Developmental Neurotoxicity of Methamidophos in the Embryo-Larval Stages of Zebrafish
Abstract
:1. Introduction
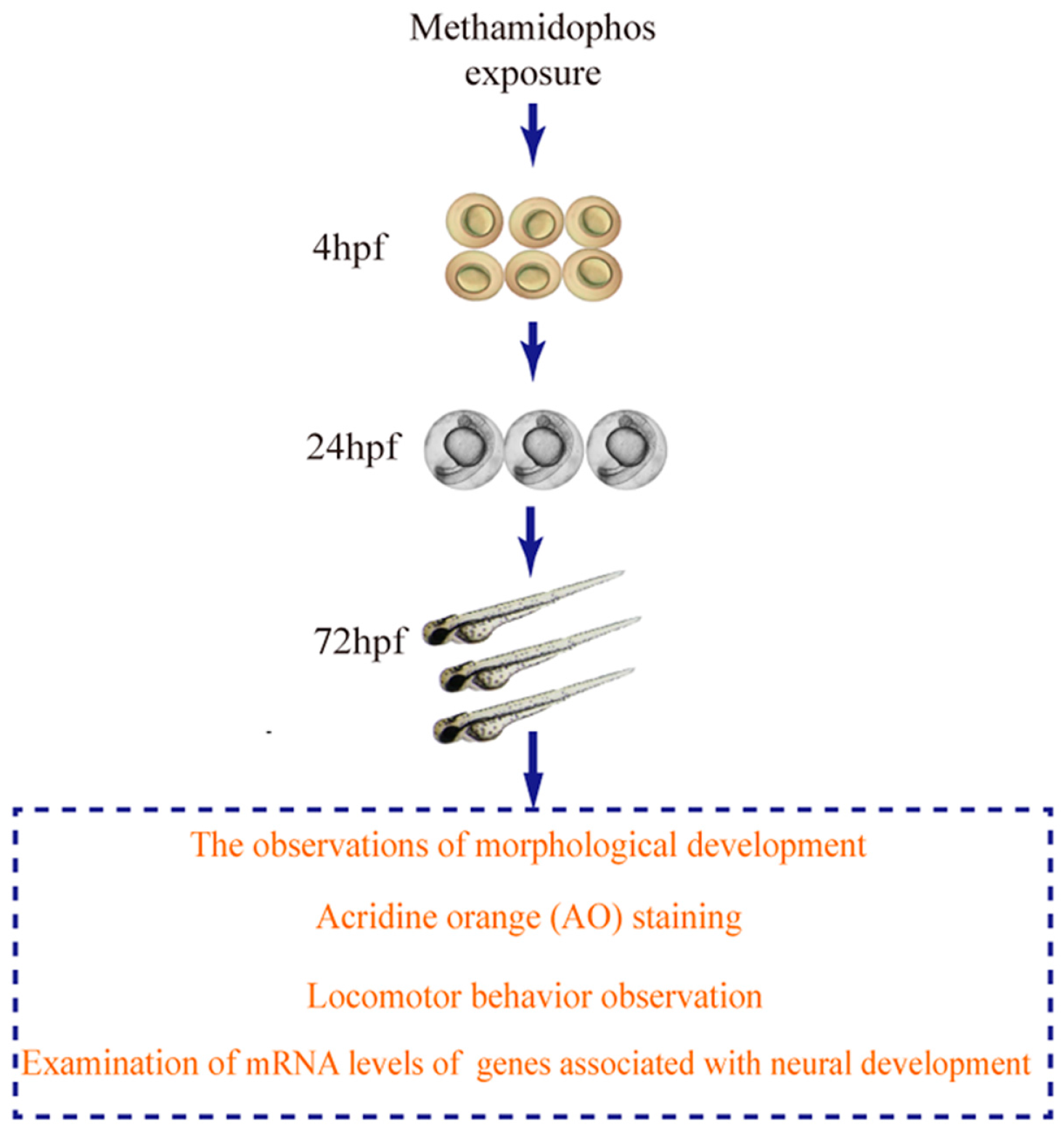
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Zebrafish Husbandry and Collection of Embryos
2.3. Methamidophos Treatment
2.4. The Observations of Morphological Development
2.5. Locomotor Behavior Observation
2.6. AO Staining
2.7. RNA Isolation and Real-Time PCR Assays
2.8. Statistical Analysis
3. Results
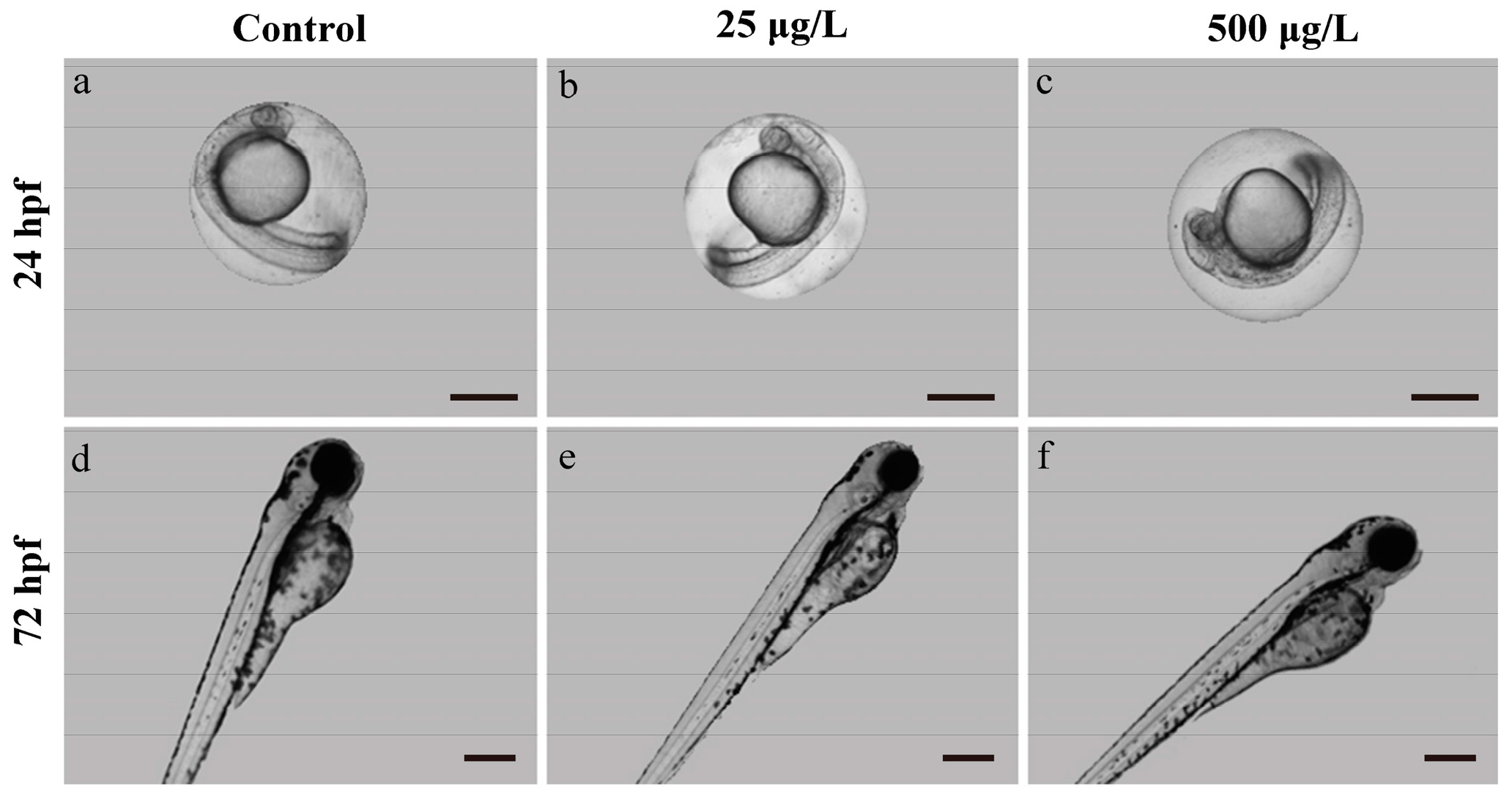
3.1. Morphological Development Observation
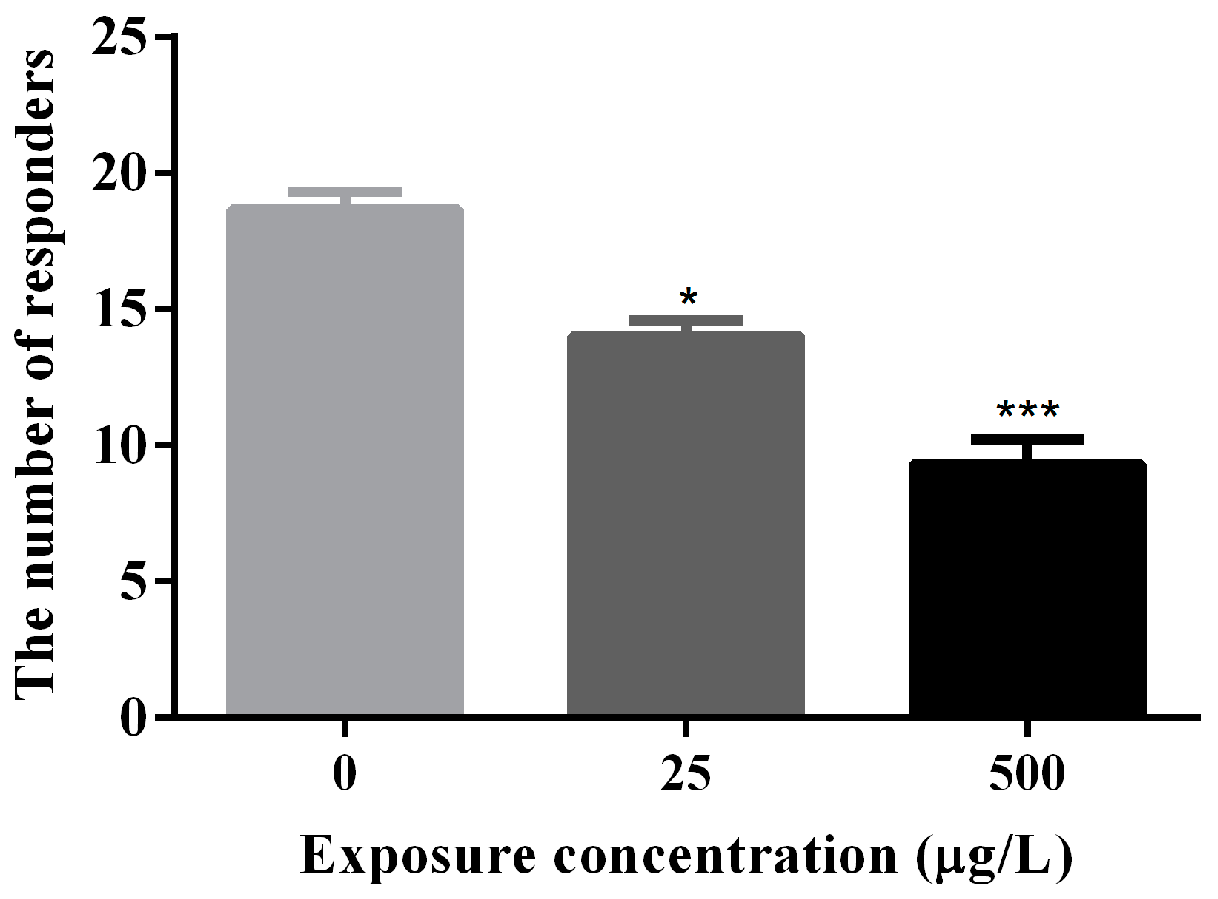
3.2. Abnormal Locomotor Behavior of Methamidophos-Exposed Zebrafish Larvae
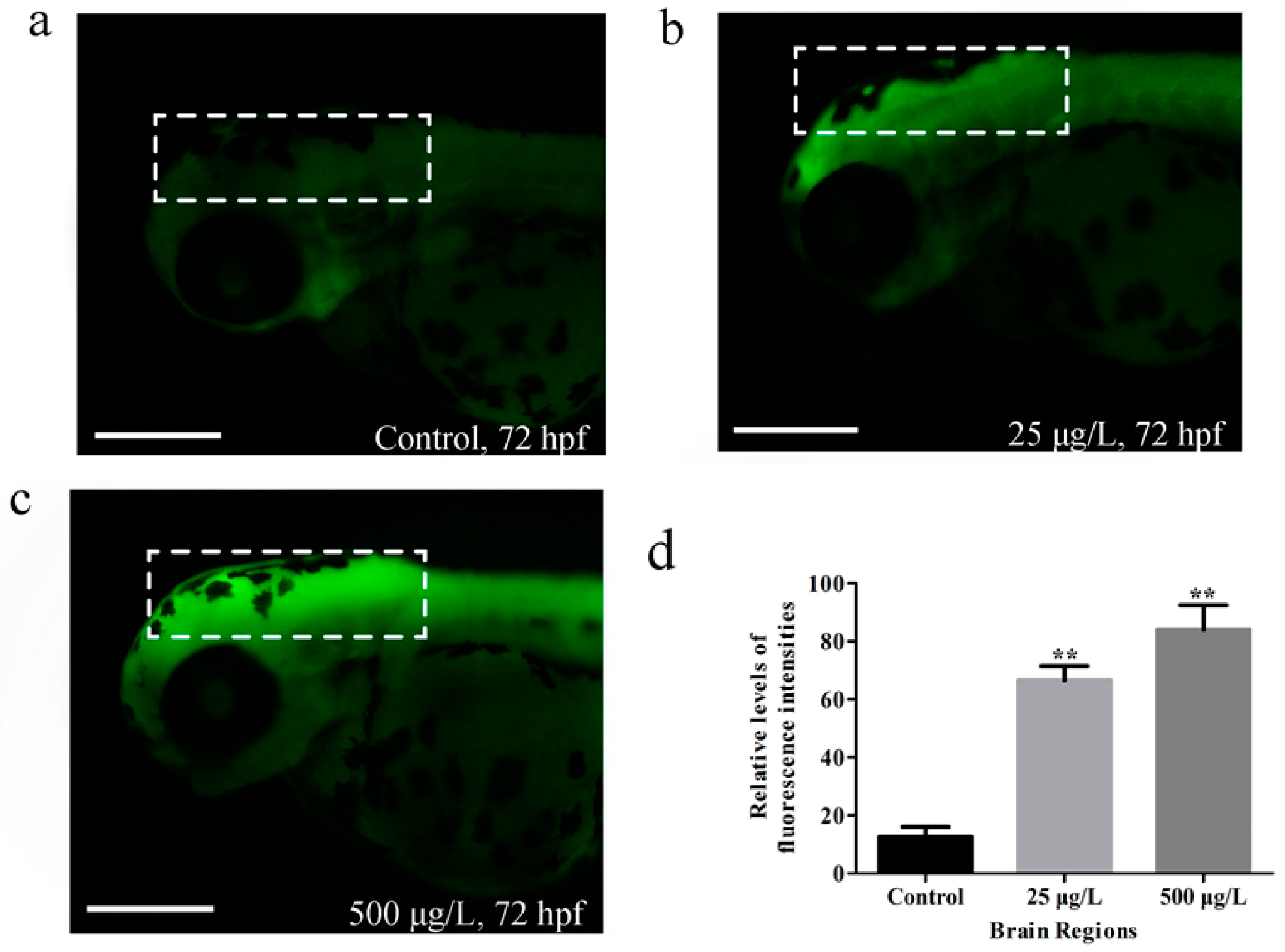
3.3. Apoptosis in Zebrafish Larvae Induced by Methamidophos Exposure
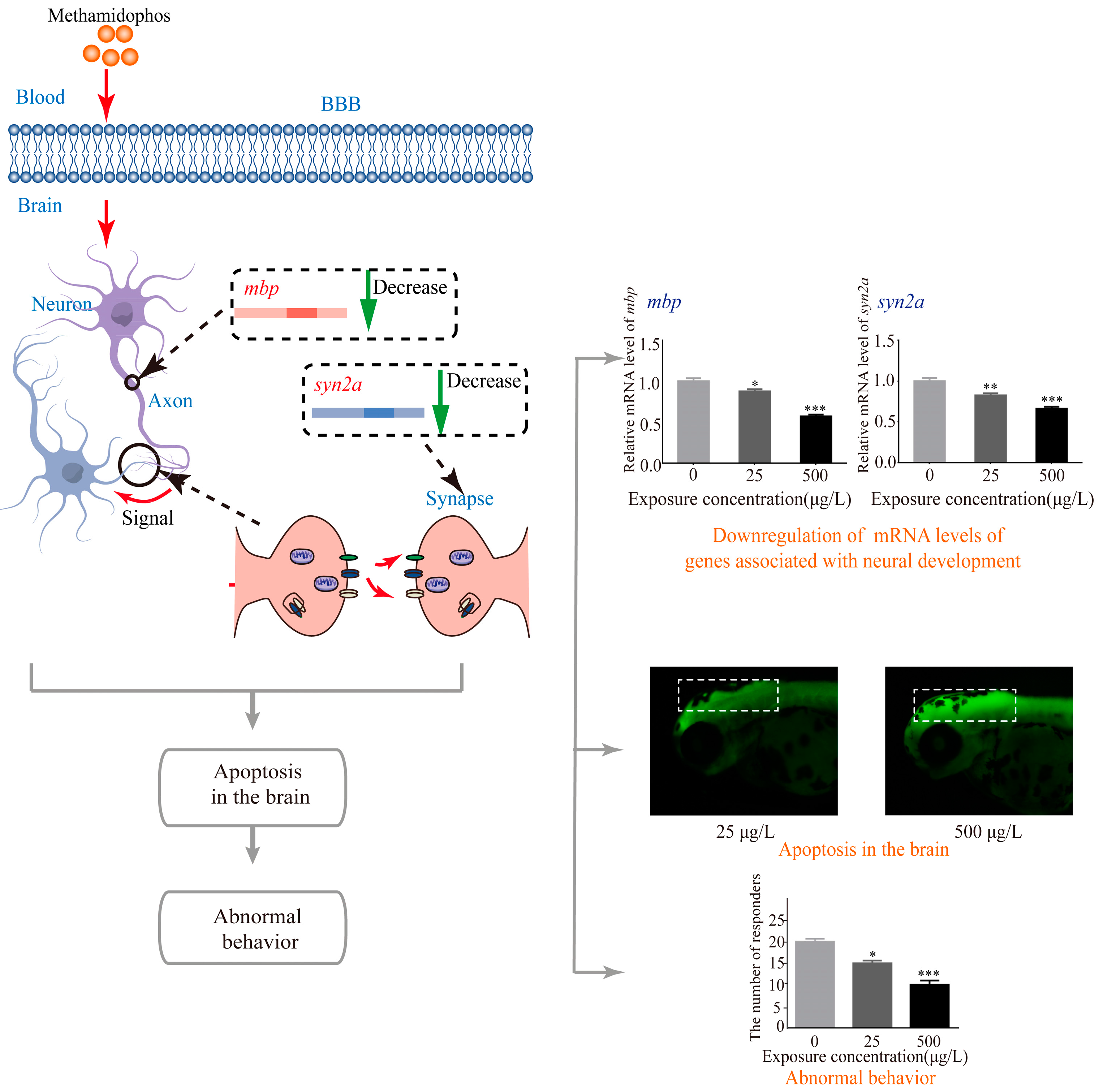
3.4. Effects of Methamidophos on the mRNA Levels of Genes Associated with Neural Development
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ibhazehiebo, K.; Iyawe, V.I.; Koibuchi, N. Effect of methamidophos on cerebellar neuronal cells. Niger. J. Physiol. Sci. 2013, 28, 115–120. [Google Scholar] [PubMed]
- Recena, M.C.; Caldas, E.D.; Pires, D.X.; Pontes, E.R. Pesticides exposure in Culturama, Brazil—Knowledge, attitudes, and practices. Environ. Res. 2006, 102, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Gubert, P.; Avila, D.S.; Bridi, J.C.; Saurin, S.; Lugokenski, T.H.; Villarinho, J.G.; Fachinetto, R.; Pereira, M.E.; Ferreira, J.; da Rocha, J.B.; et al. Low concentrations of methamidophos do not alter ache activity but modulate neurotransmitters uptake in hippocampus and striatum in vitro. Life Sci. 2011, 88, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Quinteros, E.; Ribo, A.; Mejia, R.; Lopez, A.; Belteton, W.; Comandari, A.; Orantes, C.M.; Pleites, E.B.; Hernandez, C.E.; Lopez, D.L. Heavy metals and pesticide exposure from agricultural activities and former agrochemical factory in a Salvadoran rural community. Environ. Sci. Pollut. Res. Int. 2016. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Garcia, A.E.; Medina-Diaz, I.M.; Robledo-Marenco, M.L.; Barron-Vivanco, B.S.; Giron-Perez, M.I.; Velazquez-Fernandez, J.B.; Gonzalez-Arias, C.A.; Albores-Medina, A.; Quintanilla-Vega, B.; Ostrosky-Wegman, P.; et al. Hematological, biochemical effects, and self-reported symptoms in pesticide retailers. J. Occup. Environ. Med. 2011, 53, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Liu, J.S.; Wang, Q.C.; Liu, Q.; Wang, Y. Contamination of organophosphorus pesticides residue in fresh vegetables and related health risk assessment in Changchun, China. Huan Jing Ke Xue 2015, 36, 3486–3492. (In Chinese) [Google Scholar] [PubMed]
- Syed, J.H.; Alamdar, A.; Mohammad, A.; Ahad, K.; Shabir, Z.; Ahmed, H.; Ali, S.M.; Sani, S.G.; Bokhari, H.; Gallagher, K.D.; et al. Pesticide residues in fruits and vegetables from Pakistan: A review of the occurrence and associated human health risks. Environ. Sci. Pollut. Res. Int. 2014, 21, 13367–13393. [Google Scholar] [CrossRef] [PubMed]
- Andrade, G.C.; Monteiro, S.H.; Francisco, J.G.; Figueiredo, L.A.; Botelho, R.G.; Tornisielo, V.L. Liquid chromatography-electrospray ionization tandem mass spectrometry and dynamic multiple reaction monitoring method for determining multiple pesticide residues in tomato. Food Chem. 2015, 175, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Uriostegui-Acosta, M.; Hernandez-Ochoa, I.; Sanchez-Gutierrez, M.; Pina-Guzman, B.; Rafael-Vazquez, L.; Solis-Heredia, M.J.; Martinez-Aguilar, G.; Quintanilla-Vega, B. Methamidophos alters sperm function and DNA at different stages of spermatogenesis in mice. Toxicol. Appl. Pharmacol. 2014, 279, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.F.; Bao, X.D.; Zhang, Y.; Huang, L.; Huang, H.Q. Identification of differentially expressed proteins of brain tissue in response to methamidophos in flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2015, 44, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Vargas, M.A.; Huerta-Beristain, G.; Guzman-Guzman, I.P.; Alarcon-Romero, L.D.; Flores-Alfaro, E.; Rojas-Garcia, A.E.; Moreno-Godinez, M.E. Methamidophos induces cytotoxicity and oxidative stress in human peripheral blood mononuclear cells. Environ. Toxicol. 2015, 32, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, T.; van Wendel de Joode, B.; Lindh, C.H.; Rojas, M.; Lundberg, I.; Wesseling, C. Assessment of long-term and recent pesticide exposure among rural school children in Nicaragua. Occup. Environ. Med. 2012, 69, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Maretto, G.X.; do Nascimento, C.P.; Passamani, L.M.; Schenberg, L.C.; de Andrade, T.U.; Figueiredo, S.G.; Mauad, H.; Sampaio, K.N. Acute exposure to the insecticide O,S-dimethyl phosphoramidothioate (methamidophos) leads to impairment of cardiovascular reflexes in rats. Ecotoxicol. Environ. Saf. 2012, 80, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Emerick, G.L.; DeOliveira, G.H.; dos Santos, A.C.; Ehrich, M. Mechanisms for consideration for intervention in the development of organophosphorus-induced delayed neuropathy. Chem. Biol. Interact. 2012, 199, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Noriega-Ortega, B.R.; Armienta-Aldana, E.; Cervantes-Pompa, J.A.; Hernandez-Ruiz, E.; Chaparro-Huerta, V.; Bravo-Cuellar, A.; Beas-Zarate, C. Gaba and dopamine release from different brain regions in mice with chronic exposure to organophosphate methamidophos. J. Toxicol. Pathol. 2011, 24, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Emerick, G.L.; Peccinini, R.G.; de Oliveira, G.H. Organophosphorus-induced delayed neuropathy: A simple and efficient therapeutic strategy. Toxicol. Lett. 2010, 192, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; White, T.; Spassova, D.; Jiang, Y. Physicochemical, molecular-orbital and electronic properties of acephate and methamidophos. Pharmacol. Toxicol. Endocrinol. 1998, 119, 107–117. [Google Scholar] [CrossRef]
- Bradman, A.; Barr, D.B.; Claus Henn, B.G.; Drumheller, T.; Curry, C.; Eskenazi, B. Measurement of pesticides and other toxicants in amniotic fluid as a potential biomarker of prenatal exposure: A validation study. Environ. Health Perspect. 2003, 111, 1779–1782. [Google Scholar] [CrossRef] [PubMed]
- Vidair, C.A. Age dependence of organophosphate and carbamate neurotoxicity in the postnatal rat: Extrapolation to the human. Toxicol. Appl. Pharmacol. 2004, 196, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.; Barone, S. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ. Health Perspect. 2000, 108, 511–533. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Du, Y.; Lam, P.K.; Wu, R.S.; Zhou, B. Developmental toxicity and alteration of gene expression in zebrafish embryos exposed to PFOS. Toxicol. Appl. Pharmacol. 2008, 230, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ho, N.Y.; Alshut, R.; Legradi, J.; Weiss, C.; Reischl, M.; Mikut, R.; Liebel, U.; Muller, F.; Strahle, U. Zebrafish embryos as models for embryotoxic and teratological effects of chemicals. Reprod. Toxicol. 2009, 28, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Dong, Q.; Li, S.; Guo, J.; Wang, X.; Zhu, G. Developmental toxicity of cartap on zebrafish embryos. Aquat. Toxicol. 2009, 95, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, J.R.; Jobin, C. Think small: Zebrafish as a model system of human pathology. J. Biomed. Biotechnol. 2012, 2012, 817341. [Google Scholar] [CrossRef] [PubMed]
- Canestro, C.; Yokoi, H.; Postlethwait, J.H. Evolutionary developmental biology and genomics. Nat. Rev. Genet. 2007, 8, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Murakami, S.; Ashikawa, Y.; Sasagawa, S.; Umemoto, N.; Shimada, Y.; Tanaka, T. Zebrafish as a systems toxicology model for developmental neurotoxicity testing. Congenit. Anomalies 2015, 55, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.L. Analysis of trends of the types of pesticide used, residues and related factors among farmers in the largest vegetable producing area in the Philippines. J. Rural Med. 2010, 5, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Montesano, M.A.; Barr, D.; Thomas, J.; Geller, R. Urinary elimination kinetics of acephate and its metabolite, methamidophos, in urine after acute ingestion. J. Med. Toxicol. 2009, 5, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Saera-Vila, A.; Kish, P.E.; Kahana, A. Automated scalable heat shock modification for standard aquatic housing systems. Zebrafish 2015, 12, 312–314. [Google Scholar] [CrossRef] [PubMed]
- Kari, G.; Rodeck, U.; Dicker, A.P. Zebrafish: An emerging model system for human disease and drug discovery. Clin. Pharmacol. Ther. 2007, 82, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Bernut, A.; Le Moigne, V.; Lesne, T.; Lutfalla, G.; Herrmann, J.-L.; Kremer, L. In vivo assessment of drug efficacy against mycobacterium abscessus using the embryonic zebrafish test system. Antimicrob. Agent. Chemother. 2014, 58, 4054–4063. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Gu, A.; Ji, G.; Li, Y.; Di, J.; Jin, J.; Hu, F.; Long, Y.; Xia, Y.; Lu, C.; et al. Developmental toxicity of cypermethrin in embryo-larval stages of zebrafish. Chemosphere 2011, 85, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Legradi, J.; el Abdellaoui, N.; van Pomeren, M.; Legler, J. Comparability of behavioural assays using zebrafish larvae to assess neurotoxicity. Environ. Sci. Pollut. Res. 2015, 22, 16277–16289. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.M.; Benaich, N.; Malone, C.F.; Bernardos, R.L.; Russell, A.R.; Downes, G.B.; Barresi, M.J.; Hutson, L.D. Vincristine and bortezomib cause axon outgrowth and behavioral defects in larval zebrafish. J. Peripher. Nerv. Syst. 2012, 17, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Burgess, H.A.; Granato, M. Sensorimotor gating in larval zebrafish. J. Neurosci. 2007, 27, 4984–4994. [Google Scholar] [CrossRef] [PubMed]
- Stehr, C.M.; Linbo, T.L.; Incardona, J.P.; Scholz, N.L. The developmental neurotoxicity of fipronil: Notochord degeneration and locomotor defects in zebrafish embryos and larvae. Toxicol. Sci. 2006, 92, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Mutero, C.M.; Ouma, J.H.; Agak, B.K.; Wanderi, J.A.; Copeland, R.S. Malaria prevalence and use of self-protection measures against mosquitoes in Suba District, Kenya. East. Afr. Med. J. 1998, 75, 11–15. [Google Scholar] [PubMed]
- Deng, J.; Yu, L.; Liu, C.; Yu, K.; Shi, X.; Yeung, L.W.; Lam, P.K.; Wu, R.S.; Zhou, B. Hexabromocyclododecane-induced developmental toxicity and apoptosis in zebrafish embryos. Aquat. Toxicol. 2009, 93, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Sun, H.; Xie, P.; Wang, J.; Zhang, G.; Chen, N.; Yan, W.; Li, G. The role of apoptosis in MCLR-induced developmental toxicity in zebrafish embryos. Aquat. Toxicol. 2014, 149, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Li, G.; Feng, W.; Liu, L.; Zhang, J.; Wu, W.; Xu, L.; Yan, Y. Chlorpyrifos is estrogenic and alters embryonic hatching, cell proliferation and apoptosis in zebrafish. Chem.-Biol. Interact. 2015, 239, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Gu, A.; Shi, X.; Yuan, C.; Ji, G.; Zhou, Y.; Long, Y.; Song, L.; Wang, S.; Wang, X. Exposure to fenvalerate causes brain impairment during zebrafish development. Toxicol. Lett. 2010, 197, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Kurps, J.; Broeke, J.H.; Cijsouw, T.; Kompatscher, A.; van Weering, J.R.; de Wit, H. Quantitative image analysis tool to study the plasma membrane localization of proteins and cortical actin in neuroendocrine cells. J. Neurosci. Method 2014, 236, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Casano, A.M.; Albert, M.; Peri, F. Developmental apoptosis mediates entry and positioning of microglia in the zebrafish brain. Cell Rep. 2016, 16, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Mattinen, L.; Kublbeck, J.; Rechardt, O.; Honkakoski, P.; Rautio, J. Direct and rapid transcript analysis assay for CYP mRNA expression and inducibility in human primary hepatocytes. Drug Metab. Lett. 2014, 8, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Hainzl, D.; Cole, L.M.; Casida, J.E. Mechanisms for selective toxicity of fipronil insecticide and its sulfone metabolite and desulfinyl photoproduct. Chem. Res. Toxicol. 1998, 11, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Brosamle, C.; Halpern, M.E. Characterization of myelination in the developing zebrafish. Glia 2002, 39, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Bauer, N.M.; Schafer, I.; White, R. Making myelin basic protein from mRNA transport to localized translation. Front. Cell Neurosci. 2013, 7, 169. [Google Scholar] [CrossRef] [PubMed]
- Kao, H.T.; Porton, B.; Czernik, A.J.; Feng, J.; Yiu, G.; Haring, M.; Benfenati, F.; Greengard, P. A third member of the synapsin gene family. Proc. Natl. Acad. Sci. USA 1998, 95, 4667–4672. [Google Scholar] [CrossRef] [PubMed]
- Saieva, C.; Aprea, C.; Tumino, R.; Masala, G.; Salvini, S.; Frasca, G.; Giurdanella, M.C.; Zanna, I.; Decarli, A.; Sciarra, G.; et al. Twenty-four-hour urinary excretion of ten pesticide metabolites in healthy adults in two different areas of Italy (Florence and Ragusa). Sci. Total Environ. 2004, 332, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Weppner, S.; Elgethun, K.; Lu, C.; Hebert, V.; Yost, M.G.; Fenske, R.A. The Washington aerial spray drift study: Children’s exposure to methamidophos in an agricultural community following fixed-wing aircraft applications. J. Expo. Sci. Environ. Epidemiol. 2006, 16, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Akoto, O.; Gavor, S.; Appah, M.K.; Apau, J. Estimation of human health risk associated with the consumption of pesticide-contaminated vegetables from Kumasi, Ghana. Environ. Monit. Assess. 2015, 187, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Moser, V.C. Functional assays for neurotoxicity testing. Toxicol. Pathol. 2011, 39, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Sano, K.; Isobe, T.; Yang, J.; Win-Shwe, T.T.; Yoshikane, M.; Nakayama, S.F.; Kawashima, T.; Suzuki, G.; Hashimoto, S.; Nohara, K.; et al. In utero and lactational exposure to acetamiprid induces abnormalities in socio-sexual and anxiety-related behaviors of male mice. Front. Neurosci. 2016, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Park, G.; Bae, N.; Kim, J.; Oh, M.S.; Yang, H.O. Anti-apoptotic effect of modified chunsimyeolda-tang, a traditional Korean herbal formula, on mptp-induced neuronal cell death in a Parkinson’s disease mouse model. J. Ethnopharmacol. 2015, 176, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Clift, D.E.; Thorn, R.J.; Passarelli, E.A.; Kapoor, M.; LoPiccolo, M.K.; Richendrfer, H.A.; Colwill, R.M.; Creton, R. Effects of embryonic cyclosporine exposures on brain development and behavior. Behav. Brain Res. 2015, 282, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yu, K.; Huang, C.; Yu, L.; Zhu, B.; Lam, P.K.; Lam, J.C.; Zhou, B. Prenatal transfer of polybrominated diphenyl ethers (PBDES) results in developmental neurotoxicity in zebrafish larvae. Environ. Sci. Technol. 2012, 46, 9727–9734. [Google Scholar] [CrossRef] [PubMed]
- Ulukaya, E.; Acilan, C.; Yilmaz, Y. Apoptosis: Why and how does it occur in biology? Cell Biochem. Funct. 2011, 29, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Spassova, D.; White, T.; Singh, A.K. Acute effects of acephate and methamidophos on acetylcholinesterase activity, endocrine system and amino acid concentrations in rats. Toxicol. Pharmacol. 2000, 126, 79–89. [Google Scholar] [CrossRef]
- Jung, S.H.; Kim, S.; Chung, A.Y.; Kim, H.T.; So, J.H.; Ryu, J.; Park, H.C.; Kim, C.H. Visualization of myelination in GFP-transgenic zebrafish. Dev. Dyn. 2010, 239, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.Y.; Kim, P.S.; Kim, S.; Kim, E.; Kim, D.; Jeong, I.; Kim, H.K.; Ryu, J.H.; Kim, C.H.; Choi, J.; et al. Generation of demyelination models by targeted ablation of oligodendrocytes in the zebrafish CNS. Mol Cell. 2013, 36, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liu, Z.; Peng, T.; Fu, Z. The toxicity of chlorpyrifos on the early life stage of zebrafish: A survey on the endpoints at development, locomotor behavior, oxidative stress and immunotoxicity. Fish Shellfish Immunol. 2015, 43, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, G.; Costa, S.; Pestarino, M.; Candiani, S. Differential expression of synapsin genes during early zebrafish development. Neuroscience 2014, 280, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Moser, V.C. Comparison of aldicarb and methamidophos neurotoxicity at different ages in the rat: Behavioral and biochemical parameters. Toxicol. Appl. Pharmacol. 1999, 157, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.S.; Dutra-Tavares, A.C.; Nunes, F.; Nunes-Freitas, A.L.; Ribeiro-Carvalho, A.; Filgueiras, C.C.; Manhaes, A.C.; Meyer, A.; Abreu-Villaca, Y. Methamidophos exposure during the early postnatal period of mice: Immediate and late-emergent effects on the cholinergic and serotonergic systems and behavior. Toxicol. Sci. 2013, 134, 125–139. [Google Scholar] [CrossRef] [PubMed]
- De Castro, V.L.; Chiorato, S.H.; Pinto, N.F. Relevance of developmental testing of exposure to methamidophos during gestation to its toxicology evaluation. Toxicol. Lett. 2000, 118, 93–102. [Google Scholar] [CrossRef]

| Target Gene | GenBank Accession No. | Primer Sequences |
|---|---|---|
| mbp | AY860977 | Forward: 5’-AATCAGCAGGTTCTTCGGAGGAGA-3’ Reverse: 5’-AAGAAATGCACGACAGGGTTGACG-3’ |
| syn2a | NM_001002597 | Forward: 5’-GTGACCATGCCAGCATTTC-3’ |
| Reverse: 5’-TGGTTCTCCACTTTCACCTT-3’ | ||
| β-actin | AF025305 | Forward: 5’-ACAGGGAAAAGATGACACAGATCA-3’ Reverse: 5’-CAGCCTGGATGGCAACGTA-3’ |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Gao, J.; Dong, T.; Chen, M.; Zhou, K.; Chang, C.; Luo, J.; Wang, C.; Wang, S.; Chen, D.; et al. Developmental Neurotoxicity of Methamidophos in the Embryo-Larval Stages of Zebrafish. Int. J. Environ. Res. Public Health 2017, 14, 23. https://doi.org/10.3390/ijerph14010023
He X, Gao J, Dong T, Chen M, Zhou K, Chang C, Luo J, Wang C, Wang S, Chen D, et al. Developmental Neurotoxicity of Methamidophos in the Embryo-Larval Stages of Zebrafish. International Journal of Environmental Research and Public Health. 2017; 14(1):23. https://doi.org/10.3390/ijerph14010023
Chicago/Turabian StyleHe, Xiaowei, Jiawei Gao, Tianyu Dong, Minjian Chen, Kun Zhou, Chunxin Chang, Jia Luo, Chao Wang, Shoulin Wang, Daozhen Chen, and et al. 2017. "Developmental Neurotoxicity of Methamidophos in the Embryo-Larval Stages of Zebrafish" International Journal of Environmental Research and Public Health 14, no. 1: 23. https://doi.org/10.3390/ijerph14010023





