Parity, Age at First Birth, and Risk of Death from Bladder Cancer: A Population-Based Cohort Study in Taiwan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
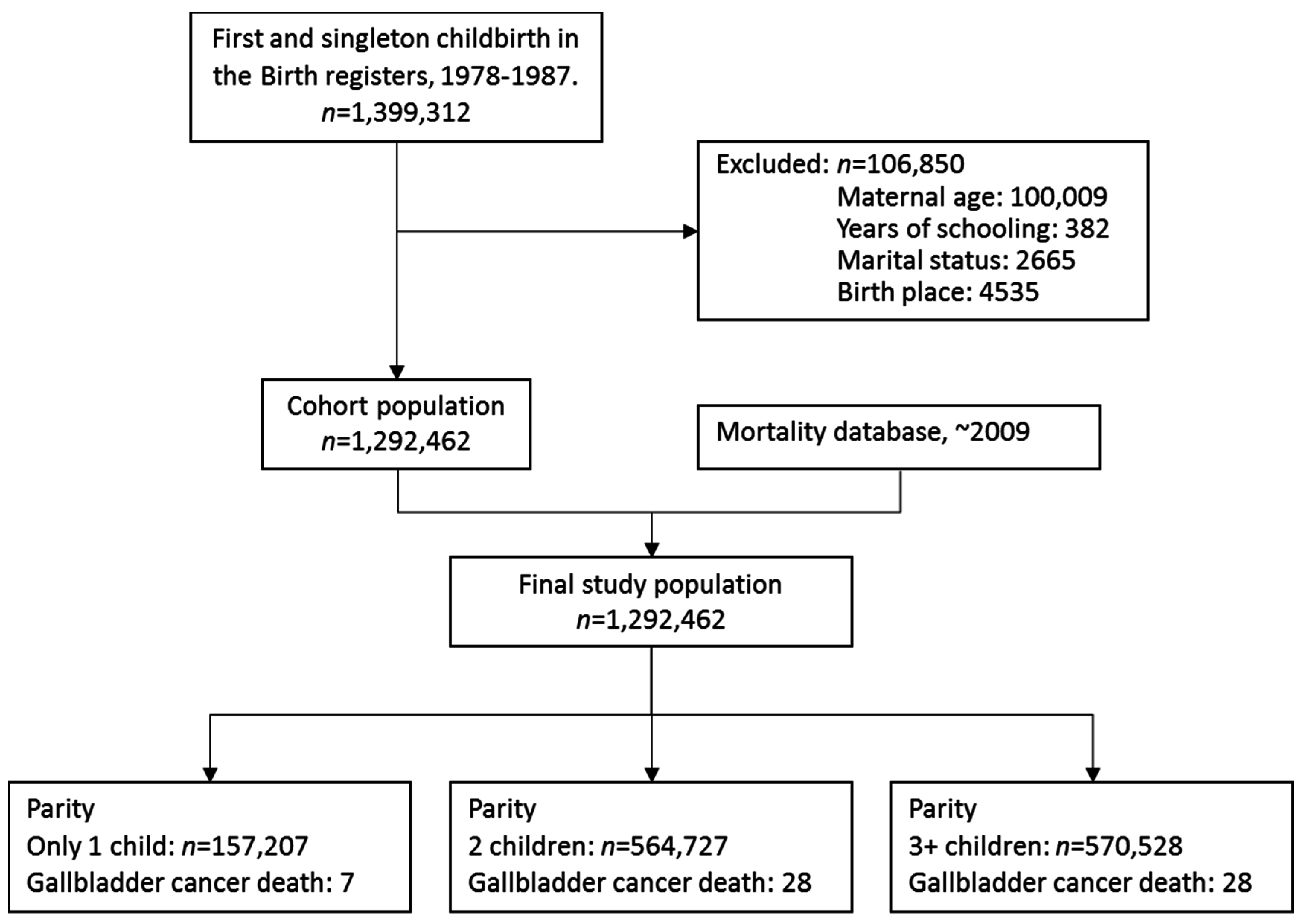
2.2. Study Population
2.3. Follow-Up
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Department of Health, Taiwan (ROC). Statistics of Causes of Death; Department of Health: Taipei, Taiwan, 2012.
- Yang, C.Y.; Hsieh, Y.L. The relationship between population density and cancer mortality in Taiwan. Jpn. J. Cancer Res. 1998, 89, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.; Bogillot, O.; Greiser, E.; Chang-Claude, J.; Wahrendorf, J.; Cordier, S.; Jöckel, K.H.; Lopez-Abente, G.; Tzonou, A.; Vineis, P.; et al. The contribution of cigarette smoking to bladder cancer in women (pooled European data). Cancer Causes Control 2001, 12, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Castelao, J.E.; Yuan, J.M.; Skipper, P.L.; Tannenbaum, S.R.; Gago-Dominguez, M.; Crowder, J.S.; Ross, R.K.; Yu, M.C. Gender- and smoking-related bladder cancer risk. J. Natl. Cancer Inst. 2001, 93, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.D.; Silverman, D.T.; Hollenbeck, A.R.; Schatzkin, A.; Abnet, C.C. Association between smoking and risk of bladder cancer among men and women. JAMA 2011, 306, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Murta-Nascimento, C.; Schmitz-Drager, B.J.; Zeegers, M.P.; Steineck, G.; Kogevinas, M.; Real, F.X.; Malats, N. Epidemiology of urinary bladder cancer from tumor development to patient’s death. World J. Urol. 2007, 25, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Pelucchi, C.; La Vecchia, C.; Negri, E.; Dal Maso, L.; Franceschi, S. Smoking and other risk factors for bladder cancer in women. Prev. Med. 2002, 35, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Scosyrev, E.; Noyes, K.; Feng, C.; Messing, E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer 2009, 115, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Hayne, D.; Arya, M.; Quinn, M.J.; Babb, P.J.; Beacock, C.J.; Patel, H.R. Current trends in bladder cancer in England and Wales. J. Urol. 2004, 172, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Kogevinas, M.; Garcia-Closas, M.; Trichopoulos, D. Urinary bladder cancer. In Textbook of Cancer Epidemiology; Adami, H.O., Hunter, D., Trichopoulos, D., Eds.; Oxford University Press: New York, NY, USA, 2008; pp. 403–445, 494–516, 573–596. [Google Scholar]
- Weibull, C.E.; Eloranta, S.; Altman, D.; Johansson, A.L.; Lambe, M. Childbearing and the risk of bladder cancer: A nationwide population-based cohort study. Eur. Urol. 2013, 63, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Hartge, P.; Harvey, E.B.; Linehan, W.M.; Silverman, D.T.; Sullivan, J.W.; Hoover, R.N.; Fraumeni, J.F., Jr. Unexplained excess risk of bladder cancer in men. J. Natl. Cancer Inst. 1990, 82, 1636–1640. [Google Scholar] [CrossRef] [PubMed]
- Cantor, K.P.; Lynch, C.F.; Johnson, D. Bladder cancer, parity, and age at first birth. Cancer Causes Control 1992, 3, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Kirkali, Z.; Chan, T.; Manoharan, M. Bladder cancer: Epidemiology, staging and grading, and diagnosis. J. Urol. 2006, 66 (Suppl. 6A), 4–34. [Google Scholar] [CrossRef] [PubMed]
- McGrath, M.; Michaud, D.S.; De Vivo, I. Hormonal and reproductive factors and the risk of bladder cancer in women. Am. J. Epidemiol. 2006, 163, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Prizment, A.E.; Anderson, K.E.; Harlow, B.L.; Folsom, A.R. Reproductive risk factors for incident bladder cancer: Iowa Women’s Health Study. Int. J. Cancer 2006, 120, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Iosif, C.S.; Batra, S.; Ek, A.; Astedt, B. Estrogen receptors in the human female lower urinary tract. Am. J. Obstet. Gynecol. 1981, 141, 817–820. [Google Scholar] [CrossRef]
- Pacchioni, D.; Revelli, A.; Casetta, G.; Cassoni, P.; Piana, P.; Tizzani, A.; Bussolati, G.; Massobrio, M. Immunohistochemical detection of estrogen and progesterone receptors in the normal urinary-bladder and in pseudomembranous trigonitis. J. Endocrinol. Investig. 1992, 15, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Blakeman, P.J.; Hilton, P.; Bulmer, J.N. Oestrogen and progesterone receptor expression in the female lower urinary tract, with reference to oestrogen status. BJU Int. 2000, 86, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.S.; Smith, C.L.; Hsieh, J.T.; Yu, J.; Kim, I.Y.; Jian, W.; Sonpavde, G.; Ayala, G.E.; Younes, M.; Lerner, S.P. Expression of estrogen receptors-alpha and -beta in bladder cancer cell lines and human bladder tumor tissue. Cancer 2006, 106, 2610–2616. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, C.; Negri, E.; Franceschi, S.; Parazzini, F. Long-term impact of reproductive factors on cancer risk. Int. J. Cancer 1993, 53, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Cantwell, M.M.; Lacey, J.V.; Schairer, C.; Schatzkin, A.; Michaud, D.S. Reproductive factors, exogenous hormone use and bladder cancer risk in a prospective study. Int. J. Cancer 2006, 119, 2398–2401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, A.; Folsom, A.R.; Anderson, K.E. Risk factors for urinary bladder carcinoma in postmenopausal women—The Iowa women’s health study. Cancer 2002, 95, 2316–2323. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, K.; Demidenko, E.; Schned, A.; Zens, M.S.; Heaney, J.; Karagas, M.R. Parity, early menopause and the incidence of bladder cancer in women: A case-control study and meta-analysis. Eur. J. Cancer 2011, 47, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Davis-Dao, C.A.; Henderson, K.D.; Sullivan-Halley, J.; Ma, H.; West, D.; Xiang, Y.B.; Gago-Dominguez, M.; Stern, M.C.; Castelao, J.E.; Conti, D.V.; et al. Lower risk in parous women suggests that hormonal factors are important in bladder cancer etiology. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1156–1170. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.T.; Kogevinas, M.; Silverman, D.T.; Malats, N.; Rothman, N.; Tardón, A.; Serra, C.; García-Closas, R.; Carrato, A.; Cantor, K.P. Bladder cancer and reproductive factors among women in Spain. Cancer Causes Control 2009, 20, 1907–1913. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.C.; Young, C.L. Study of the birth reporting system. J. Natl. Public Health Assoc. 1986, 6, 15–27. [Google Scholar]
- Yang, C.Y.; Chang, C.C.; Kuo, H.W.; Chiu, H.F. Parity and risk of death from subarachnoid hemorrhage in women: Evidence from a cohort in Taiwan. Neurology 2006, 67, 514–515. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Kuo, H.W.; Chiu, H.F. Age at first birth, parity, and risk of death from ovarian cancer in Taiwan: A country of low incidence of ovarian cancer. Int. J. Gynecol. Cancer 2007, 17, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Chan, T.F.; ChangChien, C.C.; Yang, C.Y. Parity, age at first birth, and risk of death from liver cancer: Evidence from a cohort in Taiwan. J. Gastroenterol. Hepatol. 2011, 26, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.H.; Tsai, S.S.; Chen, C.C.; Yang, C.Y. Parity and risk of death from lung cancer among a cohort of premenopausal parous women in Taiwan. J. Epidemiol. 2012, 22, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J.; Greenland, S. Modern Epidemiology, 2nd ed.; Lippincott-Raven: Philadelphia, PA, USA, 1998. [Google Scholar]
- Sandler, D.P.; Wilcox, A.J.; Horney, L.F. Age at menarche and subsequent reproductive events. Am. J. Epidemiol. 1984, 119, 765–774. [Google Scholar] [PubMed]
- Tanahashi, N.K.; Suzawa, N.; Azuma, C. Effects of sex hormones on oncogenesis in rat urinary bladder by N-butyl-N-(4-hydroxybutyl)-nitrosamine. Int. J. Clin. Pharmacol. Biopharm. 1977, 15, 101–105. [Google Scholar] [PubMed]
- Okajima, E.; Hiramatsu, T.; Iriya, K.; Ijuin, M.; Matsushima, S. Effects of sex hormones on development of urinary bladder tumors induced by N-butyl-N-(4-hydroxybutyl)-nitrosamine. Urol. Res. 1975, 3, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Reid, L.M.; Leav, I.; Kwan, P.W.; Russell, P.; Merk, F.B. Characterization of a human sex-steroid-responsive transplantable cell carcinoma maintained as a tumor line (R 198) in athymic nude mice. Cancer Res. 1984, 44, 4560–4573. [Google Scholar] [PubMed]
- Yen, S.S. Endocrinology of pregnancy. In Maternal-Fetal Medicine: Principles and Practice, 3rd ed.; Creasy, R.K., Resnik, R., Eds.; Saunders: Philadelphia, PA, USA, 1994; pp. 382–412. [Google Scholar]
- Johnson, A.M.; O’Connell, M.J.; Messing, E.M.; Reeder, J.E. Decreased bladder cancer growth in parous mice. J. Urol. 2008, 72, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Chen, K.Y.; Lin, H.C. Non-alcoholic cirrhosis and the risk of stroke: A five year follow-up study. Liver Int. 2011, 31, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E.; Gallus, S.; Bosetti, C.; Franceschi, S.; Negri, E.; La Vecchia, C. Hormone replacement therapy and cancer risk: A systematic analysis from a network of case-control studies. Int. J. Cancer 2003, 105, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Chie, W.C.; Chung, Y.L.; Huang, C.S.; Chang, K.J.; Yen, M.L.; Lin, R.S. Oral contraceptives and breast cancer risk in Taiwan, a country of low incidence of breast cancer and low use of oral contraceptives. Int. J. Cancer 1998, 77, 219–223. [Google Scholar] [CrossRef]
- Yen, M.L.; Yen, B.L.; Bai, C.H.; Lin, R.S. Risk factors for ovarian cancer in Taiwan: A case-control study in a low-incidence population. Gynecol. Oncol. 2003, 89, 318–324. [Google Scholar] [CrossRef]
- Cheng, T.Y.; Wen, C.P.; Tsai, M.C.; Tsai, S.P. The current status of smoking behavior in Taiwan: Data analysis from national health interview survey in 2001. Taiwan J. Public Health 2003, 22, 453–464. [Google Scholar]
| Variables | No. of Subjects | Follow-Up Person-Years | No. of Deaths from Bladder Cancer | Mortality Rate (per 100,000 Person-Years) |
|---|---|---|---|---|
| Age at recruitment (first birth) | ||||
| ≤23 | 551,759 | 15,312,470.08 | 21 | 0.14 |
| >23~≤26 | 433,114 | 11,592,980.92 | 19 | 0.16 |
| >26 | 307,589 | 8,074,795.00 | 23 | 0.28 |
| Parity (children) | ||||
| 1 | 157,207 | 4,170,772.33 | 7 | 0.17 |
| 2 | 564,727 | 15,124,112.33 | 28 | 0.19 |
| 3+ | 570,528 | 15,685,361.33 | 28 | 0.18 |
| Marital status | ||||
| Married | 1,260,615 | 34,115,479.25 | 63 | 0.18 |
| Not married | 31,847 | 864,766.75 | 0 | 0.00 |
| Years of schooling | ||||
| ≤9 years | 722,518 | 19,850,938.17 | 31 | 0.16 |
| >9 years | 569,944 | 15,129,307.83 | 32 | 0.21 |
| Birth place | ||||
| Hospital/clinic | 1,245,925 | 33,638,862.83 | 60 | 0.18 |
| Home/other | 46,537 | 1,341,383.17 | 3 | 0.22 |
| Variables | Crude RR (95% CI) | Multivariate-Adjusted RR * (95% CI) |
|---|---|---|
| Age at recruitment (first birth) | ||
| ≤23 | 1.00 | 1.00 |
| >23–26 | 1.29 (0.69–2.40) | 1.24 (0.66–2.35) |
| >26 | 2.33 (1.29–4.22) | 2.30 (1.21–4.39) |
| p = 0.01 for linear trend | p = 0.01 for linear trend | |
| Parity (children) | ||
| 1 | 1.00 | 1.00 |
| 2 | 1.10 (0.48–2.51) | 1.17 (0.51–2.69) |
| 3+ | 1.01 (0.44–2.31) | 1.31 (0.56–3.10) |
| p = 0.89 for linear trend | p = 0.58 for linear trend | |
| Marital status | ||
| Married | 1.00 | 1.00 |
| Not married | – | – |
| Years of schooling | ||
| ≤9 years | 1.00 | 1.00 |
| >9 years | 1.46 (0.89–2.40) | 1.25 (0.73–2.13) |
| Birth place | ||
| Hospital/clinic | 1.00 | 1.00 |
| Home/other | 1.11 (0.35–3.55) | 1.35 (0.42–4.40) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, H.-F.; Chen, B.K.; Yang, C.-Y. Parity, Age at First Birth, and Risk of Death from Bladder Cancer: A Population-Based Cohort Study in Taiwan. Int. J. Environ. Res. Public Health 2016, 13, 1197. https://doi.org/10.3390/ijerph13121197
Chiu H-F, Chen BK, Yang C-Y. Parity, Age at First Birth, and Risk of Death from Bladder Cancer: A Population-Based Cohort Study in Taiwan. International Journal of Environmental Research and Public Health. 2016; 13(12):1197. https://doi.org/10.3390/ijerph13121197
Chicago/Turabian StyleChiu, Hui-Fen, Brian K. Chen, and Chun-Yuh Yang. 2016. "Parity, Age at First Birth, and Risk of Death from Bladder Cancer: A Population-Based Cohort Study in Taiwan" International Journal of Environmental Research and Public Health 13, no. 12: 1197. https://doi.org/10.3390/ijerph13121197





