Fine Particulate Matter in Urban Environments: A Trigger of Respiratory Symptoms in Sensitive Children
Abstract
:1. Introduction
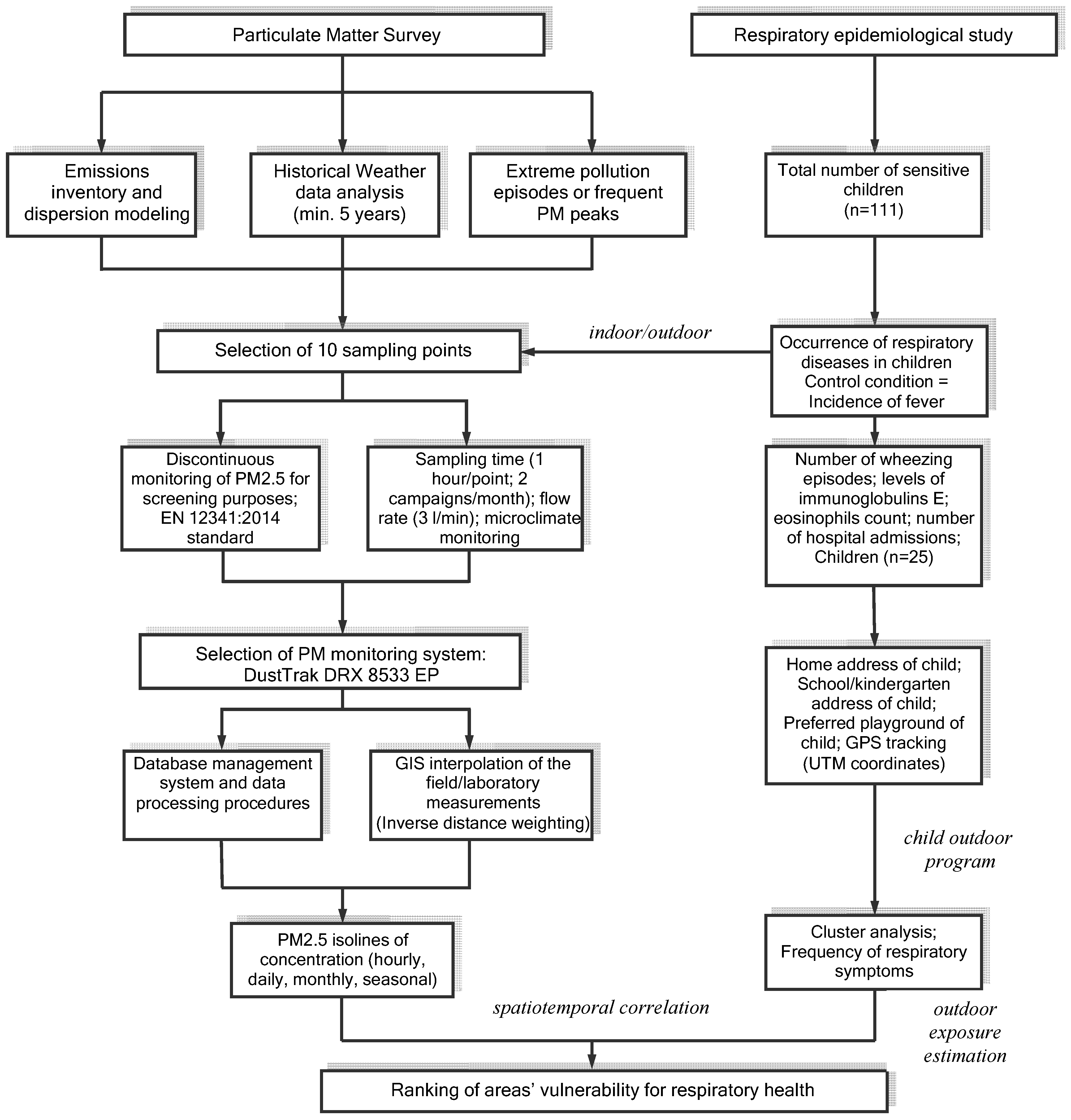
2. Materials and Methods
2.1. Site Description
2.2. Assessment of the Respiratory Symptoms in Children Due to PM2.5 in Targoviste, Romania
2.3. Particulate Matter Monitoring
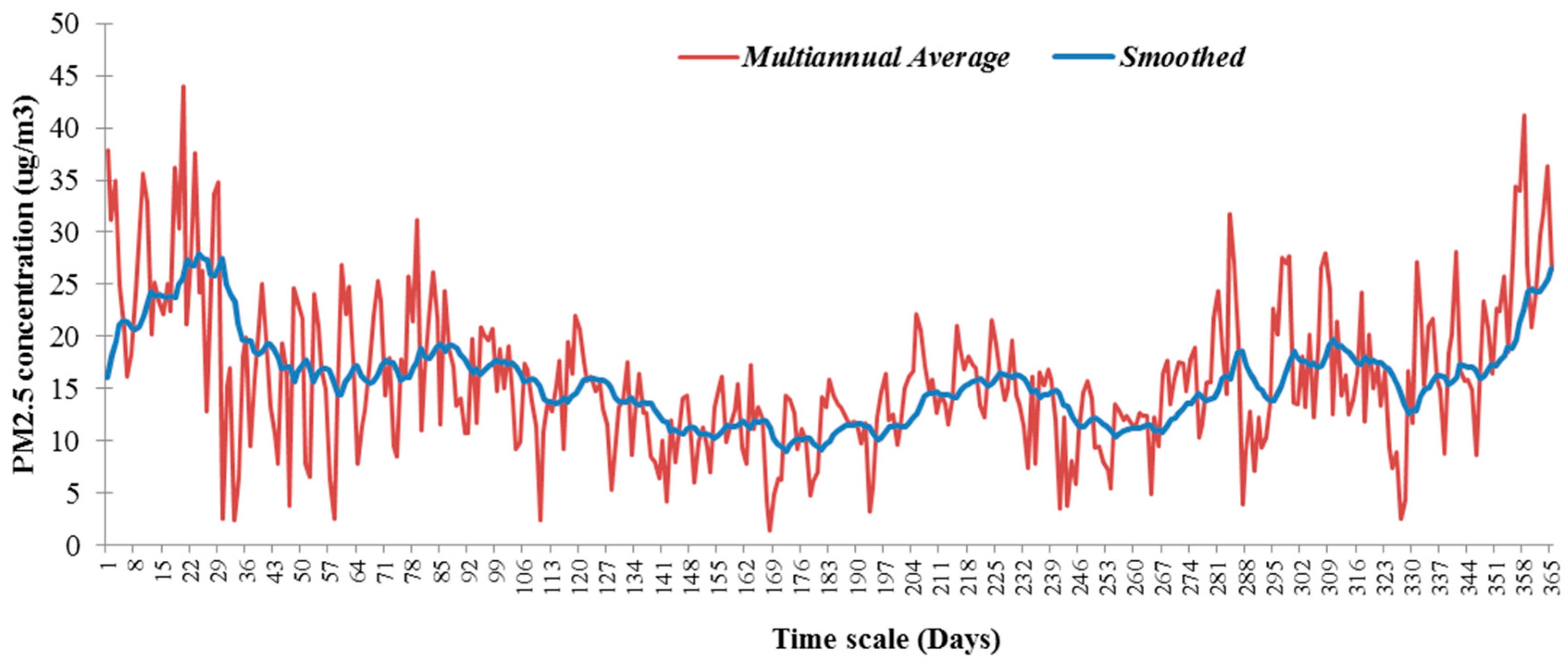
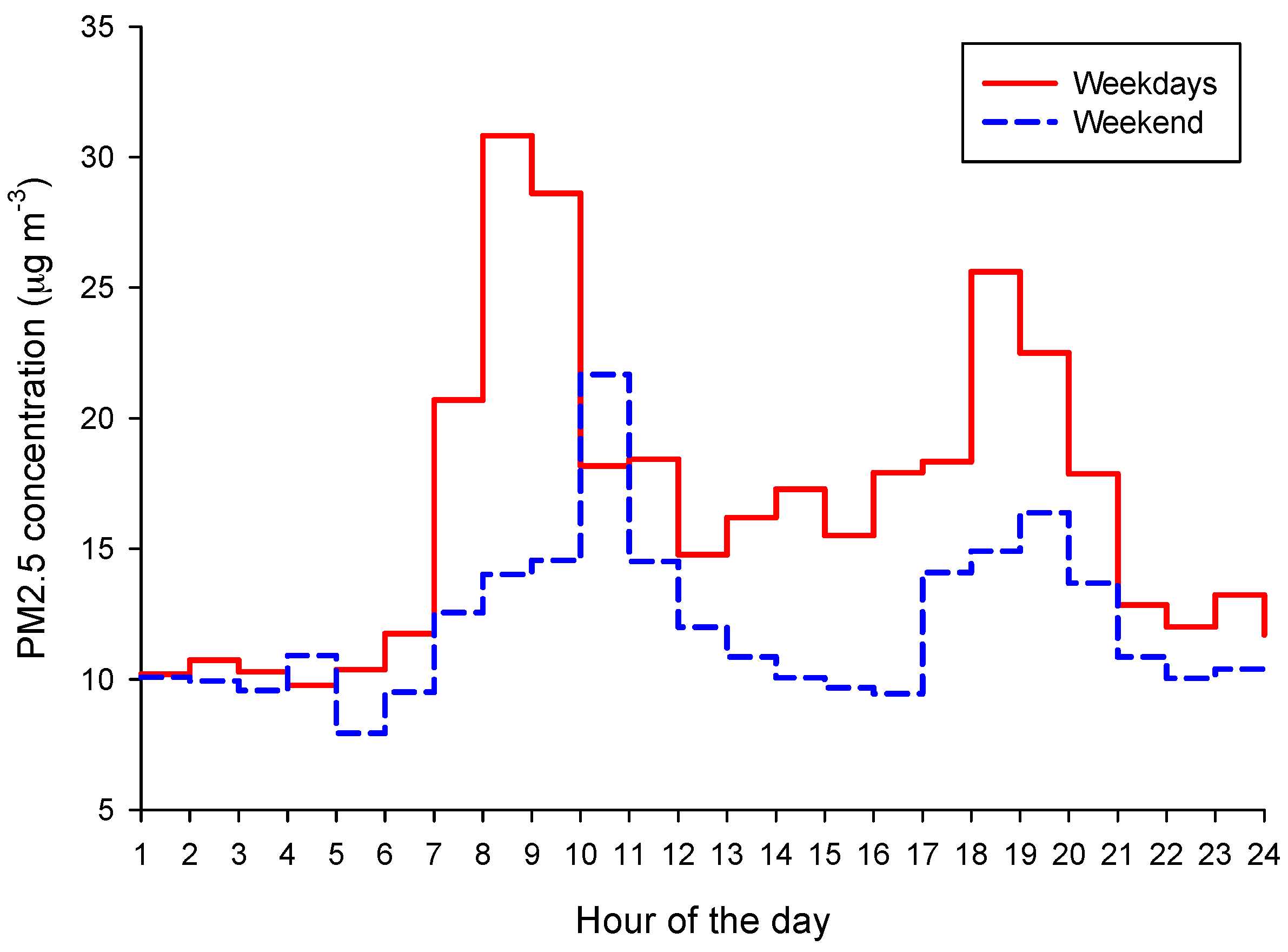
2.4. PM2.5 Time Series Analysis
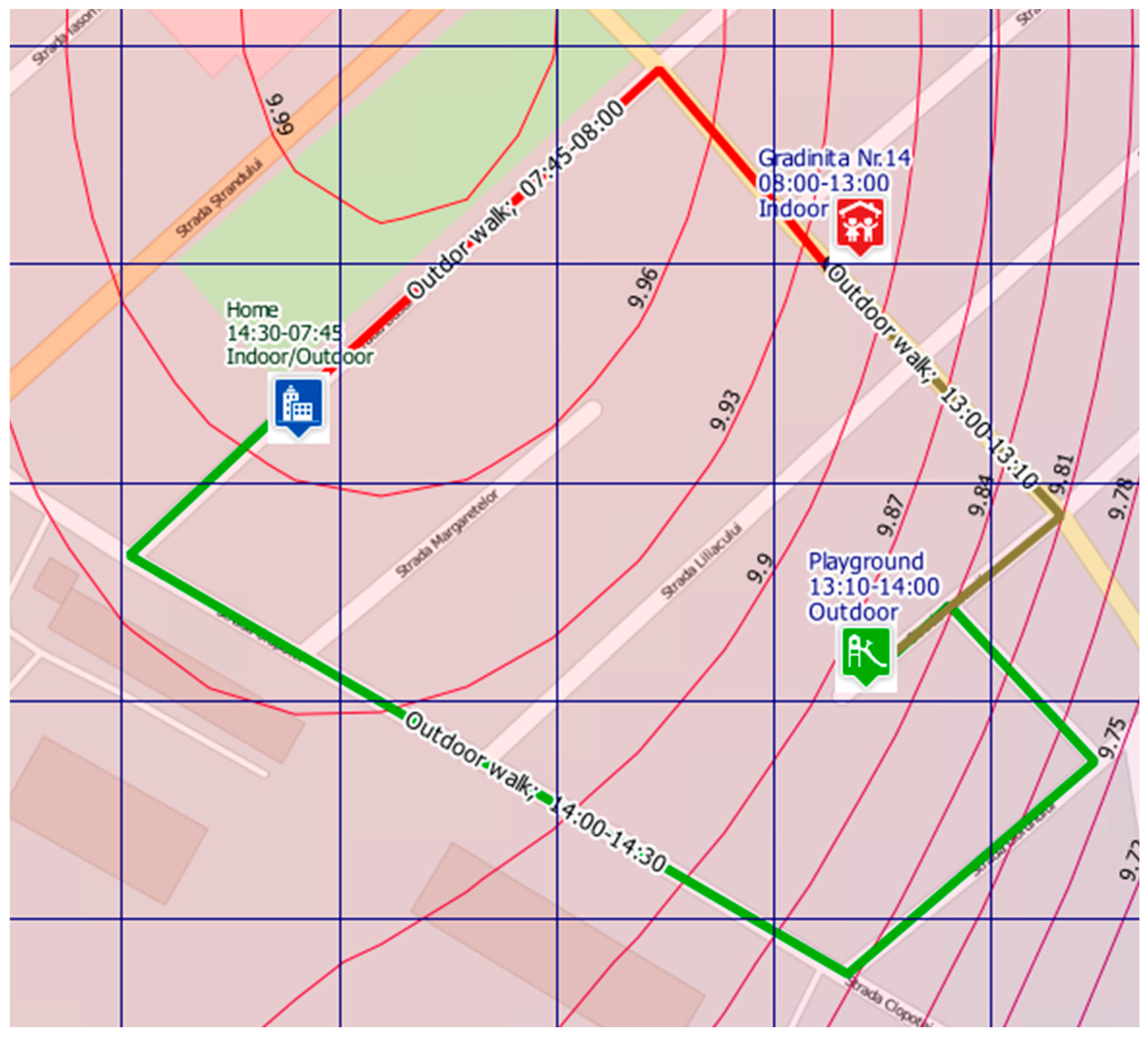
2.5. Outdoor Exposure Assessment
2.6. Statistical Analysis
3. Results and Discussion
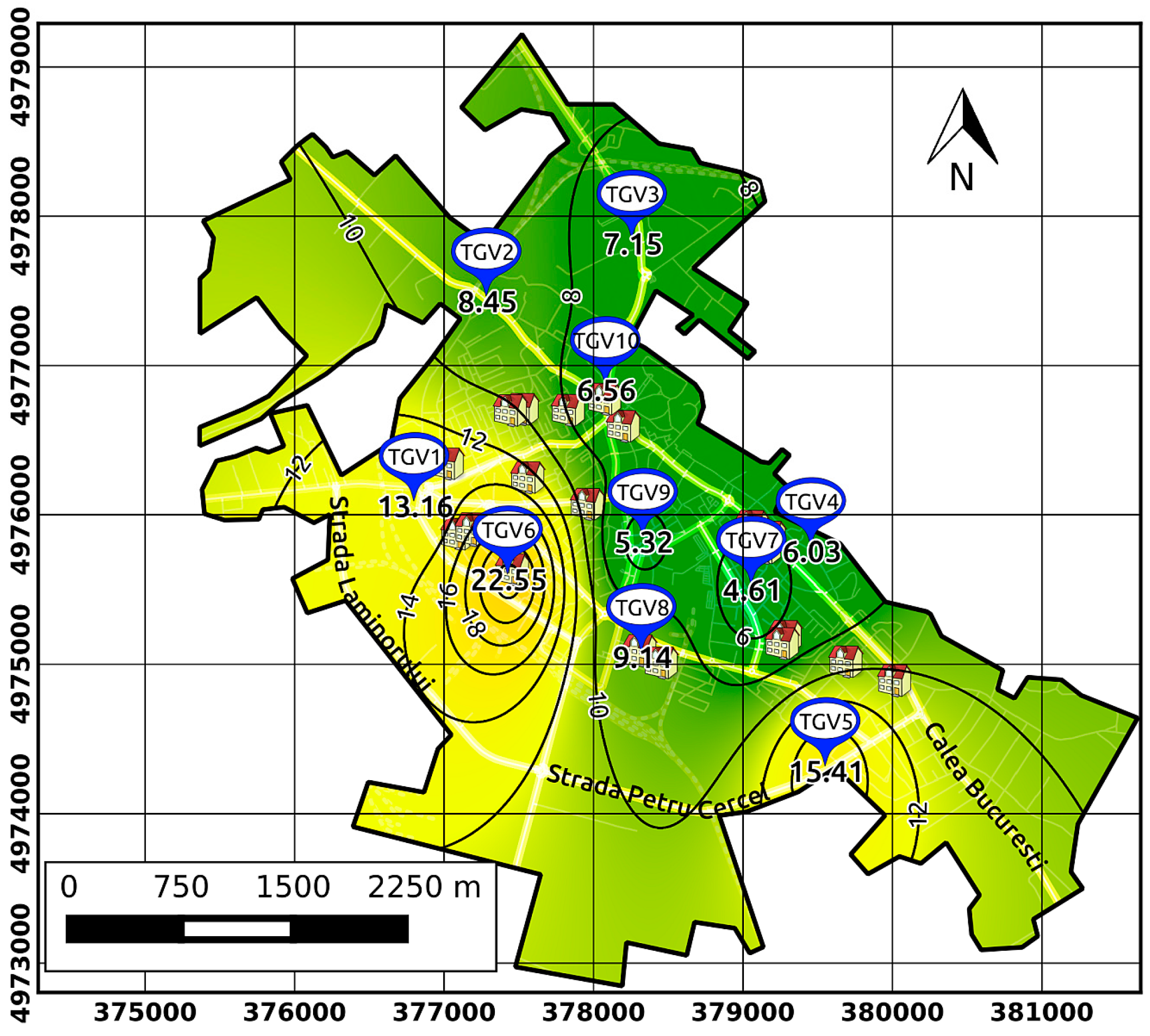
3.1. Assessment of PM2.5 Concentrations and Their Spatiotemporal Variability in Targoviste
3.2. Assessment of the PM2.5 Dose in the Analysed Group of Children
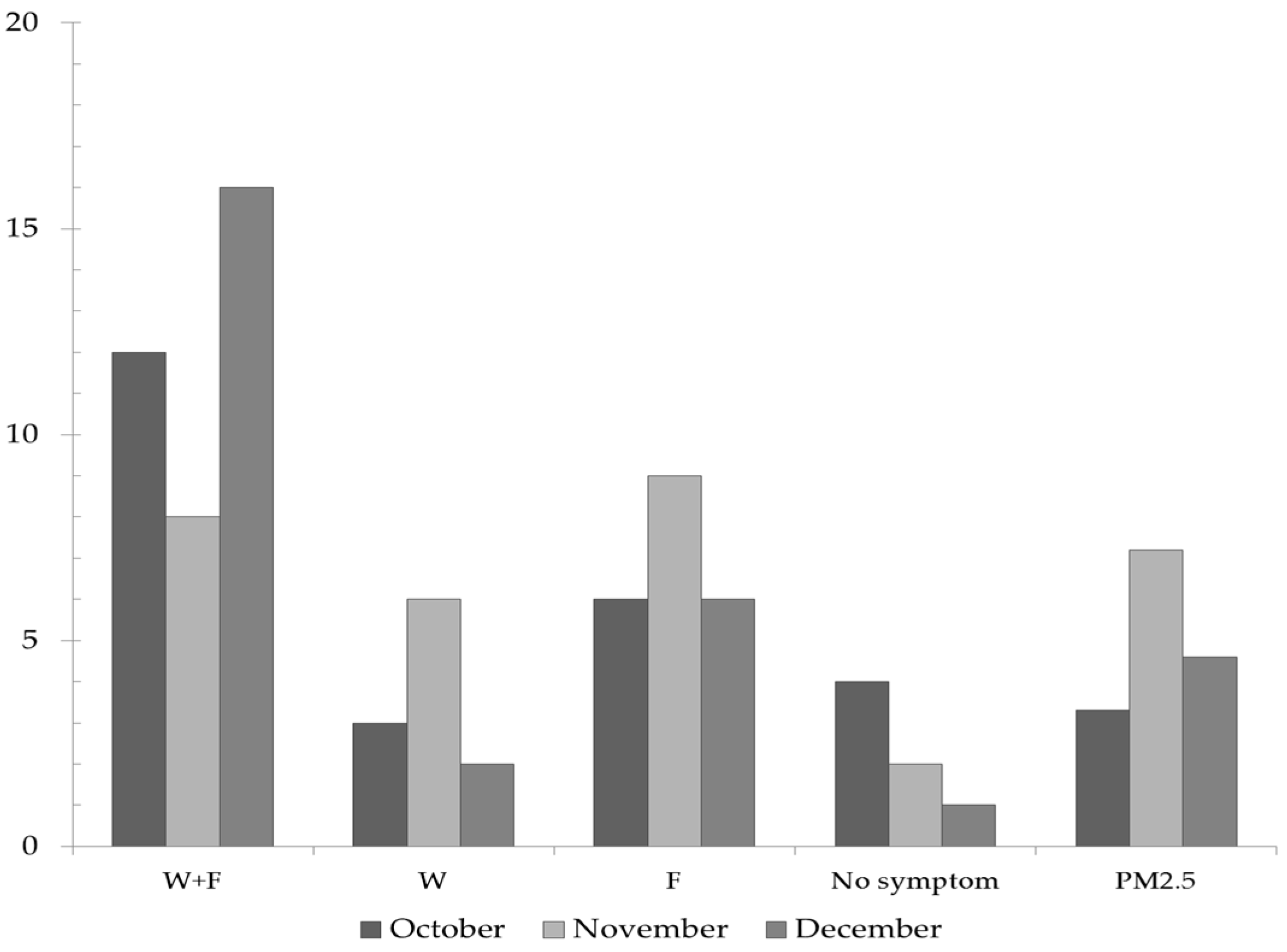
3.3. Assessment of the Respiratory Symptoms Due to Outdoor PM2.5 Concentrations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Review of Evidence on Health Aspects of Air Pollution—REVIHAAP Project Technical Report. 2013. Available online: http://www.euro.who.int/__data/assets/pdf_file/0004/193108/REVIHAAP-Final-technical-report-final-version.pdf?ua=1 (accessed on 1 August 2016).
- Pope, C.A.; Dockery, D.W. Health effects of fine particulate air pollution: Lines that connect. J. Air Waste Manag. Assoc. 2006, 56, 709–742. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J.; Etzel, R.A. Textbook of Children’s Environmental Health; Oxford University Press: New York, NY, USA, 2014. [Google Scholar]
- Tortajada, J.I.; Castell, J.I.; Andreu, J.A.L.; Dominguez, F.I.; Garcia, J.A.O.; Tornero, O.B.; Garcia, V.I.; Conesa, A.C. Diseases associated to atmospheric pollution from fossil fuels. Pediatrics aspects. Rev. Esp. Pediatr. 2001, 57, 213–249. (In Spanish) [Google Scholar]
- Zeng, X.W.; Vivian, E.; Mohammed, K.; Jakhar, S.; Vaughn, M.; Huang, J.; Zelicoff, A.; Xaverius, P.; Bai, Z.; Lin, S.; et al. Long-term ambient air pollution and lung function impairment in Chinese children from a high air pollution range area: The Seven Northeastern Cities (SNEC) study. Atmos. Environ. 2016, 138, 144–151. [Google Scholar] [CrossRef]
- Chen, C.H.; Chan, C.C.; Chen, B.-Y.; Cheng, T.-J.; Guo, Y.L. Effects of particulate air pollution and ozone on lung function in non-asthmatic children. Environ. Res. 2015, 137, 40–48. [Google Scholar]
- Hwang, B.F.; Chen, Y.H.; Lin, Y.T.; Wu, X.T.; Lee, Y.L. Relationship between exposure to fine particulates and ozone and reduced lung function in children. Environ. Res. 2015, 137, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Gehring, U.; Gruzieva, O.; Agius, R.M.; Beelen, R.; Custovic, A.; Cyrys, J.; Eeftens, M.; Flexeder, C.; Fuertes, E.; Heinrich, J.; et al. Air pollution exposure and lung function in children: The ESCAPE Project. Environ. Health Perspect. 2013, 121, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Chua, H.L.; Collis, G.G.; Newbury, A.M.; Chan, K.; Bower, G.D.; Sly, P.D.; Le Souef, P.N. The influence of age on aerosol deposition in children with cystic fibrosis. Eur. Respir. J. 1994, 7, 2185–2191. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.; Simões, E.A.; Rudan, I.; Gessner, B.D.; Azziz-Baumgartner, E.; Zhang, J.S.F.; Feikin, D.R.; Mackenzie, G.A.; Moiïsi, J.C.; Roca, A.; et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: A systematic analysis. Lancet 2013, 381, 1380–1390. [Google Scholar] [CrossRef]
- Selvaraj, K.; Chinnakali, P.; Majumdar, A.; Krishnan, I.S. Acute respiratory infections among under-5 children in India: A situational analysis. J. Nat. Sci. Biol. Med. 2014, 5, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Amarchand, R.; Gupta, V.; Lafond, K.E.; Suliankatchi, R.A.; Saha, S.; Rai, S.; Misra, P.; Purakayastha, D.R.; Wahi, A.; et al. Epidemiology of acute respiratory infections in children—Preliminary results of a cohort in a rural north Indian community. BMC Infect. Dis. 2015. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Chatterjee, A.; Johston, S. Acute Repiratory Infections. In Effects of Air Pollution on Children’s Health and Development. A Review of the Evidence; Copenhagen Denmark WHO Regional Office for Europe: Copenhagen, Denmark, 2005. [Google Scholar]
- Dunea, D.; Iordache, S.; Liu, H.-Y.; Bøhler, T.; Pohoata, A.; Radulescu, C. Quantifying the impact of PM2.5 and associated heavy metals on respiratory health of children near metallurgical facilities. Environ. Sci. Pollut. Res. 2016, 23, 15395–15406. [Google Scholar] [CrossRef] [PubMed]
- Kliegman, R.M.; Stanton, B.F.; St. Geme, J.; Schor, N.F. Nelson Textbook of Pediatrics, 20th ed.; Elsevier: Philadelphia, PA, USA, 2016. [Google Scholar]
- Buonanno, G.; Marks, G.B.; Morawska, L. Health effects of daily airborne particle dose in children: Direct association between personal dose and respiratory health effects. Environ. Pollut. 2013, 180, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Zora, J.E.; Sarnat, S.E.; Raysoni, A.U.; Johnson, B.A.; Li, W.W.; Greenwald, R.; Holguin, F.; Stock, T.H.; Sarnat, J.A. Associations between urban air pollution and pediatric asthma control in El Paso, Texas. Sci. Total Environ. 2013, 448, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.J.; Ayres, J.G. Particulate air pollution and panel studies in children: A systematic review. Occup. Environ. Med. 2004. [Google Scholar] [CrossRef]
- Gauvin, S.; Reungoat, P.; Cassadou, S.; Déchenaux, J.; Momas, I.; Just, J.; Zmirou, D. Contribution of indoor and outdoor environments to PM2.5 personal exposure of children—VESTA study. Sci. Total Environ. 2002, 297, 175–181. [Google Scholar] [CrossRef]
- Sharker, M.H.; Karimi, H.A. Computing least air pollution exposure routes. Int. J. Geogr. Inf. Sci. 2014, 28, 343–362. [Google Scholar] [CrossRef]
- Dunea, D.; Iordache, S.; Alexandrescu, D.C.; Dinca, N. Screening the weekdays/weekend patterns of air pollutant concentrations recorded in Southeastern Romania. Environ. Eng. Manag. J. 2014, 13, 3105–3114. [Google Scholar]
- Zartarian, V.G.; Ott, W.R.; Duan, N. Basic concepts and definitions of exposure and dose. In Exposure Analysis; Ott, W.R., Steinemann, A.C., Wallace, L.A., Eds.; Taylor & Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Li, L.; Zhou, X.; Kalo, M.; Piltner, R. Spatiotemporal interpolation methods for the application of estimating population exposure to fine particulate matter in the contiguous U.S. and a Real-Time web application. Int. J. Environ. Res. Public Health 2016, 13, 749. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C.I.; Phalen, R.F.; Solomon, P.A. Airborne Particulate Matter and Human Health: A Review. Aerosol Sci. Technol. 2005, 39, 737–749. [Google Scholar] [CrossRef]
- Oakes, M.; Baxter, L.; Long, T.C. Evaluating the application of multipollutant exposure metrics in air pollution health studies. Environ. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Van Roosbroeck, S.; Wichmann, J.; Janssen, N.A.H.; Hoek, G.; van Wijnen, J.H.; Lebret, E.; Brunekreef, B. Long-term personal exposure to traffic-related air pollution among school children, a validation study. Sci. Total Environ. 2006, 368, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Adani, M.; Mircea, M.; D’Isidoro, M.; Costa, M.P.; Silibello, C. Heavy metal modelling study over Italy: Effects of grid resolution, lateral boundary conditions and foreign emissions on air concentrations. Water Air Soil Pollut. 2015. [Google Scholar] [CrossRef]
- AQEG. Fine Particulate Matter (PM2.5) in the United Kingdom; Defra: London, UK, 2012.
- Rivas, I.; Viana, M.; Moreno, T.; Pandolfi, M.; Amato, F.; Reche, C.; Bouso, L.; Àlvarez-Pedrerol, M.; Alastuey, A.; Sunyer, J.; et al. Child exposure to indoor and outdoor air pollutants in schools in Barcelona, Spain. Environ. Int. 2014, 69, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, C.; Iordache, S.; Dunea, D.; Stihi, C.; Dulama, I.D. Risks assessment of heavy metals on public health associated with atmospheric exposure to PM2.5 in urban area. Rom. J. Phys. 2015, 60, 1171–1182. [Google Scholar]
- Neuberger, M.; Schimek, M.G.; Horak, F., Jr.; Moshammer, H.; Kundi, M.; Frischer, T.; Gomiscek, B.; Puxbaum, H.; Hauck, H.; AUPHEP-Team. Acute effects of particulate matter on respiratory diseases, symptoms and functions: Epidemiological results of the Austrian Project on Health Effects of Particulate Matter (AUPHEP). Atmos. Environ. 2004, 38, 3971–3981. [Google Scholar] [CrossRef]
- Meliker, J.R.; Slotnick, M.J.; AvRuskin, G.A.; Kaufmann, A.; Jacquez, G.M.; Nriagu, J.O. Improving exposure assessment in environmental epidemiology: Application of spatio-temporal visualization tools. J. Geogr. Syst. 2005, 7, 49–66. [Google Scholar] [CrossRef]
- Enkhtur, B. Geostatistical Modelling and Mapping of Air Pollution. Master's Thesis, University of Twente, Enschede, The Netherlands, 2013. [Google Scholar]
- Kanaroglou, P.S.; Soulakellis, N.A.; Sifakis, N.I. Improvement of satellite derived pollution maps with the use of a geostatistical interpolation method. J. Geogr. Syst. 2002, 4, 193–208. [Google Scholar] [CrossRef]
- Sinha, G.; Mark, D.M. Measuring similarity between geospatial lifelines in studies of environmental health. J. Geogr. Syst. 2005, 7, 115–136. [Google Scholar] [CrossRef]
- Montero, J.-M.; Chasco, C.; Larraz, B. Building an environmental quality index for a big city: A spatial interpolation approach combined with a distance indicator. J. Geogr. Syst. 2010, 12, 435–459. [Google Scholar] [CrossRef] [Green Version]
- Stanek, L.W.; Sacks, J.D.; Dutton, S.J.; Dubois, J.-J.B. Attributing health effects to apportioned components and sources of particulate; an evaluation of collective results. Atmos. Environ. 2011, 45, 5655–5663. [Google Scholar] [CrossRef]
- Castell, N.; Kobernus, M.; Liu, H.Y.; Schneider, P.; Lahoz, W.; Berre, A.J.; Noll, J. Mobile technologies and services for environmental monitoring: The Citi-Sense-MOB approach. Urban Clim. 2014. [Google Scholar] [CrossRef]
- Peters, J.; Theunis, J.; Van Poppel, M.; Berghmans, P. Monitoring PM10 and Ultrafine Particles in Urban Environments Using Mobile Measurements. Aerosol. Air Qual. Res. 2013, 13, 509–522. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Skjetne, E.; Kobernus, M. Mobile phone tracking: In support of modelling traffic-related air pollution contribution to individual exposure and its implications for public health impact assessment. Environ. Health 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- Iordache, S.; Dunea, D.; Lungu, E.; Predescu, L.; Dumitru, D.; Ianache, C.; Ianache, R. A Cyberinfrastructure for Air Quality Monitoring and Early Warnings to protect Children with Respiratory Disorders. In Proceedings of the 20th International Conference on Control Systems and Computer Science (CSCS20–2015), Bucharest, Romania, 27–29 May 2015.
- Kim, E.; Park, H.; Park, E.A.; Hong, Y.C.; Ha, M.; Kim, H.C.; Ha, E.H. Particulate matter and early childhood body weight. Environ. Int. 2016, 94, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Noma, H.; Kurai, J.; Sano, H.; Hantan, D.; Ueki, M.; Kitano, H.; Shimizu, E. Effects of short-term exposure to particulate air pollutants on the inflammatory response and respiratory symptoms: A panel study in schoolchildren from rural areas of Japan. Int. J. Environ. Res. Public Health 2016, 13, 983. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Hsu, S.-I.; Yan, B.; Moors, K.; Chillrud, S.N.; Ross, J.; Wang, S.; Perzanowski, M.S.; Kinney, P.L.; Whyatt, R.M.; et al. Childhood exposure to fine particulate matter and black carbon and the development of new wheeze between ages 5 and 7 in an urban prospective cohort. Environ. Int. 2012, 45, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.M.; Quinn, J.W.; Jung, K.H.; Hoepner, L.; Diaz, D.; Perzanowski, M.; Rundle, A.; Kinney, P.L.; Perera, F.P.; Miller, R.L. Traffic density and stationary sources of air pollution associated with wheeze, asthma, and immunoglobulin E from birth to age 5 years among New York City children. Environ. Res. 2011, 111, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Dunea, D.; Iordache, S.; Oprea, M.; Savu, T.; Pohoata, A.; Lungu, E. A relational database structure for linking air pollution levels with children’s respiratory illnesses. Bull. UASVM Agric. 2014, 71, 205–213. [Google Scholar]
- Olejnik, S.; Algina, J. Generalized eta and omega squared statistics: Measures of effect size for some common research designs. Psychol. Methods 2003, 8, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Jacquemin, B.; Lepeule, J.; Boudier, A.; Arnould, C.; Benmerad, M.; Chappaz, C.; Ferran, J.; Kauffmann, F.; Morelli, X.; Pin, I.; et al. Impact of geocoding methods on associations between long-term exposure to urban air pollution and lung function. Environ. Health Persp. 2013, 121, 1054–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popescu, V. Pathology of Respiratory System in Children (in Romanian); Teora: Bucharest, Romania, 1999. [Google Scholar]
- Zuurbier, M.; Hoek, G.; van den Hazel, P.; Brunekreef, B. Minute ventilation of cyclists, car and bus passengers: An experimental study. Environ. Health 2009, 8, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Dunea, D. An exploratory analysis of PM10 particulate matter relationships with weather data and spatial variation. In Proceedings of the 14th International Multidisciplinary Scientific GeoConference (SGEM), GeoConference on Energy and Clean Technologies, Albena, Bulgaria, 17–26 June 2014; pp. 273–280.
- Allan, M.; Richardson, G.M.; Jones-Otazo, H. Probability density functions describing 24-hour inhalation rates for use in human health risk assessments: An update and comparison. Hum. Ecol. Risk Assess. 2008, 14, 372–391. [Google Scholar] [CrossRef]
- Greene, N.A.; Morris, V.R. Assessment of public health risks associated with atmospheric exposure to PM2.5 in Washington, DC, USA. Int. J. Environ. Res. Public Health 2006, 3, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Alves de Oliveira, B.F.; Ignotti, E.; Artaxo, P.; do Nascimento Saldiva, P.H.; Junger, W.L.; Hacon, S. Risk assessment of PM2.5 to child residents in Brazilian Amazon region with biofuel production. Environ. Health 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunea, D.; Pohoata, A.; Iordache, S. Using wavelet—Feedforward neural networks to improve air pollution forecasting in urban environments. Environ. Monit. Assess. 2015. [Google Scholar] [CrossRef] [PubMed]
- Dunea, D.; Iordache, S. Time series analysis of air pollutants recorded from Romanian EMEP stations at mountain sites. Environ. Eng. Manag. J. 2015, 14, 2725–2735. [Google Scholar]
| Indicator | Descriptor |
|---|---|
| Location | Targoviste, Romania |
| Study period | Between October 2015 and December 2015 (3 months) |
| Date of birth | Between January 2005 and December 2013 |
| Age groups | Total of 25 children: Small children: 2–3 years (7); pre-schoolers: 4–5 years (7); school children: 6–8 years (7) and 9–10 (4). |
| Gender | males (50%); females (50%). |
| Grouping by home address in a region of the city (sensitive children) | Group A (14)—highest concentrations of PM2.5 (~16 µg·m−3); Group B (6)—middle concentrations (~11 µg·m−3); Group C (5)—lowest concentrations (~6.5 µg·m−3)—city centre |
| Medical records (used to select the children for the trial) | Number of wheezing episodes/asthma attacks and hospitalizations (counted starting with a child’s first consultation/admission in hospital); blood test indicators. |
| Selection criteria for sensitive children | Number of wheezing episodes; eosinophil count; immunoglobulin E (IgE) serum level; response to inhalation medication. |
| Control condition | Incidence of Fever |
| Fields in the observation sheet completed by each parent during the trial | Date of birth; Home address; School/kindergarten; Medication during the trial; height; weight; occurrence of respiratory symptoms i.e., coughing, rhinorrhoea, wheezing, and fever; physical effort |
| Routes and time spent outdoors | Activities, commuting mode between various locations and timing (diary); GPS tracks using smartphones and Garmin GPS devices |
| Indicator | Units | 1 Week | 1 Year | 3 Years | 5 Years | 8 Years | 10 Years |
|---|---|---|---|---|---|---|---|
| Height (average values) | cm | 48–52 | 75 | 96 | 100 | 130 | 140 |
| Weight (average values) | kg | 3 | 10 | 14.5 | 18 | 26 | 33 |
| FRC 1 | mL | 75 | 263 | 532 | 660 | 1174 | 1546 |
| VC 2 | mL | 100 | 475 | 910 | 1200 | 1885 | 2358 |
| Ventilation | mL/min | 550 | 1175 | 2460 | 2600 | 3240 | 3458 |
| Vt 3 | mL | 17 | 78 | 112 | 130 | 180 | 217 |
| Respiratory frequency | breaths/min | 30 | 24 | 22 | 20 | 18 | 16 |
| Pulse rates 4 (low–high) | - | 100–160 | 100–160 | 90–150 | 80–140 | 70–120 | 60–100 |
| Low-normal systolic blood pressure | - | >60 | >70 | >75 | >75 | >80 | >90 |
| Age (Years) | 2–3 | 4–5 | 6–8 | 9–10 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rng | SD | IQR | Rng | SD | IQR | Rng | SD | IQR | Rng | SD | IQR | |
| Weekdays | ||||||||||||
| Route to kindergarten/school | 0.85–1.85 | 0.7 | 0.5 | 0.90–1.95 | 0.7 | 0.5 | 1.12–2.43 | 0.9 | 0.7 | 1.19–2.59 | 1.0 | 0.7 |
| Play after school | 1.09–2.36 | 0.9 | 0.6 | 1.15–2.50 | 1.0 | 0.7 | 1.43–3.11 | 1.2 | 0.8 | 1.53–3.32 | 2.0 | 0.9 |
| Play in the afternoon | 1.36–2.95 | 1.1 | 0.8 | 1.44–5.72 | 3.0 | 2.1 | 1.79–7.78 | 4.2 | 3.0 | 1.91–8.65 | 4.8 | 3.4 |
| Estimated dose (day) | 3.29–7.16 | 2.7 | 1.9 | 3.48–10.2 | 4.7 | 3.3 | 4.34–13.32 | 6.3 | 4.5 | 4.63–14.56 | 7.0 | 5.0 |
| Weekend | ||||||||||||
| Morning walk | 0.41–2.51 | 1.5 | 1.1 | 0.43–2.65 | 1.6 | 1.1 | 0.54–3.30 | 2.0 | 1.4 | 0.57–3.53 | 2.1 | 1.5 |
| Play in the afternoon | 0.68–1.48 | 0.6 | 0.4 | 1.01–1.56 | 0.4 | 0.3 | 1.61–2.92 | 0.9 | 0.7 | 1.72–3.11 | 1.0 | 0.7 |
| Estimated dose (day) | 1.09–3.99 | 2.0 | 1.4 | 1.44–4.21 | 1.9 | 1.3 | 2.15–6.22 | 2.8 | 2.0 | 2.29–6.64 | 3.1 | 2.1 |
| Age (Years) | 2–3 (n = 7) | 4–5 (n = 7) | 6–8 (n = 7) | 9–10 (n = 4) | Group Average | SD |
|---|---|---|---|---|---|---|
| Persistent Coughing | ||||||
| October | 8.7 | 9.1 | 6.0 | 5.6 | 7.4 | 1.8 |
| SD | 4.6 | 3.7 | 1.8 | 3.5 | - | - |
| IQR | 4.5 | 4.0 | 2.5 | 3.7 | - | - |
| November | 9.3 | 9.0 | 6.9 | 4.7 | 7.5 | 2.1 |
| SD | 3.9 | 5.5 | 3.2 | 2.3 | - | - |
| IQR | 6.0 | 2.0 | 1.5 | 2.9 | - | - |
| December | 7.3 | 7.9 | 6.9 | 5.8 | 6.9 | 0.9 |
| SD | 5.4 | 2.7 | 1.8 | 3.0 | - | - |
| IQR | 2.0 | 3.5 | 2.0 | 2.1 | - | - |
| Total average | 8.4 | 8.7 | 6.6 | 5.4 | 7.3 | - |
| Rhinorrhoea | ||||||
| October | 4.6 | 5.7 | 5.4 | 3.5 | 4.8 | 1.0 |
| SD | 3.0 | 4.6 | 2.8 | 2.2 | - | - |
| IQR | 3.5 | 5.5 | 3.0 | 2.0 | - | - |
| November | 5.6 | 4.3 | 4.7 | 2.1 | 4.2 | 1.5 |
| SD | 3.7 | 2.0 | 4.5 | 1.6 | - | - |
| IQR | 1.5 | 2.0 | 1.5 | 3.2 | - | - |
| December | 6.3 | 5.0 | 5.6 | 3.1 | 5.0 | 1.4 |
| SD | 5.4 | 3.3 | 4.5 | 1.6 | - | - |
| IQR | 3.0 | 2.0 | 3.0 | 2.0 | - | - |
| Total average | 5.5 | 5.0 | 5.2 | 2.9 | 4.7 | - |
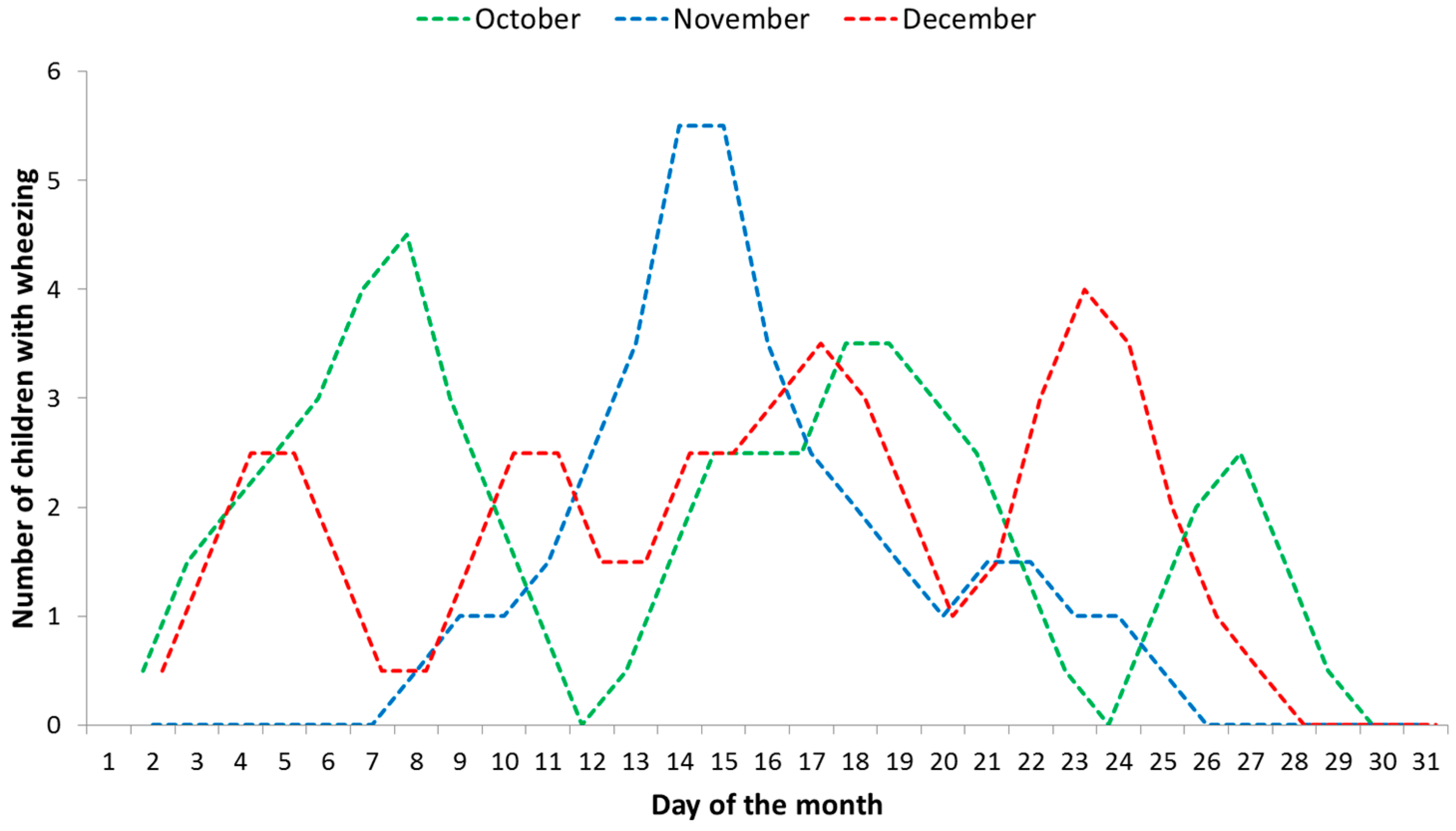
| Wheezing | ||||||
| October | 2.7 | 1.4 | 2.4 | 1.2 | 1.9 | 0.7 |
| SD | 1.6 | 1.9 | 2.4 | 1.0 | - | - |
| IQR | 1.5 | 3.0 | 4.5 | 1.6 | - | - |
| November | 1.7 | 2.3 | 1.4 | 0.7 | 1.5 | 0.7 |
| SD | 2.9 | 1.3 | 1.8 | 0.6 | - | - |
| IQR | 2.0 | 1.0 | 3.0 | 0.9 | - | - |
| December | 1.6 | 3.1 | 2.6 | 0.7 | 2.0 | 1.1 |
| SD | 1.5 | 0.7 | 2.0 | 0.6 | - | - |
| IQR | 3.0 | 0.5 | 3.0 | 0.9 | - | - |
| Total average | 2.0 | 2.3 | 2.1 | 0.9 | 1.8 | - |
| Fever | ||||||
| October | 2.1 | 2.9 | 1.6 | 1.1 | 1.9 | 0.8 |
| SD | 1.6 | 1.7 | 1.5 | 1.0 | - | - |
| IQR | 2.0 | 2.0 | 2.0 | 1.6 | - | - |
| November | 3.3 | 1.9 | 1.4 | 0.5 | 1.8 | 1.2 |
| SD | 1.7 | 1.9 | 1.4 | 0.2 | - | - |
| IQR | 2.5 | 3.5 | 2.5 | 0.2 | - | - |
| December | 2.0 | 3.3 | 2.1 | 1.9 | 2.3 | 0.7 |
| SD | 1.2 | 1.6 | 1.6 | 1.0 | - | - |
| IQR | 1.5 | 2.0 | 2.0 | 1.6 | - | - |
| Total average | 2.5 | 2.7 | 1.7 | 1.2 | 2.0 | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dunea, D.; Iordache, S.; Pohoata, A. Fine Particulate Matter in Urban Environments: A Trigger of Respiratory Symptoms in Sensitive Children. Int. J. Environ. Res. Public Health 2016, 13, 1246. https://doi.org/10.3390/ijerph13121246
Dunea D, Iordache S, Pohoata A. Fine Particulate Matter in Urban Environments: A Trigger of Respiratory Symptoms in Sensitive Children. International Journal of Environmental Research and Public Health. 2016; 13(12):1246. https://doi.org/10.3390/ijerph13121246
Chicago/Turabian StyleDunea, Daniel, Stefania Iordache, and Alin Pohoata. 2016. "Fine Particulate Matter in Urban Environments: A Trigger of Respiratory Symptoms in Sensitive Children" International Journal of Environmental Research and Public Health 13, no. 12: 1246. https://doi.org/10.3390/ijerph13121246







