Volatile Organic Compounds (VOCs) in Conventional and High Performance School Buildings in the U.S.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection and Characteristics of School Buildings
2.2. Air Quality Sampling
2.3. VOC Sampling and Analysis
2.4. Air Exchange Rates
2.5. Data Analysis
3. Results
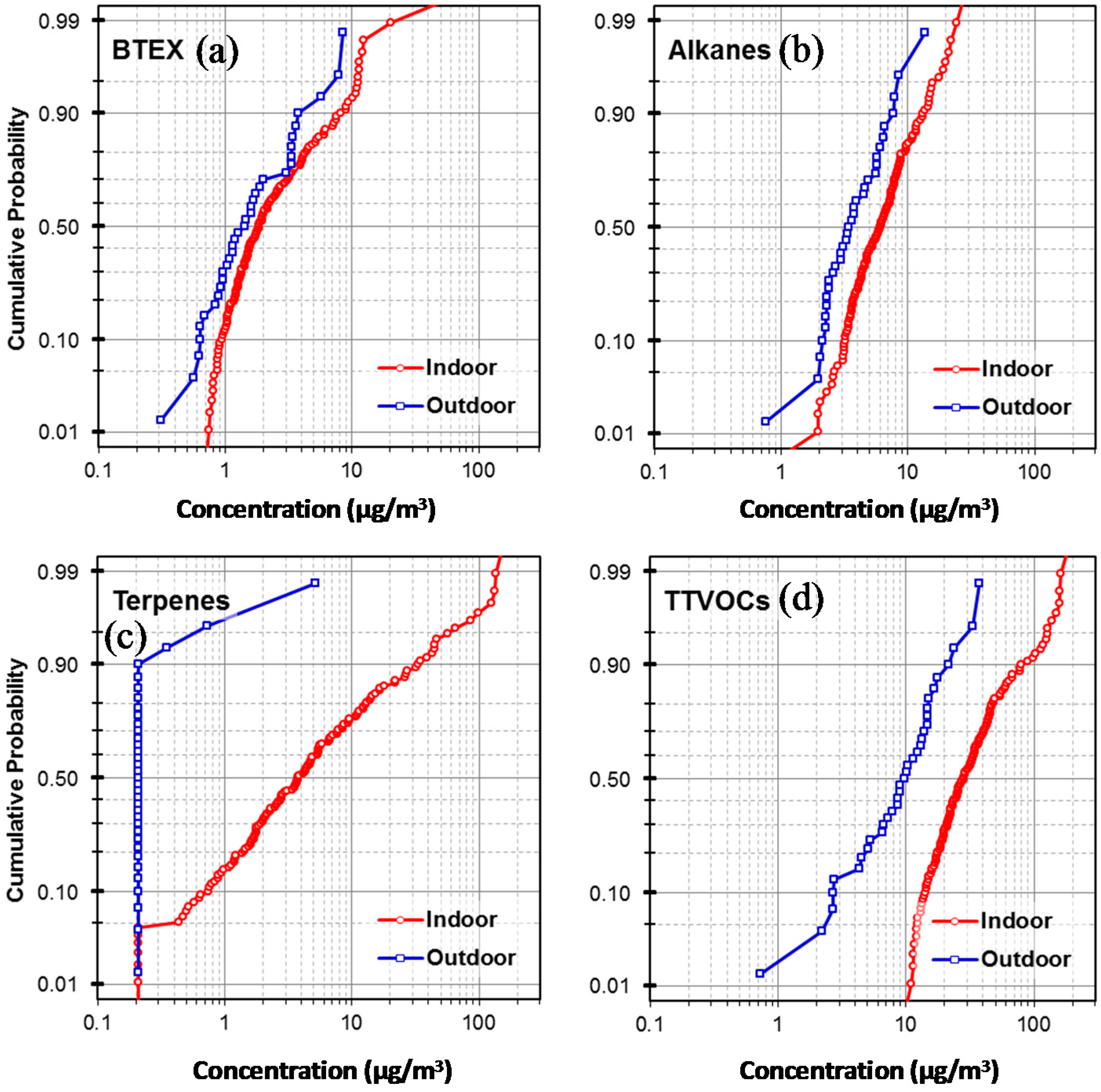
3.1. Indoor and Outdoor VOC Concentrations
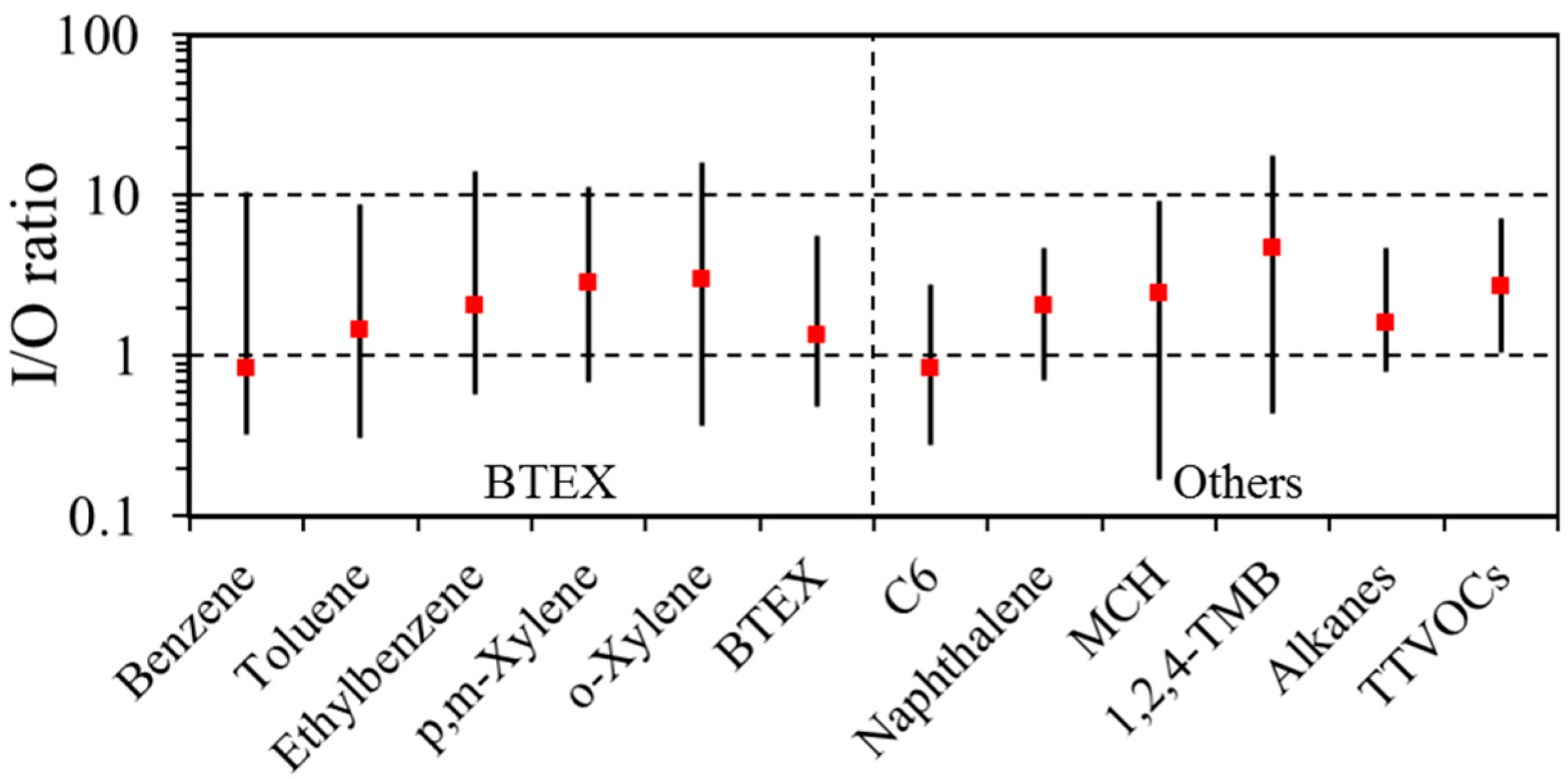
3.2. Indoor/Outdoor (I/O) Relationship
4. Discussion
4.1. Effect of Indoor Environment Parameters
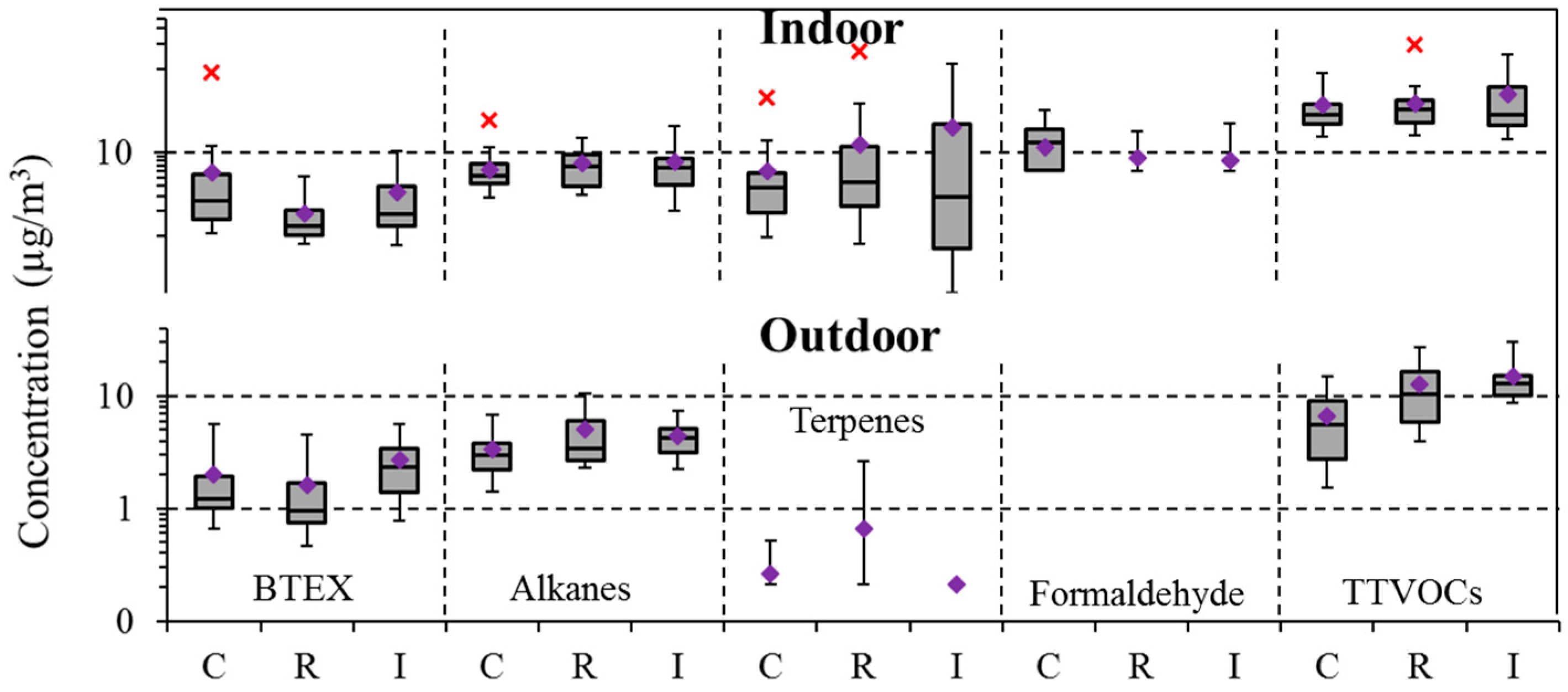
4.2. Within- and Between-School Comparisons
4.3. Seasonal Variation
4.4. Community Variation
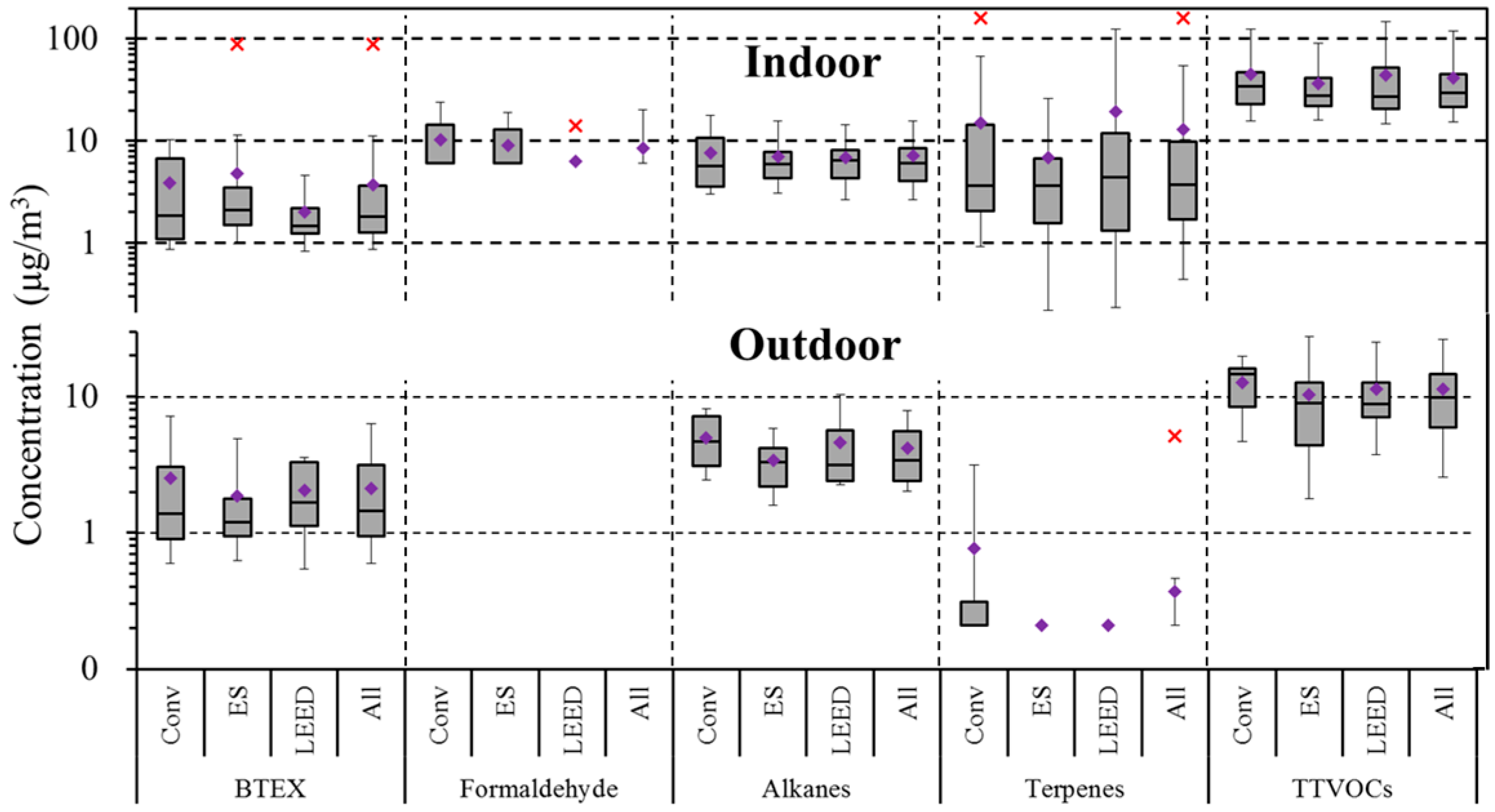
4.5. Comparison of Conventional and High Performance School Buildings
4.6. Comparison with the Literature
4.7. Limitations
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mendell, M.J.; Heath, G.A. Do indoor pollutants and thermal conditions in schools influence student performance? A critical review of the literature. Indoor Air 2005, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Stafford, T.M. Indoor air quality and academic performance. J. Environ. Econ. Manag. 2015, 70, 34–50. [Google Scholar] [CrossRef]
- Annesi-Maesano, I.; Baiz, N.; Banerjee, S.; Rudnai, P.; Rive, S.; Grp, S. Indoor air quality and sources in schools and related health effects. J. Toxicol. Environ. Health 2013, 16, 491–550. [Google Scholar] [CrossRef] [PubMed]
- Salthammer, T.; Uhde, E.; Schripp, T.; Schieweck, A.; Morawska, L.; Mazaheri, M.; Clifford, S.; He, C.; Buonanno, G.; Querol, X.; et al. Children’s well-being at schools: Impact of climatic conditions and air pollution. Environ. Inter. 2016, 94, 196. [Google Scholar] [CrossRef] [PubMed]
- Daisey, J.M.; Angell, W.J.; Apte, M.G. Indoor air quality, ventilation and health symptoms in schools: An analysis of existing information. Indoor Air 2003, 13, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Shendell, D.G.; Barnett, C.; Boese, S. Science-based recommendations to prevent or reduce potential exposure to biological, chemical, and physical agents in schools. J. School Health 2004, 74, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Fraga, S.; Ramos, E.; Martins, A.; Samúdio, M.J.; Silva, G.; Guedes, J.; Fernandes, E.O.; Barros, H. Indoor air quality and respiratory symptoms inporto schools. Revista Portuguesa de Pneumologia 2008, 14, 487–507. [Google Scholar] [CrossRef]
- Madureira, J.; Paciência, I.; Rufo, J.; Ramos, E.; Barros, H.; Teixeira, J.P.; de Oliveira Fernandes, E. Indoor air quality in schools and its relationship with children’s respiratory symptoms. Atmos. Environ. 2015, 118, 145–156. [Google Scholar] [CrossRef]
- Pekey, H.; Arslanbaş, D. The relationship between indoor, outdoor and personal VOC concentrations in homes, offices and schools in the metropolitan region of Kocaeli, Turkey. Water Air Soil Poll. 2008, 191, 113–129. [Google Scholar] [CrossRef]
- Geiss, O.; Giannopoulos, G.; Tirendi, S.; Barrero-Moreno, J.; Larsen, B.R.; Kotzias, D. The airmex study—VOC measurements in public buildings and schools/kindergartens in eleven European cities: Statistical analysis of the data. Atmos. Environ. 2011, 45, 3676–3684. [Google Scholar] [CrossRef]
- Pegas, P.N.; Alves, C.A.; Evtyugina, M.G.; Nunes, T.; Cerqueira, M.; Franchi, M.; Pio, C.A.; Almeida, S.M.; Verde, S.C.; Freitas, M.C. Seasonal evaluation of outdoor/indoor air quality in primary schools in Lisbon. J. Environ. Monit. 2011, 13, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Chatzidiakou, L.; Mumovic, D.; Summerfield, A.J.; Hong, S.M.; Altamirano-Medina, H. A victorian school and a low carbon designed school: Comparison of indoor air quality, energy performance, and student health. Indoor Built Environ. 2014, 23, 417–432. [Google Scholar] [CrossRef]
- De Gennaro, G.; Farella, G.; Marzocca, A.; Mazzone, A.; Tutino, M. Indoor and outdoor monitoring of volatile organic compounds in school buildings: Indicators based on health risk assessment to single out critical issues. Int. J. Environ. Res. Public Health 2013, 10, 6273–6291. [Google Scholar] [CrossRef] [PubMed]
- Godwin, C.; Batterman, S. Indoor air quality in michigan schools. Indoor Air 2007, 17, 109. [Google Scholar] [CrossRef] [PubMed]
- Pegas, P.N.; Alves, C.A.; Evtyugina, M.G.; Nunes, T.; Cerqueira, M.; Franchi, M.; Pio, C.A.; Almeida, S.M.; Freitas, M.C. Indoor air quality in elementary schools of Lisbon in spring. Environ. Geochem. Health 2011, 33, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Adgate, J.L.; Eberly, L.E.; Stroebel, C.; Pellizzari, E.D.; Sexton, K. Personal, indoor, and outdoor VOC exposures in a probability sample of children. J. Expos. Anal. Environ. Epidemiol. 2004, 14, S4–S13. [Google Scholar] [CrossRef] [PubMed]
- Shendell, D.G.; Winer, A.M.; Stock, T.H.; Zhang, L.; Zhang, J.F.; Maberti, S.; Colome, S.D. Air concentrations of vocs in portable and traditional classrooms: Results of a pilot study in Los Angeles county. J. Expos. Anal. Environ. Epidemiol. 2004, 14, 44–59. [Google Scholar] [CrossRef] [PubMed]
- USGBC. U.S. Green Building Council: Our History. Available online: http://www.usgbc.org/about (accessed on 30 July 2016).
- Devine, A.; Kok, N. Green certification and building performance: Implications for tangibles and intangibles. J. Portf. Manage. 2015, 41, 151–163. [Google Scholar] [CrossRef]
- Chen, Q.; Kleinman, L.; Dial, A. Energy performance of campus leed (R) buildings: Implications for green building and energy policy. J. Green Build. 2015, 10, 137–160. [Google Scholar] [CrossRef]
- Wei, W.; Ramalho, O.; Mandin, C. Indoor air quality requirements in green building certifications. Build. Environ. 2015, 92, 10–19. [Google Scholar] [CrossRef]
- Persily, A. Challenges in developing ventilation and indoor air quality standards: The story of ASHRAE standard 62. Build. Environ. 2015, 91, 61–69. [Google Scholar] [CrossRef]
- American Society of Heating Refrigeration and Ventilation Engineers. ASHRAE standard 62—1989 energy, cost, and program implications. In Ventilation for Acceptable Indoor Air Quality; ASHRAE: Washington, DC, USA, 2010. [Google Scholar]
- Dinh, T.V.; Kim, S.Y.; Son, Y.S.; Choi, I.Y.; Park, S.R.; Sunwoo, Y.; Kim, J.C. Emission characteristics of VOCs emitted from consumer and commercial products and their ozone formation potential. Environ. Sci. Pollut. Res. 2015, 22, 9345–9355. [Google Scholar] [CrossRef] [PubMed]
- Schieweck, A.; Bock, M.C. Emissions from low-VOC and zero-VOC paints—Valuable alternatives to conventional formulations also for use in sensitive environments? Build. Environ. 2015, 85, 243–252. [Google Scholar] [CrossRef]
- Zhao, P.; Cheng, Y.H.; Lin, C.C.; Cheng, Y.L. Effect of resin content and substrate on the emission of BTEX and carbonyls from low-VOC water-based wall paint. Environ. Sci. Pollut. Res. 2016, 23, 3799–3808. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, A.I.; Destaillats, H.; Sullivan, D.P.; Fisk, W.J. Performance of ultraviolet photocatalytic oxidation for indoor air cleaning applications. Indoor Air 2007, 17, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Batterman, S.; Metts, T.; Kalliokoski, P.; Barnett, E. Low-flow active and passive sampling of VOCs using thermal desorption tubes: Theory and application at an offset printing facility. J. Environ. Monit. 2002, 4, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-Y.; Batterman, S. Performance evaluation of a sorbent tube sampling method using short path thermal desorption for volatile organic compounds. J. Environ. Monitor. 2000, 2, 313–324. [Google Scholar] [CrossRef]
- Batterman, S.A.; Su, F.-C.; Wald, A.; Watkins, F.; Godwin, C.; Geofrey, T. Ventilation rates in recently constructed U.S. schools. Unpublished work. 2016. [Google Scholar]
- Haverinen-Shaughnessy, U.; Moschandreas, D.J.; Shaughnessy, R.J. Association between substandard classroom ventilation rates and students’ academic achievement. Indoor Air 2011, 21, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Batterman, S.; Godwin, C. VOCs in industrial, urban and suburban neighborhoods, part 1: Indoor and outdoor concentrations, variation, and risk drivers. Atmos. Environ. 2008, 42, 2083. [Google Scholar] [CrossRef]
- Curci, G.; Palmer, P.I.; Kurosu, T.P.; Chance, K.; Visconti, G. Estimating european volatile organic compound emissions using satellite observations of formaldehyde from the ozone monitoring instrument. Atmos. Chem. Phys. 2010, 10, 11501–11517. [Google Scholar] [CrossRef] [Green Version]
- Hellen, H.; Tykka, T.; Hakola, H. Importance of monoterpenes and isoprene in urban air in northern Europe. Atmos. Environ. 2012, 59, 59–66. [Google Scholar] [CrossRef]
- Kitanovski, Z.; Grgic, I.; Vermeylen, R.; Claeys, M.; Maenhaut, W. Liquid chromatography tandem mass spectrometry method for characterization of monoaromatic nitro-compounds in atmospheric particulate matter. J. Chromatogr. A 2012, 1268, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Hinks, M.L.; Brady, M.V.; Lignell, H.; Song, M.; Grayson, J.W.; Bertram, A.K.; Lin, P.; Laskin, A.; Laskin, J.; Nizkorodov, S.A. Effect of viscosity on photodegradation rates in complex secondary organic aerosol materials. Phys. Chem. Chem. Phys. 2016, 18, 8785–8793. [Google Scholar] [CrossRef] [PubMed]
- Karl, M.; Tsigaridis, K.; Vignati, E.; Dentener, F. Formation of secondary organic aerosol from isoprene oxidation over Europe. Atmos. Chem. Phys. 2009, 9, 7003–7030. [Google Scholar] [CrossRef]
- Noe, S.M.; Hüve, K.; Niinemets, Ü.; Copolovici, L. Seasonal variation in vertical volatile compounds air concentrations within a remote hemiboreal mixed forest. Atmos. Chem. Phys. 2012, 12, 3909–3926. [Google Scholar] [CrossRef]
- Franco, J.F.; Pacheco, J.; Behrentz, E.; Belalcázar, L.C. Characterization and source identification of VOC species in Bogotá, Colombia. Atmósfera 2015, 28, 1–11. [Google Scholar] [CrossRef]
- Cometto-Muniz, J.E.; Abraham, M.H. Compilation and analysis of types and concentrations of airborne chemicals measured in various indoor and outdoor human environments. Chemosphere 2015, 127, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Kimbrough, S.; Palma, T.; Baldauf, R.W. Analysis of mobile source air toxics (MSATS)-near-road VOC and carbonyl concentrations. J. Air Waste Manage. Assoc. 2014, 64, 349–359. [Google Scholar] [CrossRef]
- Ho, K.F.; Ho, S.S.H.; Lee, S.C.; Louie, P.K.K.; Cao, J.J.; Deng, W.J. Volatile organic compounds in roadside environment of Hong Kong. Aerosol Air Qual. Res. 2013, 13, 1331–1347. [Google Scholar] [CrossRef]
- Su, F.C.; Mukherjee, B.; Batterman, S. Determinants of personal, indoor and outdoor VOC concentrations: An analysis of the riopa data. Environ. Res. 2013, 126, 192–203. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Air Quality Trends. Available online: https://www3.epa.gov/airtrends/aqtrends.html#comparison (accessed on 1 August 2016).
- United States Environmental Protection Agency. Ozone Pollution. Available online: https://www.epa.gov/ozone-pollution (accessed on 6 August 2016).
- United States Environmental Protection Agency: Mobile Source Air Toxics. Available online: https://www3.epa.gov/otaq/toxics.htm (accessed on 7 August 2016).
- United States Environmental Protection Agency. Sale and Use of Aftermarket Catalytic Converters. Available online: https://www.epa.gov/enforcement/sale-and-use-aftermarket-catalytic-converters-federal-register-vol51no-150-tuesday (accessed on 5 August 2016).
- United States Environmental Protection Agency. Volatile Organic Compound (VOC) Control Regulations. Available online: https://www3.epa.gov/region1/airquality/voc.html (accessed on 2 August 2016).
- Propper, R.; Wong, P.; Bui, S.; Austin, J.; Vance, W.; Alvarado, A.; Croes, B.; Luo, D. Ambient and emission trends of toxic air contaminants in California. Environ. Sci. Technol. 2015, 49, 11329–11339. [Google Scholar] [CrossRef] [PubMed]
- Sather, M.E.; Cavender, K. Trends analyses of 30 years of ambient 8 hour ozone and precursor monitoring data. Environ. Sci. Process Impact. 2016, 18, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Adgate, J.L.; Church, T.R.; Ryan, A.D.; Ramachandran, G.; Fredrickson, A.L.; Stock, T.H.; Morandi, M.T.; Sexton, K. Outdoor, indoor, and personal exposure to VOCs in children. Environ. Health Perspect. 2004, 112, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Batterman, S.; Godwin, C.; Chernyak, S.; Jia, C.R.; Charles, S. Brominated flame retardants in offices in Michigan, USA. Environ. Intern. 2010, 36, 548–556. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Background Indoor Air Concentrations of Volatile Organic Compounds in North American Residences (1990–2005): A Compilation of Statistics for Assessing Vapor Intrusion EPA 530-R-10–001 2011; USEPA: Washington, DC, USA.
- Su, F.C.; Mukherjee, B.; Batterman, S. Trends of VOC exposures among a nationally representative sample: Analysis of the NHANES 1988 through 2004 data sets. Atmos. Environ. 2011, 45, 4858–4867. [Google Scholar] [CrossRef] [PubMed]

| VOC | Outdoors (n = 35) | Indoors (n = 144) | p-Value * | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DF (%) | Mean (µg/m3) | Median (µg/m3) | Max (µg/m3) | DF (%) | Mean (µg/m3) | Median (µg/m3) | Max (µg/m3) | Paired t-Test | Signed Rank | |
| Aromatics | ||||||||||
| Benzene | 86 | 0.5 | 0.5 | 2.1 | 93 | 0.5 | 0.3 | 4.4 | 0.90 | 0.58 |
| Toluene | 97 | 1.0 | 0.4 | 7.0 | 100 | 1.8 | 0.7 | 83.8 | 0.22 | 0.01 |
| Ethylbenzene | 17 | 0.1 | 0.1 | 0.9 | 36 | 0.2 | 0.1 | 2.0 | 0.05 | 0.00 |
| p,m-Xylene | 46 | 0.4 | 0.1 | 3.6 | 81 | 0.9 | 0.4 | 7.4 | 0.01 | 0.00 |
| o-Xylene | 23 | 0.1 | 0.1 | 1.3 | 51 | 0.3 | 0.1 | 2.2 | 0.01 | 0.00 |
| 1,2,4-Trimethylbenzene | 29 | 0.2 | <0.03 | 3.8 | 59 | 0.3 | 0.2 | 4.2 | 0.22 | 0.00 |
| BTEX | 97 | 2.0 | 1.3 | 8.5 | 100 | 3.7 | 1.8 | 89.1 | 0.05 | 0.01 |
| Alkanes | ||||||||||
| n-Hexane | 97 | 3.4 | 2.4 | 12.9 | 95 | 3.2 | 2.2 | 27.2 | 0.86 | 0.65 |
| n-Heptane | 9 | 0.2 | 0.1 | 2.3 | 35 | 0.9 | 0.1 | 9.9 | 0.00 | 0.00 |
| n-Undecane | 0 | <0.06 | <0.06 | <0.06 | 17 | 0.3 | 0.1 | 4.3 | 0.01 | 0.00 |
| n-Dodecane | 11 | 0.2 | 0.2 | 0.5 | 51 | 0.6 | 0.3 | 3.0 | 0.00 | 0.00 |
| n-Tridecane | 3 | 0.1 | 0.1 | 0.5 | 17 | 0.2 | 0.1 | 1.5 | 0.01 | 0.01 |
| n-Tetradecane | 6 | 0.1 | 0.1 | 0.3 | 85 | 1.3 | 1.0 | 8.2 | 0.00 | 0.00 |
| n-Pentadecane | 0 | <0.04 | <0.04 | <0.04 | 35 | 0.5 | <0.04 | 5.8 | 0.00 | 0.00 |
| n-Hexadecane | 0 | <0.06 | <0.06 | <0.06 | 28 | 0.3 | 0.1 | 2.6 | 0.00 | 0.00 |
| Methyl cyclohexane | 15 | 2.1 | 1.3 | 11.8 | 28 | 3.0 | 1.3 | 53.1 | 0.20 | 0.13 |
| Alkanes (C6–C7, C11–C16) | 97 | 4.0 | 3.4 | 13.5 | 99 | 7.2 | 6.1 | 28.8 | 0.00 | 0.00 |
| Terpenes | ||||||||||
| α-Pinene | 6 | 0.2 | 0.1 | 5.0 | 47 | 1.5 | 0.1 | 55.7 | 0.19 | 0.00 |
| d-Limonene | 3 | 0.1 | 0.1 | 0.7 | 94 | 11.3 | 3.5 | 158.5 | 0.00 | 0.00 |
| Terpenes | 9 | 0.3 | 0.2 | 5.1 | 95 | 12.8 | 3.8 | 159.7 | 0.00 | 0.00 |
| Other | ||||||||||
| Ethyl acetate | 0 | <0.25 | <0.25 | <0.25 | 26 | 0.8 | 0.3 | 7.9 | 0.00 | 0.00 |
| Methyl isobutyl ketone | 0 | <0.04 | <0.04 | <0.04 | 24 | 0.2 | <0.04 | 4.3 | 0.01 | 0.00 |
| Naphthalene | 11 | 0.2 | 0.1 | 1.7 | 40 | 0.2 | 0.1 | 2.9 | 0.28 | 0.00 |
| Methylene chloride | 29 | 2.4 | 0.8 | 13.9 | 20 | 2.8 | 0.8 | 47.7 | 0.65 | 1.00 |
| Chloroform | 37 | 2.3 | 0.1 | 19.8 | 31 | 1.7 | 0.1 | 15.3 | 0.42 | 0.71 |
| 1,4-Dichlorobenzene | 0 | <0.03 | <0.03 | <0.03 | 15 | 0.1 | <0.03 | 2.9 | 0.04 | 0.00 |
| Formaldehyde | - | - | - | - | 23 | 8.6 | 6.0 | 32.0 | - | - |
| TTVOCs | 100 | 24.2 | 21.6 | 76.5 | 100 | 41.5 | 29.3 | 196.6 | 0.00 | 0.00 |
| VOC | Benzene | Toluene | Ethyl-Benzene | m,p-Xylene | o-Xylene | BTEX | Alkanes | Terpenes | Formaldehyde | TTVOCs | Occupancy | Rh (%) | Avg. CO2 | Daytime Avg. CO2 | Avg. Aer | Daytime Avg. Aer | Nighttime Avg. Aer |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Benzene | 1.000 | ||||||||||||||||
| Toluene | 0.133 | 1.000 | |||||||||||||||
| Ethylbenzene | 0.460 * | 0.427 * | 1.000 | ||||||||||||||
| m,p-Xylene | 0.363 * | 0.586 * | 0.985 * | 1.000 | |||||||||||||
| o-Xylene | 0.450 * | 0.452 * | 0.940 * | 0.938 * | 1.000 | ||||||||||||
| BTEX | 0.250 * | 0.857 * | 0.908 * | 0.839 * | 0.819 * | 1.000 | |||||||||||
| Alkanes | 0.143 | 0.368 * | 0.269 * | 0.238 * | 0.178 | 0.330 * | 1.000 | ||||||||||
| Terpenes | 0.175 * | 0.317 * | 0.303 * | 0.354 * | 0.328 * | 0.354 * | 0.425 * | 1.000 | |||||||||
| Formaldehyde | 0.178 * | 0.245 * | 0.529 * | 0.283 * | 0.340 * | 0.275 * | 0.028 | 0.353 * | 1.000 | ||||||||
| TTVOCs | 0.222 * | 0.466 * | 0.472 * | 0.484 * | 0.493 * | 0.555 * | 0.617 * | 0.753 * | 0.561 * | 1.000 | |||||||
| Occupancy | −0.086 | 0.041 | −0.145 | −0.181 | −0.214 | −0.037 | −0.022 | 0.058 | 0.082 | 0.053 | 1.000 | ||||||
| RH (%) | −0.011 | 0.331 * | 0.588 * | 0.428 * | 0.440 * | 0.405 * | 0.129 | 0.332 * | 0.490 * | 0.399 * | −0.089 | 1.000 | |||||
| Avg. CO2 | −0.116 | 0.370 * | 0.185 | 0.169 | 0.110 | 0.296 * | 0.452 * | 0.397 * | 0.260 * | 0.416 * | 0.326 * | 0.423 * | 1.000 | ||||
| Daytime avg. CO2 | −0.019 | 0.316 * | 0.305 * | 0.183 | 0.183 | 0.233 * | 0.109 | 0.414 * | 0.470 * | 0.448 * | 0.179 * | 0.411 * | 0.506 * | 1.000 | |||
| Avg. AER | −0.035 | −0.132 | −0.153 | −0.149 | −0.239 * | −0.167 * | −0.106 | −0.202 * | −0.363 * | −0.238 * | 0.137 | −0.225 * | −0.285 * | −0.154 | 1.000 | ||
| Day avg. AER | −0.034 | −0.126 | −0.136 | −0.128 | −0.206 * | −0.174 * | −0.074 | −0.208 * | −0.307 * | −0.238 * | 0.125 | −0.220 * | −0.262 * | −0.164 | 0.993 * | 1.000 | |
| Night avg. AER | 0.047 | −0.132 | −0.130 | −0.204 * | −0.213 * | −0.152 | −0.125 | −0.135 | −0.541 * | −0.123 | 0.153 | −0.217 * | −0.150 | 0.146 | 0.310 * | 0.227 * | 1.000 |
| VOC | Percent of Variation (%) | p-Value * | |
|---|---|---|---|
| Within-School | Between-School | ||
| Aromatics | |||
| Benzene | 89.1 | 10.9 | 0.06 |
| Toluene | 91.8 | 8.2 | 0.11 |
| Ethylbenzene | 14.2 | 85.8 | 0.00 |
| p,m-Xylene | 16.5 | 83.5 | 0.00 |
| o-Xylene | 28.6 | 71.4 | 0.00 |
| 1,2,4-TMB | 41.7 | 58.3 | 0.00 |
| BTEX | 83.6 | 16.4 | 0.01 |
| Alkanes | |||
| C6 | 82.0 | 18.0 | 0.01 |
| C7 | 60.9 | 39.1 | 0.00 |
| C11 | 62.4 | 37.6 | 0.00 |
| C12 | 70.0 | 30.0 | 0.00 |
| C13 | 77.9 | 22.1 | 0.00 |
| C14 | 82.2 | 17.8 | 0.00 |
| C15 | 59.2 | 40.8 | 0.00 |
| C16 | 73.3 | 26.7 | 0.00 |
| MCH | 84.3 | 15.7 | 0.01 |
| Alkanes | 69.8 | 30.2 | 0.00 |
| Terpenes | |||
| α-Pinene | 15.7 | 84.3 | 0.00 |
| d-Limonene | 36.1 | 63.9 | 0.00 |
| Terpenes | 25.6 | 74.4 | 0.00 |
| Other | |||
| Ethyl acetate | 58.0 | 42.0 | 0.00 |
| MIBK | 57.5 | 42.5 | 0.00 |
| Naphthalene | 76.7 | 23.3 | 0.00 |
| DCM | 48.6 | 51.4 | 0.00 |
| Chloroform | 52.1 | 47.9 | 0.00 |
| p-DCB | 63.2 | 36.8 | 0.00 |
| Formaldehyde | 37.2 | 62.8 | 0.00 |
| TTVOCs | 35.4 | 64.6 | 0.00 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, L.; Su, F.-C.; Batterman, S. Volatile Organic Compounds (VOCs) in Conventional and High Performance School Buildings in the U.S. Int. J. Environ. Res. Public Health 2017, 14, 100. https://doi.org/10.3390/ijerph14010100
Zhong L, Su F-C, Batterman S. Volatile Organic Compounds (VOCs) in Conventional and High Performance School Buildings in the U.S. International Journal of Environmental Research and Public Health. 2017; 14(1):100. https://doi.org/10.3390/ijerph14010100
Chicago/Turabian StyleZhong, Lexuan, Feng-Chiao Su, and Stuart Batterman. 2017. "Volatile Organic Compounds (VOCs) in Conventional and High Performance School Buildings in the U.S." International Journal of Environmental Research and Public Health 14, no. 1: 100. https://doi.org/10.3390/ijerph14010100






