Decreases in Smoking-Related Cancer Mortality Rates Are Associated with Birth Cohort Effects in Korean Men
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Statistical Analysis
3. Results
3.1. Number and Proportion of Deaths from Smoking Related Cancer
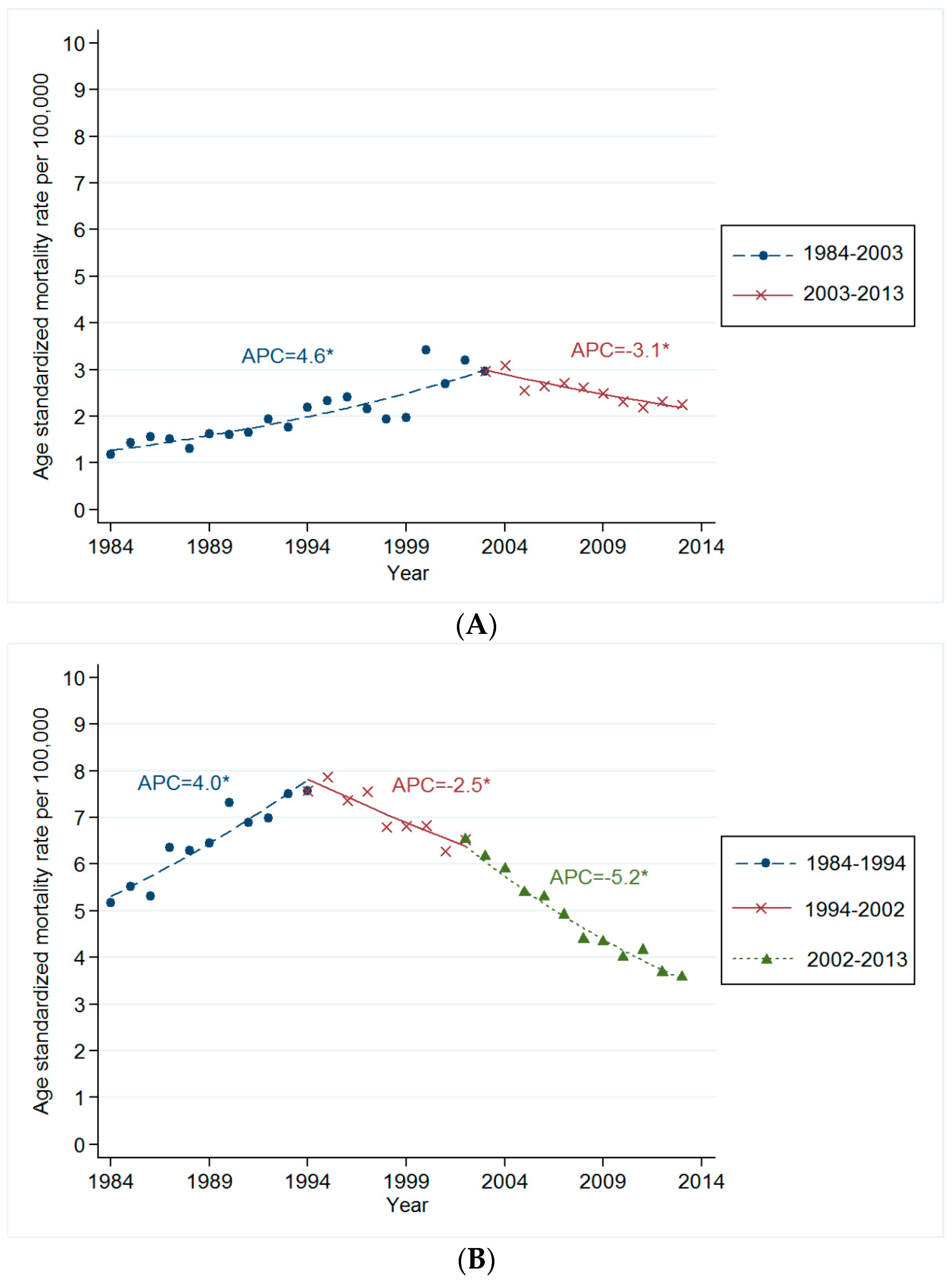
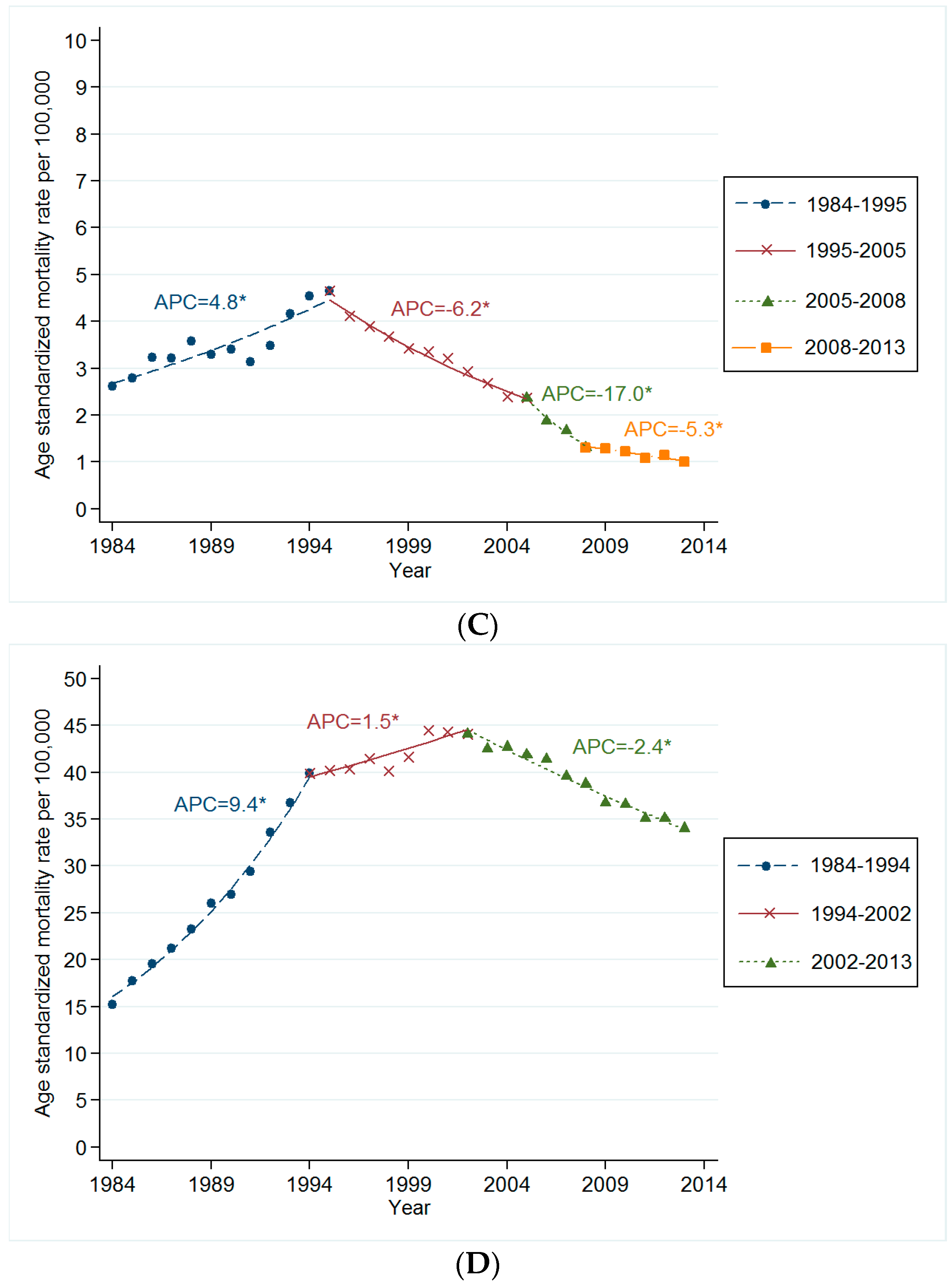
3.2. Trends in Age-Standardized Mortality Rates for Smoking Related Cancer
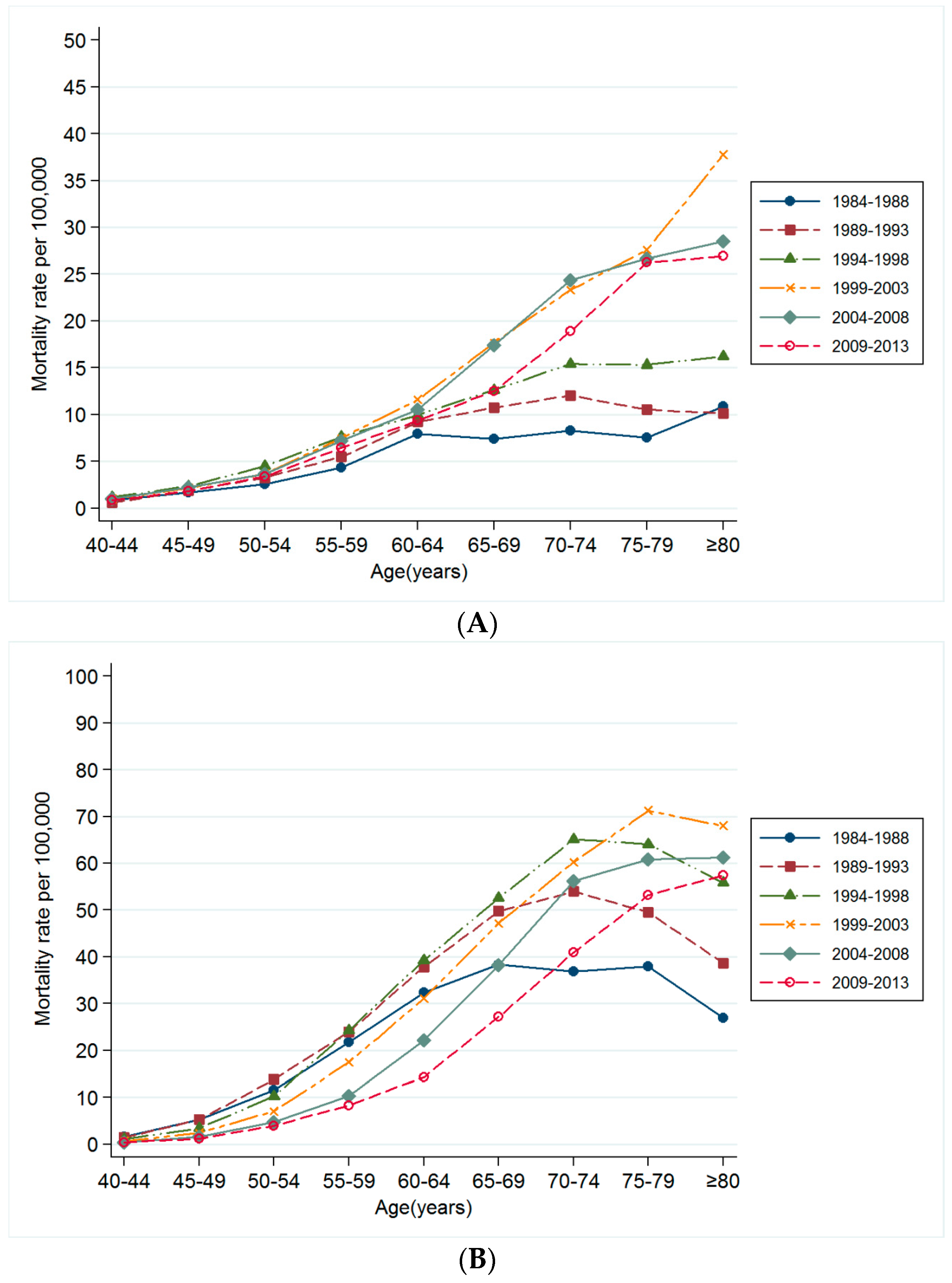
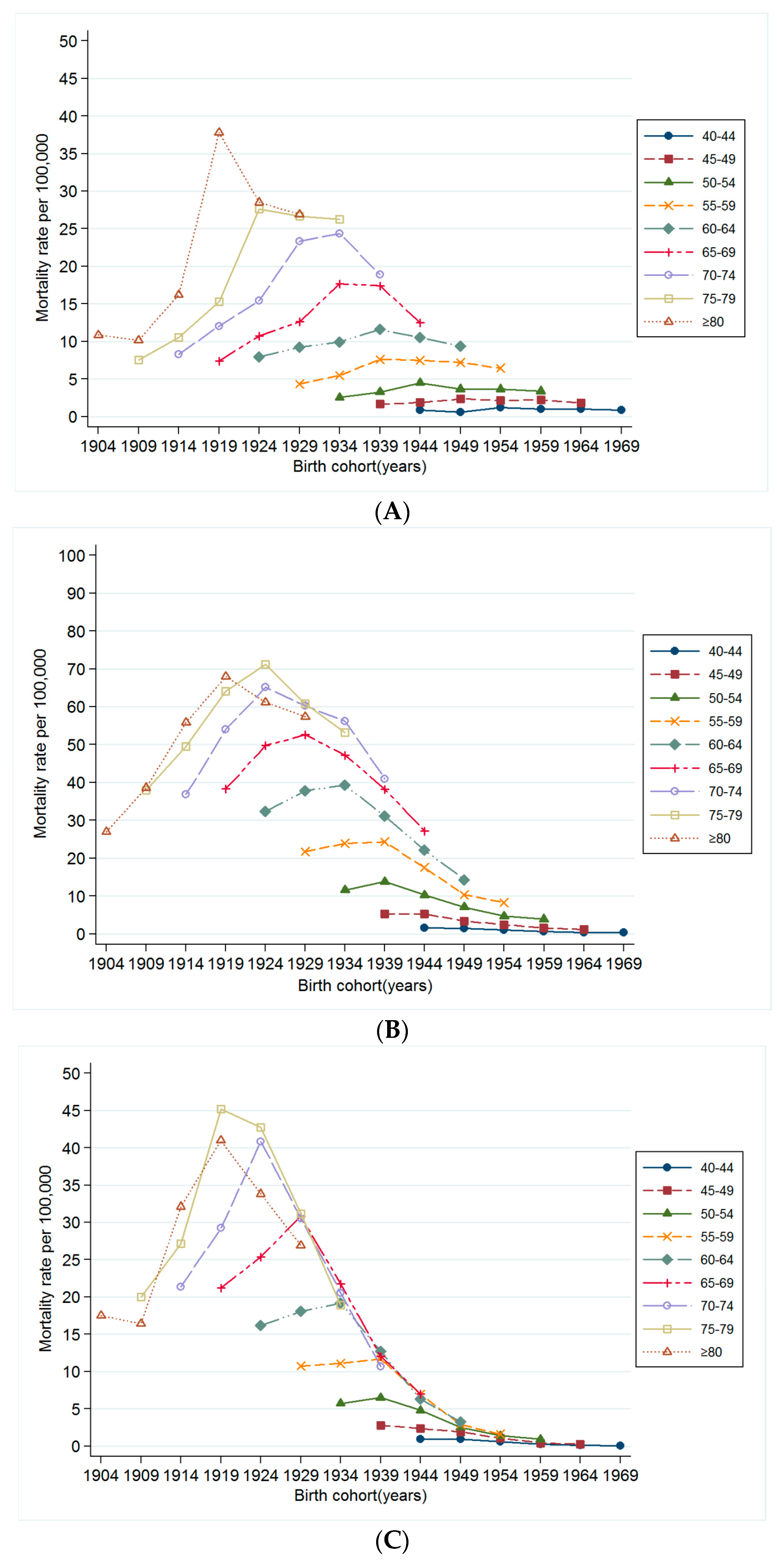
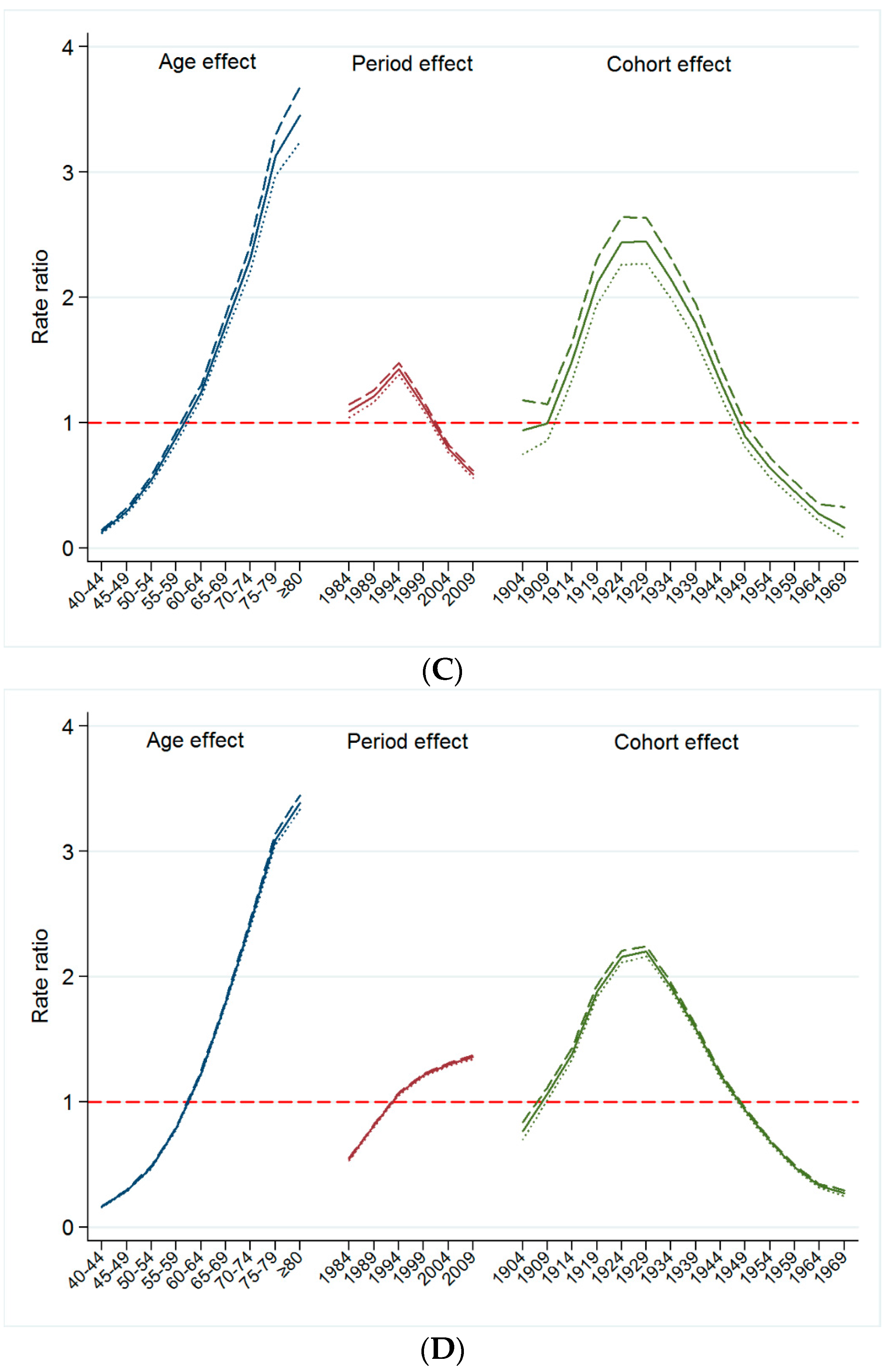
3.3. Changes in Mortality Rates for Smoking Related Cancer by Time Period and Birth Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization (WHO). Enforcing Bans on Tobacco Advertising, Promotion and Sponsorship; Report on the Global Tobacco Epidemic; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Jee, S.H.; Samet, J.M.; Ohrr, H.; Kim, J.H.; Kim, I.S. Smoking and cancer risk in Korean men and women. Cancer Causes Control 2004, 15, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Koh, H.K.; Kwon, J.W.; Suh, M.K.; Kim, H.; Cho, S.I. Secular trends in adult male smoking from 1992 to 2006 in South Korea: Age-specific changes with evolving tobacco-control policies. Public Health 2009, 123, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Statistics Korea. Available online: http://kosis.kr/ (accessed on 30 August 2015).
- Alexandrov, L.B.; Ju, Y.S.; Haase, K.; van Loo, P.; Martincorena, I.; Nik-Zainal, S.; Totoki, Y.; Fujimoto, A.; Nakagawa, H.; Shibata, T. Mutational signatures associated with tobacco smoking in human cancer. Science 2016, 354, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jee, S.H.; Shin, H.R.; Park, E.H.; Shin, A.; Jung, K.W.; Hwang, S.S.; Cha, E.S.; Yun, Y.H.; Park, S.K.; et al. Attributable fraction of tobacco smoking on cancer using population-based nationwide cancer incidence and mortality data in Korea. BMC Cancer 2014, 14, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Yoon, S.J.; Ahn, H.S. Measuring the burden of major cancers due to smoking in Korea. Cancer. Sci. 2006, 97, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.H.; Yoon, S.J.; Yoon, T.Y.; Choi, J.M.; Choe, B.K.; Kim, E.J.; Kim, Y.A.; Seo, H.Y.; Park, Y.H. Health and economic burden of major cancers due to smoking in Korea. Asian Pac. J. Cancer Prev. 2012, 13, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Nadler, D.L.; Zurbenko, I.G. Estimating cancer latency times using a Weibull model. Adv. Epidemiol. 2014, 2014, 746769. [Google Scholar] [CrossRef]
- Rosenberg, P.S.; Anderson, W.F. Age-period-cohort models in cancer surveillance research: Ready for prime time? Cancer Epidemiol. Biomark. Prev. 2011, 20, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.C.; Chang, C.J.; Hsu, C.; Cheng, C.C.; Chiu, C.F.; Cheng, A.L. Significant difference in the trends of female breast cancer incidence between Taiwanese and Caucasian Americans: Implications from age-period-cohort analysis. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1986–1990. [Google Scholar] [CrossRef] [PubMed]
- Keyes, K.M.; Utz, R.L.; Robinson, W.; Li, G. What is a cohort effect? Comparison of three statistical methods for modeling cohort effects in obesity prevalence in the United States, 1971–2006. Soc. Sci. Med. 2010, 70, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Weiderpass, E. Lung cancer mortality trends in 36 European countries: Secular trends and birth cohort patterns by sex and region 1970–2007. Int. J. Cancer 2010, 126, 1454–1466. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Tobacco Atlas. Available online: http://www.tobaccoatlas.org/country-data/south-korea/ (accessed on 8 November 2016).
- International Agency for Research on Cancer. Attributable Causes of Cancer in France in the Year 2000; IARC: Lyon, France, 2007. [Google Scholar]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems; 10th Revision; World Health Organization: Geneva, Switzerland, 1994. [Google Scholar]
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef]
- Yang, Y.; Fu, W.J.; Land, K.C. A methodological comparison of age-period-cohort models: The intrinsic estimator and conventional generalized linear models. Sociol. Methodol. 2004, 34, 75–110. [Google Scholar] [CrossRef]
- Oh, C.M.; Jung, K.W.; Won, Y.J.; Shin, A.; Kong, H.J.; Lee, J.S. Age-period-cohort analysis of thyroid cancer incidence in Korea. Cancer Res. Treat. 2015, 47, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, A.; Rodríguez-Domínguez, S.; López-Campos, J.L.; Vigil, E. Age-period-cohort analysis of lung cancer mortality rates in Andalusia, 1975–2004. Lung Cancer 2007, 57, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Holford, T.R.; Ebisu, K.; McKay, L.; Oh, C.; Zheng, T. Yale lung cancer model. Risk Anal. 2012, 32 (Suppl. 1), S151–S165. [Google Scholar] [CrossRef] [PubMed]
- Du, J.L.; Lin, X.; Zhang, L.F.; Li, Y.H.; Xie, S.H.; Yang, M.J.; Guo, J.; Lin, E.H.; Liu, Q.; Hong, M.H.; et al. Secular trend analysis of lung cancer incidence in Sihui city, China between 1987 and 2011. Chin. J. Cancer 2015, 34, 33. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Ioka, A.; Nakayama, T.; Tsukuma, H.; Nakamura, T. Comparison of trends in cancer incidence and mortality in Osaka, Japan, using an age-period-cohort model. Asian Pac. J. Cancer Prev. 2011, 12, 879–888. [Google Scholar] [PubMed]
- Funatogawa, I.; Funatogawa, T.; Yano, E. Trends in smoking and lung cancer mortality in Japan, by birth cohort, 1949–2010. Bull. World Health Organ. 2013, 91, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.P.; Mackay, D.F.; Morrison, D.; Pell, J.P. Smoking-related cancer in military veterans: Retrospective cohort study of 57,000 veterans and 173,000 matched non-veterans. BMC Cancer 2016, 16, 311. [Google Scholar] [CrossRef] [PubMed]
- Vokers, E.E.; Agrawal, N.; Seiwert, T.Y. HPV-associated head and neck cancer. J. Natl. Cancer Inst. 2015, 107, djv344. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Simard, E.P.; Dorell, C.; Noone, A.M.; Markowitz, L.E.; Kohler, B.; Eheman, C.; Saraiya, M.; Bandi, P.; Saslow, D.; et al. Annual report to the nation on the status of cancer, 1975–2009, featuring the burden and trends in Human Papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. J. Natl. Cancer Inst. 2013, 105, 175–201. [Google Scholar] [CrossRef] [PubMed]
- Sturgis, E.M.; Cinciripini, P.M. Trends in head and neck cancer incidence in relation to smoking prevalence. Cancer 2007, 110, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J. The status and future challenges of tobacco control policy in Korea. J. Prev. Med. Public Health 2014, 47, 129–135. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Mortality Database; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Kim, D.S.; Kim, Y.S.; Jung, K.; Chang, J.H.; Lim, C.; Lee, J.H.; Uh, S.; Shim, J.J.; Lew, W.J. Prevalence of chronic obstructive pulmonary disease in Korea: A population-based spirometry survey. Am. J. Respir. Crit. Care Med. 2005, 172, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Park, T.H.; Ko, Y.; Lee, S.J.; Lee, K.B.; Lee, J.; Han, M.K.; Park, J.M.; Cho, Y.J.; Hong, K.S.; Kim, D.H.; et al. Identifying target risk factors using population attributable risks of ischemic stroke by age and sex. J. Stroke 2015, 17, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, M.A.; Hayes, L.; Durkin, S.; Borland, R. Introduction effects of the Australian plain packaging policy on adult smokers: A cross-sectional study. BMJ Open 2013, 3, e003175. [Google Scholar] [CrossRef] [PubMed]
- Hammond, D.; Fong, G.T.; McDonald, P.W.; Cameron, R.; Brown, K.S. Impact of the graphic Canadian warning labels on adult smoking behaviour. Tob. Control 2003, 12, 391–395. [Google Scholar] [CrossRef] [PubMed]



| Categories | Total Cancer Deaths * | Tumor Sites | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Oropharynx | Esophageal | Larynx | Lung | |||||||
| N | % | N | % | N | % | N | % | N | % | |
| Total | 1,024,976 | 100.0 | 14,679 | 1.4 | 34,782 | 3.3 | 15,356 | 1.5 | 222,097 | 22.7 |
| Age group | ||||||||||
| <40 years | 52,415 | 5.1 | 604 | 4.1 | 234 | 0.7 | 217 | 1.4 | 3214 | 1.4 |
| 40–49 years | 99,598 | 9.7 | 1429 | 9.7 | 1707 | 4.9 | 764 | 5.0 | 10,632 | 4.8 |
| 50–59 years | 214,102 | 20.9 | 3347 | 22.8 | 7764 | 22.3 | 3123 | 20.3 | 35,164 | 15.8 |
| 60–69 years | 300,422 | 29.3 | 4619 | 31.5 | 12,970 | 37.3 | 5400 | 35.2 | 73,694 | 33.2 |
| ≥70 years | 358,439 | 35.0 | 4680 | 31.9 | 12,107 | 34.8 | 5852 | 38.1 | 99,393 | 44.8 |
| Year of death | ||||||||||
| 1984–1993 | 235,172 | 23.0 | 2212 | 15.1 | 8754 | 25.2 | 4512 | 29.4 | 34,586 | 15.6 |
| 1994–2003 | 351,795 | 34.3 | 5000 | 34.0 | 12,923 | 37.1 | 6582 | 42.9 | 77,197 | 34.7 |
| 2004–2013 | 438,009 | 42.7 | 7467 | 50.8 | 13,105 | 37.7 | 4262 | 27.7 | 110,314 | 49.7 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jee, Y.H.; Shin, A.; Lee, J.-K.; Oh, C.-M. Decreases in Smoking-Related Cancer Mortality Rates Are Associated with Birth Cohort Effects in Korean Men. Int. J. Environ. Res. Public Health 2016, 13, 1208. https://doi.org/10.3390/ijerph13121208
Jee YH, Shin A, Lee J-K, Oh C-M. Decreases in Smoking-Related Cancer Mortality Rates Are Associated with Birth Cohort Effects in Korean Men. International Journal of Environmental Research and Public Health. 2016; 13(12):1208. https://doi.org/10.3390/ijerph13121208
Chicago/Turabian StyleJee, Yon Ho, Aesun Shin, Jong-Keun Lee, and Chang-Mo Oh. 2016. "Decreases in Smoking-Related Cancer Mortality Rates Are Associated with Birth Cohort Effects in Korean Men" International Journal of Environmental Research and Public Health 13, no. 12: 1208. https://doi.org/10.3390/ijerph13121208






