Association among Complement Factor H Autoantibodies, Deletions of CFHR, and the Risk of Atypical Hemolytic Uremic Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Studies
3.2. CFHR1 Deficiency and aHUS Risk
3.3. CFHR1/R3 Deficiency with aHUS Risk
3.4. CFHR1 Deficiency, Anti-CFH, and aHUS Risk
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Noris, M.; Remuzzi, G. Atypical hemolytic-uremic syndrome. N. Engl. J. Med. 2009, 361, 1676–1687. [Google Scholar] [CrossRef] [PubMed]
- Bresin, E.; Rurali, E.; Caprioli, J.; Sanchezcorral, P.; Fremeauxbacchi, V.; De Cordoba, S.R.; Pinto, S.; Albert, M.; Ribes, D.; Valoti, E.; et al. Combined complement gene mutations in atypical hemolytic uremic syndrome influence clinical phenotype. JASN 2013, 24, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Loirat, C.; Fremeauxbacchi, V. Atypical hemolytic uremic syndrome. Orphanet. J. Rare Dis. 2011, 6, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Westra, D.; Wetzels, J.F.; Volokhina, E.B.; van den Heuvel, L.P.; van de Kar, N.C. A new era in the diagnosis and treatment of atypical hemolytic uremic syndrome. Neth. J. Med. 2012, 70, 121–129. [Google Scholar] [PubMed]
- Warwicker, P.; Goodship, T.H.; Donne, R.L.; Pirson, Y.; Nicholls, A.; Ward, R.; Turnpenny, P.D.; Goodship, J.A. Genetic studies into inherited and sporadic hemolytic uremic syndrome. Kidney Int. 1998, 53, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Skerka, C.; Qian, C.; Fremeauxbacchi, V.; Roumenina, L.T. Complement factor H related proteins (CFHRs). Mol. Immunol. 2013, 56, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Harboe, M.; Thorgersen, E.B.; Mollnes, T.E. Advances in assay of complement function and activation. Adv. Drug Deliv. Rev. 2011, 63, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Schramm, E.C.; Roumenina, L.T.; Rybkine, T.; Chauvet, S.; Vieiramartins, P.; Hue, C.; Maga, T.; Valoti, E.; Wilson, V.; Jokiranta, T.S.; et al. Mapping interactions between complement C3 and regulators using mutations in atypical haemolytic uraemic syndrome. Blood 2015, 125, 2359–2369. [Google Scholar] [CrossRef] [PubMed]
- Noris, M.; Remuzzi, G. Glomerular diseases dependent on complement activation, including atypical hemolytic uremic syndrome, membranoproliferative glomerulonephritis, and C3 glomerulopathy: Core curriculum 2015. Am. J. Kidney Dis. 2015, 66, 359–375. [Google Scholar] [CrossRef] [PubMed]
- Abarrategui-Garrido, C.; Martínez-Barricarte, R.; López-Trascasa, M.; de Córdoba, S.R.; Sánchez-Corral, P. Characterization of complement factor H-related (CFHR) proteins in plasma reveals novel genetic variations of CFHR1 associated with atypical hemolytic uremic syndrome. Blood 2009, 114, 4261–4271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valoti, E.; Alberti, M.; Tortajada, A.; Garciafernandez, J.; Gastoldi, S.; Besso, L.; Bresin, E.; Remuzzi, G.; De Cordoba, S.R.; Noris, M. A novel atypical hemolytic uremic syndrome–associated hybrid CFHR1/CFH gene encoding a fusion protein that antagonizes factor H–dependent complement regulation. JASN 2015, 26, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Bu, F.; Maga, T.; Meyer, N.C.; Wang, K.; Thomas, C.P.; Nester, C.M.; Smith, R.J. Comprehensive genetic analysis of complement and coagulation genes in atypical hemolytic uremic syndrome. JASN 2014, 5, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, P.F.; Edey, M.; Heinen, S.; Jozsi, M.; Richter, H.; Misselwitz, J.; Hoppe, B.; Routledge, D.; Strain, L.; Hughes, A.E.; et al. Deletion of complement factor H–related genes CFHR1 and CFHR3 is associated with atypical hemolytic uremic syndrome. PLoS Genet. 2007, 3, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Hofer, J.; Janecke, A.; Zimmerhackl, L.B.; Riedl, M.; Rosales, A.; Giner, T.; Cortina, G.; Haindl, C.J.; Petzelberger, B.; Pawlik, P.; et al. Complement factor H-related protein 1 deficiency and factor H antibodies in pediatric patients with atypical hemolytic uremic syndrome. Clin. J. Am. Soc. Nephrol. 2013, 8, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Dragon-Durey, M.; Blanc, C.; Marliot, F. The high frequency of complement factor H related CFHR1 gene deletion is restricted to specific subgroups of patients with atypical hemolytic uremic syndrome. J. Med. Genet. 2009, 46, 447–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noris, M.; Remuzzi, G. Overview of complement activation and regulation. Semin. Nephrol. 2013, 33, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Zadura, A.F.; Zipfel, P.F.; Bokarewa, M.; Sturfelt, G.; Jonsen, A.; Nilsson, S.; Hillarp, A.; Saxne, T.; Trouw, L.A.; Blom, A.M. Factor H autoantibodies and deletion of complement factor H-related protein-1 in rheumatic diseases in comparison to atypical hemolytic uremic syndrome. Arthritis Res. Ther. 2012, 14, R185. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, D.; Roberton, J.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in the Meta-Analysis. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 20 September 2016).
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C.E. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Moore, I.; Strain, L.; Pappworth, I.Y.; Kavanagh, D.J.; Barlow, P.N.; Herbert, A.P.; Schmidt, C.Q.; Staniforth, S.J.; Holmes, L.V.V.; Ward, R.; et al. Association of factor H autoantibodies with deletions of CFHR1, CFHR3, CFHR4, and with mutations in CFH, CFI, CD46, and C3 in patients with atypical hemolytic uremic syndrome. Blood 2010, 115, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, Y.S.; Lee, J.H.; Park, S.J.; Shin, J.I.; Park, Y.; Yoo, K.H.; Cho, M.H.; Kim, S.; Kim, S.H.; et al. Atypical hemolytic uremic syndrome: Korean pediatric series. Pediatr. Int. 2015, 57, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Gulati, A.; Saini, S.; Blanc, C.; Gupta, A.; Gurjar, B.S.; Saini, H.; Kotresh, S.; Ali, U.S.; Bhatia, D.; et al. Prompt plasma exchanges and immunosuppressive treatment improves the outcomes of anti-factor H autoantibody-associated hemolytic uremic syndrome in children. Kidney Int. 2014, 85, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Carneirosampaio, M.; Liphaus, B.L.; Jesus, A.A.; Silva, C.A.; Oliveria, J.B.; Kiss, M.H. Understanding systemic lupus erythematosus physiopathology in the light of primary immunodeficiencies. J. Clin. Immunol. 2008, 28, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Hyvärinen, S.; Uchida, K.; Varjosalo, M.; Jokela, R.; Jokiranta, T.S. Recognition of malondialdehyde-modified proteins by the C terminus of complement factor H is mediated via the polyanion binding site and impaired by mutations found in atypical hemolytic uremic syndrome. J. Biol. Chem. 2014, 289, 4295–4306. [Google Scholar] [CrossRef] [PubMed]
- Mele, C.; Remuzzi, G.; Noris, M. Hemolytic uremic syndrome. Semin. Immunopathol. 2014, 36, 399–420. [Google Scholar] [CrossRef] [PubMed]
- Venables, J.P.; Strain, L.; Routledge, D.; Bourn, D.; Powell, H.M.; Warwicker, P.; Diaztorres, M.L.; Sampson, A.; Mead, P.A.; Webb, M.; et al. Atypical hemolytic uremic syndrome associated with a hybrid complement gene. PLoS Med. 2006, 3, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, P.F.; Skerka, C. Complement regulators and inhibitory proteins. Nat. Rev. Immunol. 2009, 9, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Tortajada, A.; Yebenes, H.; Abarrateguigarrido, C.; Anter, J.; Garciafernandez, J.; Martinezbarricarte, R.; Albadominguez, M.; Malik, T.H.; Bedoya, R.; Perez, R.C.; et al. C3 glomerulopathy–associated CFHR1 mutation alters FHR oligomerization and complement regulation. J. Clin. Investig. 2013, 123, 2434–2446. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Reuter, S.; Trojnar, E.; Kolodziejczyk, R.; Seeberger, H.; Hyvarinen, S.; Uzonyi, B.; Szilagyi, A.; Prohaszka, Z.; Goldman, A.; et al. The major autoantibody epitope on Factor H in atypical Hemolytic Uremic Syndrome is structurally different from its homologous site in Factor H related protein 1 supporting a novel model for induction of autoimmunity in this disease. J. Biol. Chem. 2015, 290, 9500–9510. [Google Scholar] [CrossRef] [PubMed]
- Blanc, C.; Roumenina, L.T.; Ashraf, Y.; Hyvarinen, S.; Sethi, S.K.; Ranchin, B.; Niaudet, P.; Loirat, C.; Gulati, A.; Bagga, A.; et al. Overall Neutralization of Complement Factor H by autoantibodies in the acute phase of the autoimmune form of atypical hemolytic uremic syndrome. J. Immunol. 2012, 189, 3528–3537. [Google Scholar] [CrossRef] [PubMed]
- Vernon, K.A.; Ruseva, M.M.; Cook, H.T.; Botto, M.; Malik, T.H.; Pickering, M.C. Partial complement factor H deficiency associates with C3 glomerulopathy and thrombotic microangiopathy. J. Am. Soc. Nephrol. 2015, 27, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- Pickering, M.C.; De Jorge, E.G.; Martinezbarricarte, R.; Recalde, S.; Garcialayana, A.; Rose, K.L.; Moss, J.; Walport, M.J.; Cook, H.T.; De Cordoba, S.R.; et al. Spontaneous hemolytic uremic syndrome triggered by complement factor H lacking surface recognition domains. J. Exp. Med. 2007, 204, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Francis, N.J.; Mcnicholas, B.; Awan, A.; Waldron, M.; Reddan, D.N.; Sadlier, D.M.; Kavanagh, D.J.; Strain, L.; Marchbank, K.J.; Harris, C.L.; et al. A novel hybrid CFH/CFHR3 gene generated by a microhomology-mediated deletion in familial atypical hemolytic uremic syndrome. Blood 2012, 119, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Strobel, S.; Abarrategui-Garrido, C.; Fariza-Requejo, E.; Seeberger, H.; Sánchez-Corral, P.; Józsi, M. Factor H-related protein 1 neutralizes anti-factor H autoantibodies in autoimmune hemolytic uremic syndrome. Kidney Int. 2011, 80, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Heinen, S.; Hartmann, A.; Lauer, N.; Wiehl, U.; Dahse, H.; Schirmer, S.; Gropp, K.; Enghardt, T.; Wallich, R.; Halbich, S.; et al. Factor H-related protein 1 (CFHR-1) inhibits complement C5 convertase activity and terminal complex formation. Blood 2009, 114, 2439–2447. [Google Scholar] [CrossRef] [PubMed]
- Jozsi, M.; Licht, C.; Strobel, S.; Zipfel, S.L.H.; Richter, H.; Heinen, S.; Zipfel, P.F.; Skerka, C. Factor H autoantibodies in atypical hemolytic uremic syndrome correlate with CFHR1/CFHR3 deficiency. Blood 2008, 111, 1512–1514. [Google Scholar] [CrossRef] [PubMed]
- Dragondurey, M.A.; Loirat, C.; Cloarec, S.; Macher, M.; Blouin, J.; Nivet, H.; Weiss, L.; Fridman, W.H.; Fremeauxbacchi, V. Anti-Factor H autoantibodies associated with atypical hemolytic uremic syndrome. J. Am. Soc. Nephrol. 2005, 16, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Strobel, S.; Hoyer, P.F.; Mache, C.J.; Sulyok, E.; Liu, W.; Richter, H.; Oppermann, M.; Zipfel, P.F.; Jozsi, M. Functional analyses indicate a pathogenic role of factor H autoantibodies in atypical haemolytic uraemic syndrome. Nephrol. Dial. Trans. 2010, 25, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Legendre, C.; Licht, C.; Muus, P.; Greenbaum, L.A.; Babu, S.; Bedrosian, C.L.; Bingham, C.; Cohen, D.J.; Delmas, Y.; Douglas, K.; et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N. Engl. J. Med. 2013, 368, 2169–2181. [Google Scholar] [CrossRef] [PubMed]
- Nozal, P.; Bernabeuherrero, M.E.; Uzonyi, B.; Szilagyi, A.; Hyvarinen, S.; Prohaszka, Z.; Jokiranta, T.S.; Sanchezcorral, P.; Lopeztrascasa, M.; Jozsi, M. Heterogeneity but individual constancy of epitopes, isotypes and avidity of factor H autoantibodies in atypical hemolytic uremic syndrome. Mol. Immunol. 2016, 70, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Holmes, L.V.; Strain, L.; Staniforth, S.J.; Moore, I.; Marchbank, K.J.; Kavanagh, D.J.; Goodship, J.A.; Cordell, H.J.; Goodship, T.H.J. Determining the population frequency of the CFHR3/CFHR1 deletion at 1q32. PLoS ONE 2013, 8, e60352. [Google Scholar] [CrossRef] [PubMed]
- Dragondurey, M.; Sethi, S.K.; Bagga, A.; Blanc, C.; Blouin, J.; Ranchin, B.; Andre, J.; Takagi, N.; Cheong, H.I.; Hari, P.; et al. Clinical features of anti-factor H autoantibody-associated hemolytic uremic syndrome. J. Am. Soc. Nephrol. 2010, 21, 2180–2187. [Google Scholar] [CrossRef] [PubMed]
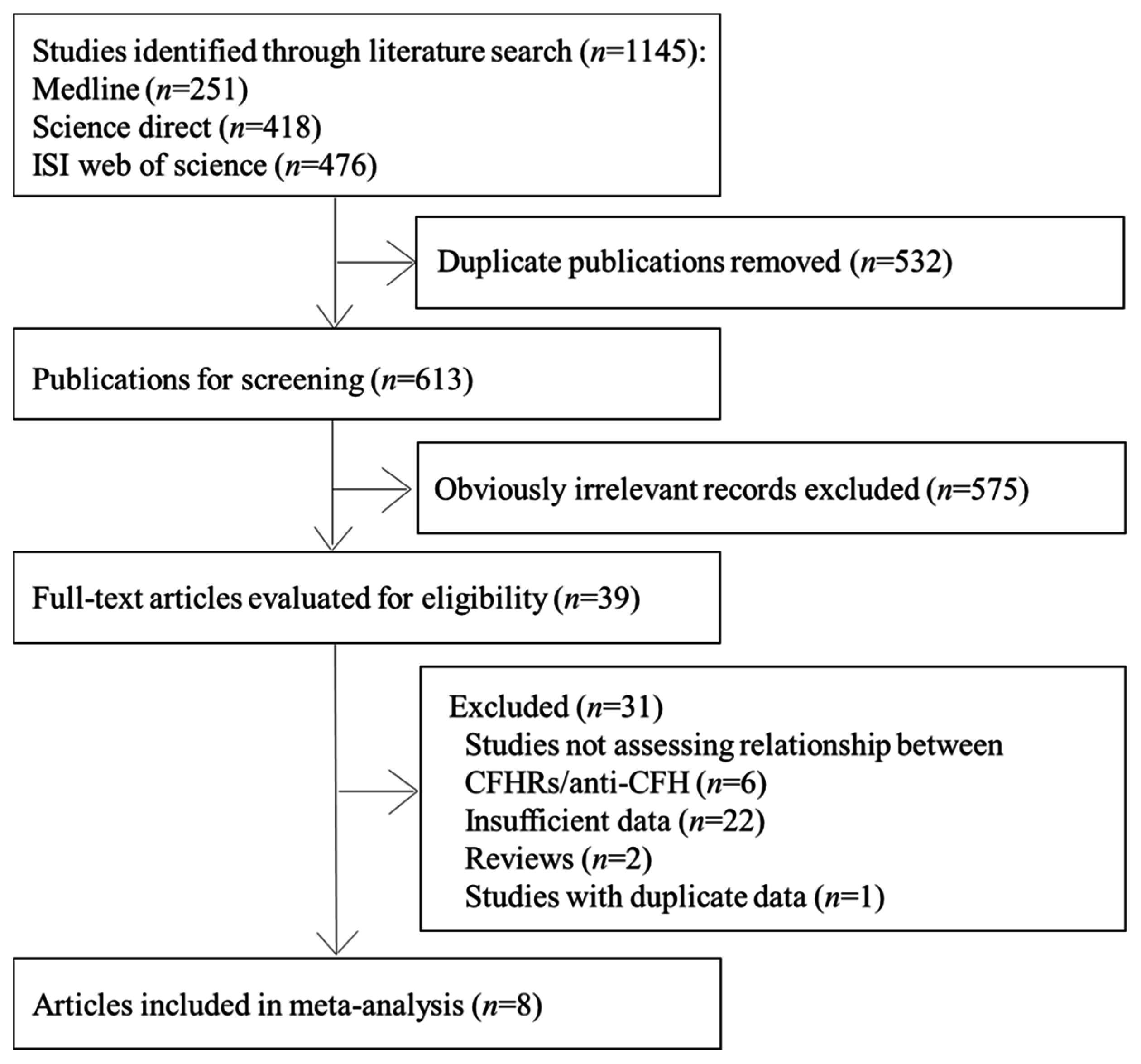
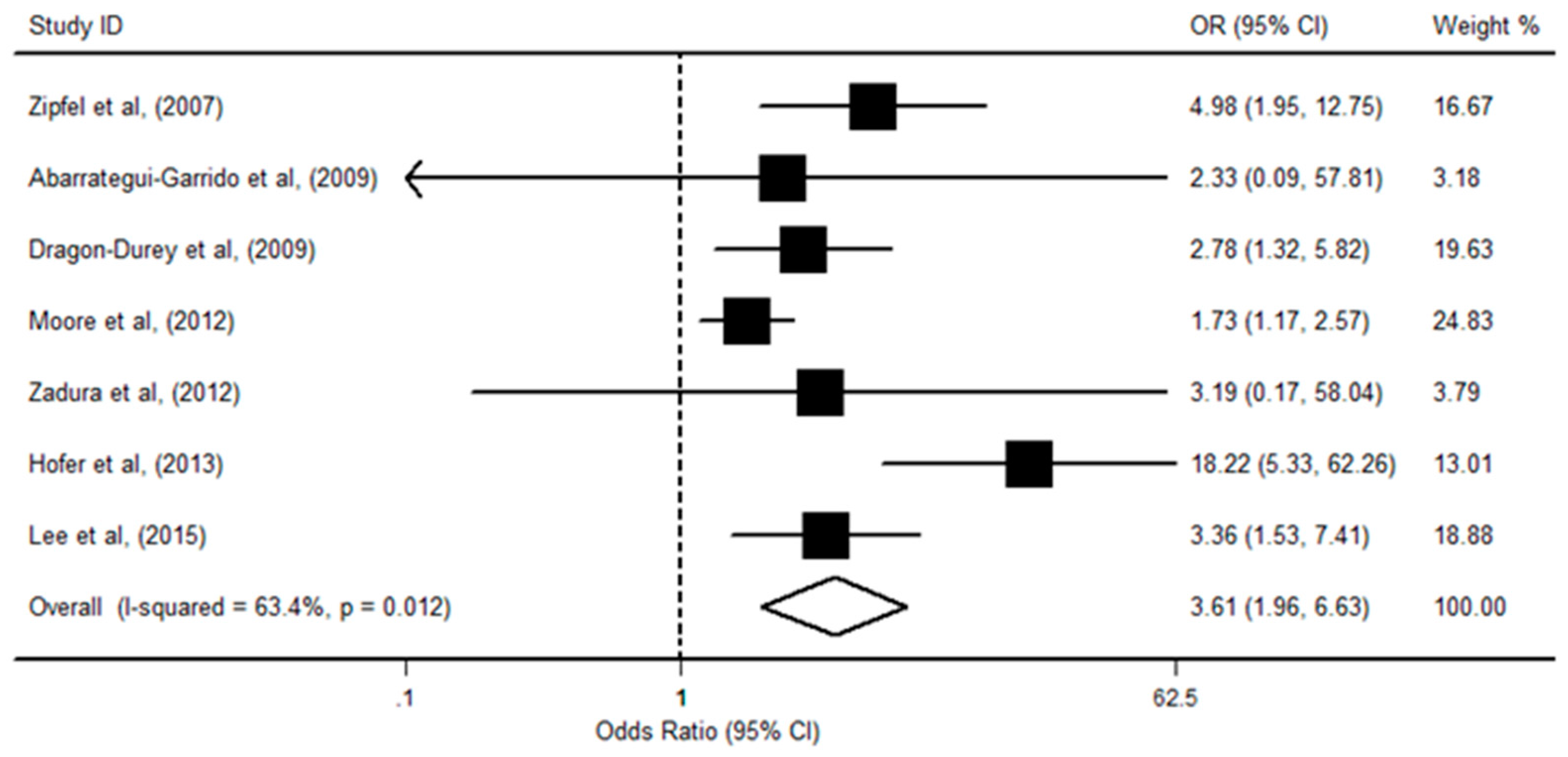
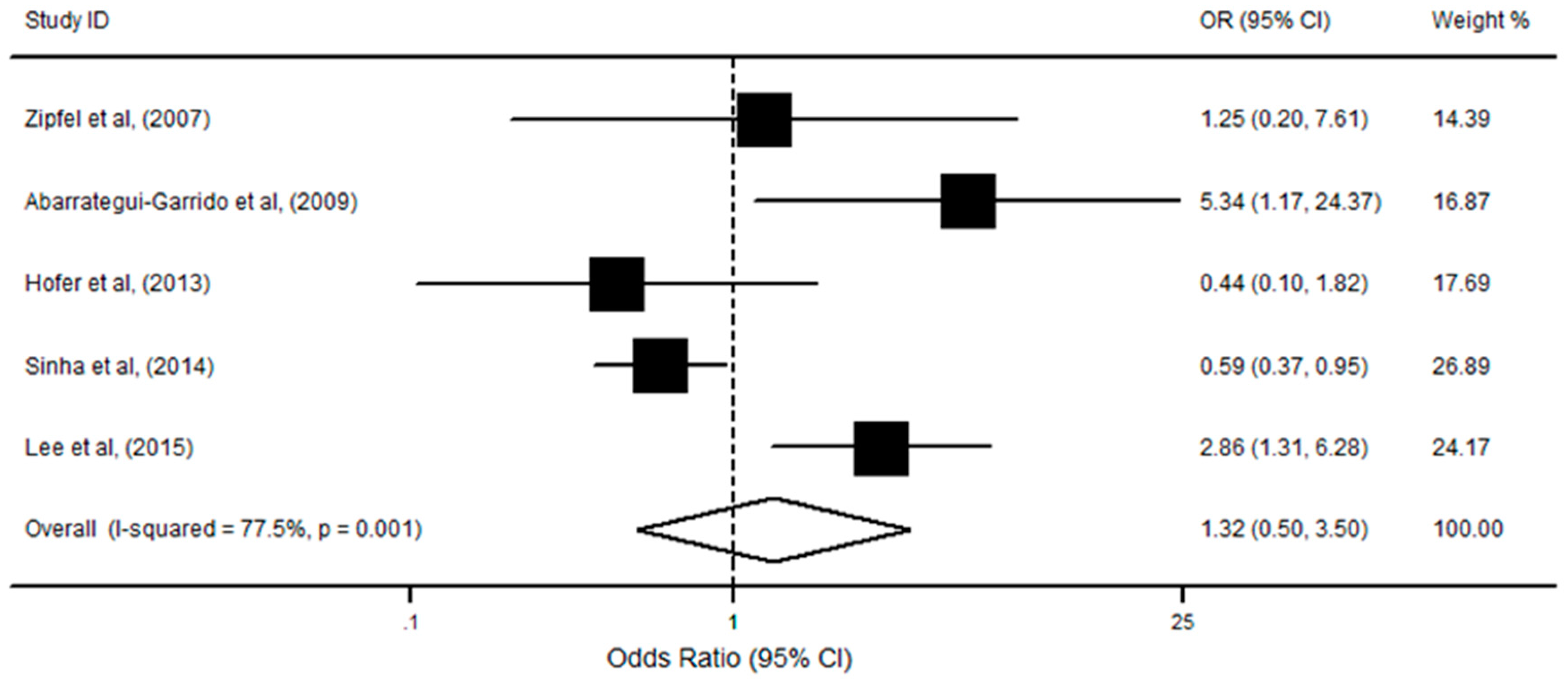
| First Author (y) | Country of Origin | Study Type | Sample Size (Cases) | Sample Size (Controls) | Source of Controls | Gene Detection Methods | Protein Detection Methods | Study Quality * |
|---|---|---|---|---|---|---|---|---|
| Lee et al., 2015 | Korea | Case-Control | 51 | 100 | HB | MLPA | ELISA | High |
| Sinha et al., 2014 | Indian | Case-Control | 138 | 84 | PB | MLPA | Western blot | High |
| Hofer et al., 2013 | European | Case-Control | 116 | 118 | PB | CGH | ELISA | Low |
| Moore et al., 2012 | UK | Case-Control | 142 | 100 | PB | MLPA | ELISA | High |
| Zadura et al., 2012 | German | Case-Control | 103 | 345 | PB | - | ELISA | High |
| Abarrategui-Garrid et al., 2009 | Spain | Case-Control | 151 | 230 | HB + PB | MLPA | ELISA | High |
| Dragon-Durey et al., 2009 | France | Case-Control | 177 | 70 | PB | MLPA | ELISA | High |
| Zipfel et al., 2007 | Germany | Case-Control | 121 | 100 | PB | PCR | NR | Low |
| Zipfel et al., 2007 | UK | Case-Control | 66 | 119 | PB | MLPA | Western Blot | Low |
| Subgroup | N | Pooled OR | 95% CI | p | |
|---|---|---|---|---|---|
| Heterogeneity | Meta-Regression | ||||
| CFHR1 Deficiency | - | - | - | - | - |
| Study Quality | 7 | - | - | - | 0.059 |
| High | 5 | 2.12 | 1.55, 2.90 | 0.572 | |
| Low | 2 | 3.61 | 1.96, 6.63 | 0.095 | |
| Ethnicity | - | - | - | - | 0.703 |
| Caucasians | 6 | 1.88 | 1.22, 2.54 | 0.005 | |
| Asians | 1 | 3.36 | 0.42, 6.30 | NA | |
| Gene Detection Methods | 6 | - | - | - | - |
| MLPA | 5 | 2.58 | 1.70, 3.91 | <0.001 | 0.001 |
| CGH | 1 | 18.22 | 5.33, 62.26 | NA | |
| CFHR1/R3 Deficiency | - | - | - | - | - |
| Study Quality | 5 | - | - | - | 0.763 |
| High | 4 | 1.35 | 0.44, 4.15 | 0.001 | |
| Low | 1 | 1.25 | 0.20, 7.61 | NA | |
| Ethnicity | 5 | - | - | - | 0.694 |
| Caucasians | 3 | 1.41 | 0.31, 6.52 | 0.06 | |
| Asians | 2 | 1.26 | 0.27, 5.09 | 0.001 | |
| Gene Detection Methods | 5 | - | - | - | - |
| MLPA | 3 | 1.86 | 0.47, 7.35 | 0.376 | 0.461 |
| CGH | 1 | 0.44 | 0.10, 1.82 | NA | |
| PCR | 1 | 1.25 | 0.20, 761 | NA | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Fan, M.-N.; Yang, M.; Lu, C.; Zhang, M.; Liu, X.-H.; Ma, L. Association among Complement Factor H Autoantibodies, Deletions of CFHR, and the Risk of Atypical Hemolytic Uremic Syndrome. Int. J. Environ. Res. Public Health 2016, 13, 1209. https://doi.org/10.3390/ijerph13121209
Jiang H, Fan M-N, Yang M, Lu C, Zhang M, Liu X-H, Ma L. Association among Complement Factor H Autoantibodies, Deletions of CFHR, and the Risk of Atypical Hemolytic Uremic Syndrome. International Journal of Environmental Research and Public Health. 2016; 13(12):1209. https://doi.org/10.3390/ijerph13121209
Chicago/Turabian StyleJiang, Hong, Meng-Nan Fan, Min Yang, Chao Lu, Ming Zhang, Xiao-Hong Liu, and Le Ma. 2016. "Association among Complement Factor H Autoantibodies, Deletions of CFHR, and the Risk of Atypical Hemolytic Uremic Syndrome" International Journal of Environmental Research and Public Health 13, no. 12: 1209. https://doi.org/10.3390/ijerph13121209






