ijerph13121190
Zhang X, Zhang X, Yuan B, Ren L, Zhang T, Lu G. Subchronic Toxicities of HZ1006, a Hydroxamate-Based Histone Deacetylase Inhibitor, in Beagle Dogs and Sprague-Dawley Rats. International Journal of Environmental Research and Public Health. 2016; 13(12):1190. https://doi.org/10.3390/ijerph13121190
Chicago/Turabian StyleZhang, Xiaofang, Xiaodong Zhang, Bojun Yuan, Lijun Ren, Tianbao Zhang, and Guocai Lu. 2016. "Subchronic Toxicities of HZ1006, a Hydroxamate-Based Histone Deacetylase Inhibitor, in Beagle Dogs and Sprague-Dawley Rats" International Journal of Environmental Research and Public Health 13, no. 12: 1190. https://doi.org/10.3390/ijerph13121190





