Determinants of Quality of Life in High-Dose Benzodiazepine Misusers
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Patients
2.2. Quality of Life Measures
2.3. Statistical Analysis
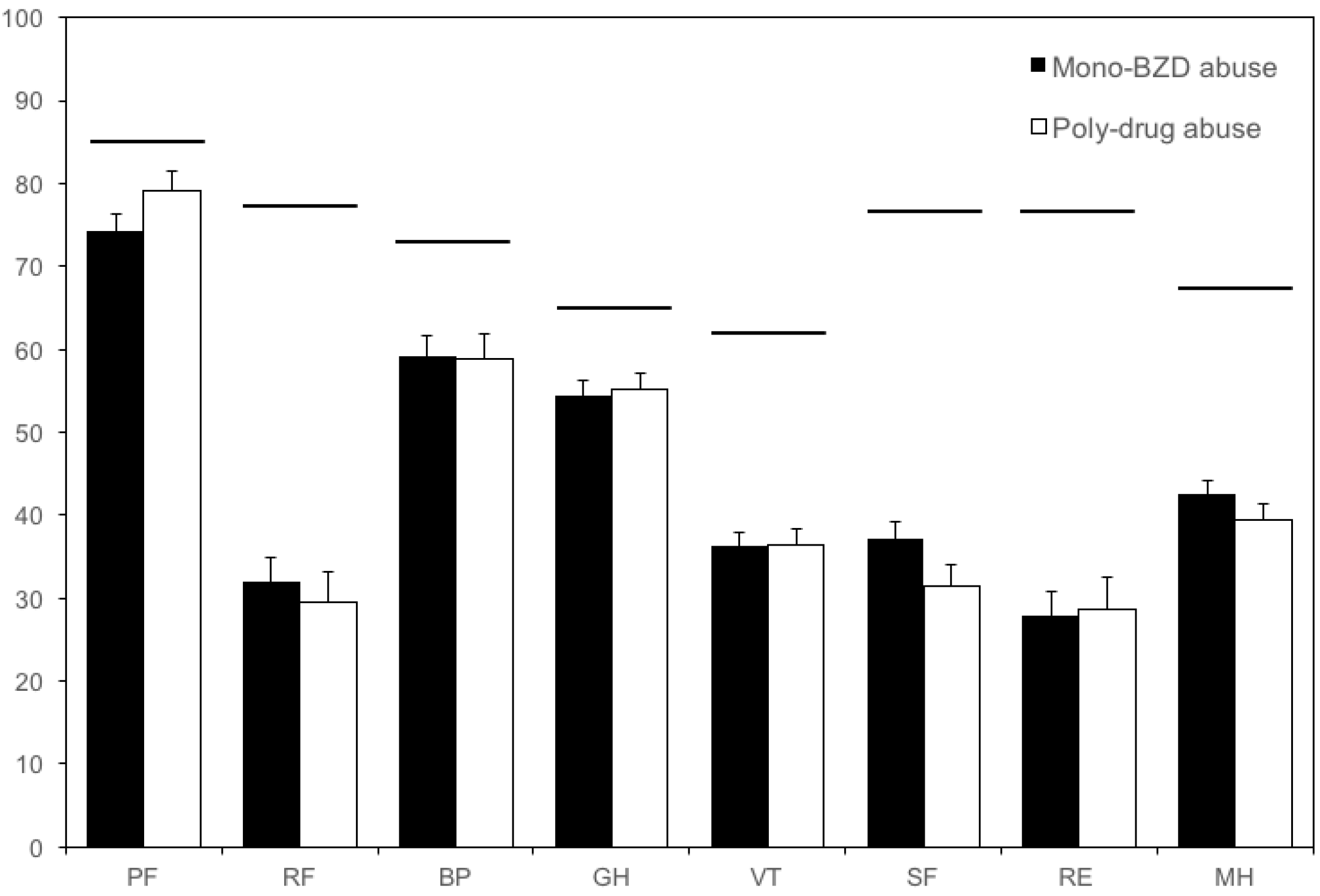
3. Results
3.1. Patients
3.2. Quality of Life Measures
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Lader, M. Benzodiazepines revisited-will we ever learn? Addiction 2011, 106, 2086–2109. [Google Scholar] [CrossRef] [PubMed]
- Kurko, T.A.; Saastamoinen, L.K.; Tähkäpää, S.; Tuulio-Henriksson, A.; Taiminen, T.; Tiihonen, J.; Airaksinen, M.S.; Hietala, J. Long-term use of benzodiazepines: Definitions, prevalence and usage patterns—A systematic review of register-based studies. Eur. Psychiatry 2015, 30, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.Y.; Chen, C.Y.; Chang, I.S.; Wu, E.C.; Chang, C.M.; Lin, K.M. Predictors of the incidence and discontinuation of long-term use of benzodiazepines: A population-based study. Drug Alcohol Depend. 2009, 104, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Faccini, M.; Leone, R.; Pajusco, B.; Quaglio, G.; Casari, R.; Albiero, A.; Donati, M.; Lugoboni, F. Lormetazepam addiction: Data analysis from an Italian medical unit for addiction. Risk Manag. Healthc. Policy 2012, 5, 43–48. [Google Scholar] [PubMed]
- Ohayon, M.M.; Lader, M.H. Use of psychotropic medication in the general population of France, Germany, Italy, and the United Kingdom. J. Clin. Psychiatry 2002, 63, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Petitjean, S.; Ladewig, D.; Meier, C.R.; Amrein, R.; Wiesbeck, G.A. Benzodiazepine prescribing to the Swiss adult population: Results from a national survey of community pharmacies. Int. Clin. Psychopharmacol. 2007, 22, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Liebrenz, M.; Schneider, M.; Buadze, A.; Gehring, M.T.; Dube, A.; Caflisch, C. High-dose benzodiazepine dependence: A qualitative study of patients’ perceptions on initiation, reasons for use, and obtainment. PLoS ONE 2015, 10, e0142057. [Google Scholar] [CrossRef] [PubMed]
- Lugoboni, F.; Mirijello, A.; Faccini, M.; Casari, R.; Cossari, A.; Musi, G.; Bissoli, G.; Quaglio, G.; Addolorato, G. Quality of life in a cohort of high-dose benzodiazepine dependent patients. Drug Alcohol Depend. 2014, 142, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Fiumana, V.; Zamboni, L.; Mazza, M.; Janiri, L.; Cibin, M.; GICS; Lugoboni, F. Quality of life on heroin users attending substitution treatment: A multicenter study in Italy. Health 2016, 8, 1195–1208. [Google Scholar] [CrossRef]
- Lozano, O.M.; Domingo-Salvany, A.; Martinez-Alonso, M.; Brugal, M.T.; Alonso, J.; Fuente, L. Health-related quality of life in young cocaine users and associated factors. Qual. Life Res. 2008, 17, 977–985. [Google Scholar] [CrossRef] [PubMed]
- De Maeyer, J.; Vanderplasschen, W.; Broekaert, E. Quality of life among opiate-dependent individuals: A review of literature. Int. J. Drug Policy 2010, 21, 364–380. [Google Scholar] [CrossRef] [PubMed]
- Domingo-Salvany, A.; Brugal, M.T.; Barrio, G.; González-Saiz, F.; Bravo, M.J.; Fuente, L.; ITINERE Investigators. Gender differences in health related quality of life of young heroin users. Health Qual. Life Outcomes 2010, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Beccaria, F.; Rolando, S.; Ascani, P. Alcohol consumption and quality of life among young adults: A comparison among three European countries. Subst. Use Misuse 2012, 47, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Zubaran, C.; Emerson, J.; Sud, R.; Zolfaghari, E.; Foresti, K. The application of the drug user quality of life scale (DUQOL) in Australia. Health Qual. Life Outcomes 2012, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Marini, M.; Schnornberger, T.M.; Brandalise, G.B.; Bergozza, M.; Heldt, E. Quality of life determinants in patients of a Psychosocial Care Center for alcohol and other drug users. Ment. Health Nurs. 2013, 34, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Brands, B.; Blake, J.; Marsh, D.C.; Sproule, B.; Jeyapalan, R.; Li, S. The impact of benzodiazepine use on methadone maintenance treatment outcomes. J. Addict. Dis. 2008, 27, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.W.; Berger, C.C.; Forde, D.P.; D’Adamo, C.; Weintraub, E.; Gandhi, D. Benzodiazepine use and misuse among patient in a methadone program. BMC Psychiatry 2011, 11, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Vorma, H.; Naukkarinen, H.; Sarna, S.; Kuoppasalmi, K. Symptom severity and quality of life after benzodiazepine withdrawal treatment in participants with complicated dependence. Addict. Behav. 2004, 29, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Brunette, M.F.; Noordsy, D.L.; Xie, H.; Drake, R.E. Benzodiazepine use and abuse among patients with severe mental illness and co-occurring substance use disorders. Psychiatr. Serv. 2003, 54, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Colpaert, K.; De Maeyer, J.; Broekaert, E.; Vanderplasschen, W. Impact of addiction severity and psychiatric comorbidity on the quality of life of alcohol-, drug- and dual-dependent persons in residential treatment. Eur. Addict. Res. 2012, 19, 173–183. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Press: Washington, DC, USA, 2000. [Google Scholar]
- Quaglio, G.; Lugoboni, F.; Fornasiero, A.; Lechi, A.; Gerra, G.; Mezzelani, P. Dependence on zolpidem: Two case reports of detoxification with flumazenil infusion. Int. Clin. Psychopharmacol. 2005, 20, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Brazier, J.E.; Harper, R.; Jones, N.M.; O’Cathain, A.; Thomas, K.J.; Usherwood, T.; West-lake, L. Validating the SF-36 health survey questionnaire: New outcome measure for primary care. BMJ 1992, 305, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Apolone, G.; Mosconi, P. The Italian SF-36 Health Survey: Translation, validation and norming. Health-related quality of life in chronic disorders: A comparison across studies using the MOS SF-36. J. Clin. Epidemiol. 1998, 51, 1025–1036. [Google Scholar] [CrossRef]
- Piccinelli, M.; Bisoffi, G.; Bon, M.G.; Cunico, L.; Tansella, M. Validity and test-retest reliability of the Italian version of the 12-item General Health Questionnaire in general practice: A comparison between three scoring methods. Compr. Psychiatry 1993, 34, 198–205. [Google Scholar] [CrossRef]
- Goldberg, D.P.; Hillier, V.F. A scaled version of the General Health Questionnaire. Psychol. Med. 1979, 9, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression, 2nd ed.; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Metz, V.E.; Comer, S.D.; Wuerzl, J.; Pribasnig, A.; Fischer, G. Characteristics and quality of life of opioid-dependent pregnant women in Austria. Arch. Womens Ment. Health 2014, 17, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Zubaran, C.; Foresti, K. Quality of life and substance use: Concepts and recent tendencies. Curr. Opin. Psychiatry 2009, 22, 281–286. [Google Scholar] [CrossRef] [PubMed]
- McLellan, A.T. Have we evaluated addiction treatment correctly? Implications from a chronic care perspective. Addiction 2002, 97, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, H.; Watts, J. Quality of life in substance abuse and dependency. Int. Rev. Psychiatry 2002, 14, 190–197. [Google Scholar] [CrossRef]
- Valderas, J.M.; Kotzeva, A.; Espallargues, M.; Guyatt, G.; Ferrans, C.E.; Halyard, M.Y.; Revicki, D.A.; Symonds, T.; Parada, A.; Alonso, J. The impact of measuring patient-reported outcomes in clinical practice: A systematic review of the literature. Qual. Life Res. 2008, 17, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.C.; Shih, S.F.; Tasi, W.D.; Li, C.R.; Xu, K.; Lee, T.S. Improvement of quality of life in methadone treatment patients in northern Taiwan: A follow-up study. BMC Psychiatry 2013, 13, 190. [Google Scholar] [CrossRef] [PubMed]
- Ugochukwu, C.; Bagot, K.S.; Delaloye, S.; Pi, S.; Vien, L.; Garvey, T.; Bolotaulo, N.I.; Kumar, N.; Ishak, W.W. The importance of quality of life in patients with alcohol abuse and dependence. Harv. Rev. Psychiatry 2013, 21, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, N.; Kruse, J.; Kugler, J. Smoking and its association with disability in chronic conditions: Results from the Canadian Community and Health Survey 2.1. Nicotine Tob. Res. 2007, 9, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Erickson, S.R.; Thomas, L.A.; Blitz, S.G.; Pontius, L.R. Smoking cessation: A pilot study of the effects on health-related quality of life and perceived work performance one week into the attempt. Ann. Pharmacother. 2004, 38, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.F.; Alessi, S.M.; Petry, N.M. The impact of contingency management on quality of life among cocaine abusers with and without alcohol dependence. Am. J. Addict. 2012, 21, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Lugoboni, F.; Carli, S.; Bissoli, G.; Musi, G.; Florio, E.; Civitelli, G.; Brizio, M.; Smacchia, C.; Biasin, C.; Cifelli, G.; et al. Evaluation of the quality of life in 171 patients undergoing methadone maintenance treatment and in 46 monodependent benzodiazepine patients. Heroin Addict. Relat. Clin. Probl. 2014, 16, 5–14. [Google Scholar]
- Prigent, A.; Auraaen, A.; Kamendje-Tchokobou, B.; Durand-Zaleski, I.; Chevreul, K. Health-related quality of life and utility scores in people with mental disorders: A comparison with the non-mentally ill general population. Int. J. Environ. Res. Public Health 2014, 11, 2804–2817. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Guo, C.; Ping, W.; Tan, Z.; Guo, Y.; Zheng, J. A community-based study of quality of life and depression among older adults. Int. J. Environ. Res. Public Health 2016, 13, 693. [Google Scholar] [CrossRef] [PubMed]
- Astals, M.; Domingo-Salvany, A.; Buenaventura, C.C.; Tato, J.; Vazquez, J.M.; Martín-Santos, R.; Torrens, M. Impact of substance dependence and dual diagnosis on the quality of life of heroin users seeking treatment. Subst. Use Misuse 2008, 43, 612–632. [Google Scholar] [CrossRef] [PubMed]
- Lozano, O.M.; Rojas, A.J.; Fernández Calderón, F. Psychiatric comorbidity and severity of dependence on substance users: How it impacts on their health-related quality of life? J. Ment. Health 2016. [Google Scholar] [CrossRef] [PubMed]
- Addolorato, G.; Mirijello, A.; D’Angelo, C.; Leggio, L.; Ferrulli, A.; Abenavoli, L.; Vonghia, L.; Cardone, S.; Leso, V.; Cossari, A.; et al. State and trait anxiety and depression in patients affected by gastrointestinal diseases: Psycho-metric evaluation of 1641 patients referred to an internal medicine outpatient setting. Int. J. Clin. Pract. 2008, 62, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Wieder-Huszla, S.; Szkup, M.; Jurczak, A.; Samochowiec, A.; Samochowiec, J.; Stanisławska, M.; Rotter, I.; Karakiewicz, B.; Grochans, E. Effects of socio-demographic, personality and medical factors on quality of life of postmenopausal women. Int. J. Environ. Res. Public Health 2014, 11, 6692–6708. [Google Scholar] [CrossRef] [PubMed]
- Van Hulten, R.; Teeuw, B.; Bakker, A.; Leufkens, H.G. Initial benzodiazepine use and improved health-related quality of life. Pharm. World Sci. 2005, 27, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Laudet, A.B.; Becker, J.B.; White, W.L. Don’t wanna go through that madness no more: Quality of life satisfaction as predictor of sustained remission from illicit drug misuse. Subst. Use Misuse 2009, 44, 227–252. [Google Scholar] [CrossRef] [PubMed]
| Variable | Type of BZD Misuse | p Value | ||
|---|---|---|---|---|
| Only BZD Misuse (n = 166) | Previous Poly-Drug Misuse (n = 52) | Active Poly-Drug Misuse (n = 49) | ||
| Sex (M, F) | 33%, 67% | 82%, 18% | 67%, 33% | <0.001 * |
| Age | 45.7 ± 10.7 | 44.2 ± 8.9 | 41.4 ± 9.0 | 0.03 * |
| Education † | 23%, 51%, 26% | 33%, 54%, 13% | 31%, 53%, 16% | n.s. |
| Employment ‡ | 37%, 63% | 48%, 52% | 45%, 55% | n.s. |
| Marital status § | 54%, 46% | 69%, 31% | 61%, 39% | n.s. |
| Variable | Type of BZD Misuse | p Value | ||
|---|---|---|---|---|
| All Patients (n = 267) | Active BZD ^ (n = 218) | Active Poly-Drug (n = 49) | ||
| Active principle † | 0.015 * | |||
| Lormetazepam | 187 (70.0%) | 156 (71.6%) | 31 (63.3%) | |
| Alprazolam | 22 (8.2%) | 17 (7.8%) | 5 (10.2%) | |
| Zolpidem | 22 (8.2%) | 20 (9.2%) | 2 (4.1%) | |
| Lorazepam | 12 (4.5%) | 6 (2.8%) | 6 (12.2%) | |
| Bromazepam | 10 (3.7%) | 7 (3.2%) | 3 (6.1%) | |
| Triazolam | 4 (1.5%) | 2 (0.9%) | 2 (4.1%) | |
| Other BZDs | 10 (3.7%) | 10 (4.5%) | 0 (0.0%) | |
| DDDE (mg) ‡ | 394.5 ± 392.0 | 406.2 ± 401.1 | 365.9 ± 324.5 | n.s. |
| Misuse duration (mos) | 74.9 ± 69.8 | 75.7 ± 72.1 | 71.4 ± 58.3 | n.s. |
| Other drugs of misuse § | n.a. | |||
| Alcohol | 39/33/29 | 25/27/0 | 14/6/29 | |
| Opioids | 70/26/5 | 37/15/0 | 33/11/5 | |
| Cocaine | 47/43/11 | 29/23/0 | 18/20/11 | |
| Cannabinoids | 57/35/9 | 32/20/0 | 25/15/9 | |
| Barbiturates | 96/3/1 | 50/1/0 | 46/2/1 | |
| Psychiatric diseases ¶ | 164 (61.4%) | 125 (57.3%) | 39 (79.6%) | 0.004 * |
| Major depression | 145 (54.3%) | 125 (45.9%) | 20 (40.8%) | n.s. |
| Other psychoses | 28 (10.5%) | 15 (11.7%) | 13 (26.5%) | <0.001 * |
| Personality disorders | 22 (8.2%) | 13 (6.0%) | 9 (18.4%) | 0.009 * |
| SF-36 Dimensions and Significant Covariates | β | 95% CI | p Value |
|---|---|---|---|
| Physical functioning (PF) | |||
| Age (year) | 1.19 | 1.01; 1.37 | <0.001 |
| Education | 8.96 | 3.81; 14.12 | 0.001 |
| Employment | 10.94 | 3.70; 18.18 | 0.003 |
| Marital status | 8.53 | 0.84; 16.22 | 0.030 |
| Poly-drug misuse (either active or previous) | 14.69 | 7.58; 21.80 | <0.001 |
| Role physical (RF) | |||
| Age (year) | 0.68 | 0.58; 0.79 | <0.001 |
| Bodily pain (BP) | |||
| Sex | −9.66 | −17.70; −1.60 | 0.019 |
| Age (year) | 1.10 | 0.92; 1.28 | <0.001 |
| Education | 9.98 | 4.18; 15.79 | 0.001 |
| General health (GH) | |||
| Age (year) | 0.90 | 0.75; 1.04 | <0.001 |
| Education | 5.61 | 1.37; 9.84 | 0.010 |
| Employment | 7.06 | 1.18; 12.94 | 0.019 |
| Vitality (VT) | |||
| Sex | −9.55 | −14.70; −4.40 | <0.001 |
| Age (year) | 0.87 | 0.75; 0.99 | <0.001 |
| Education | 4.32 | 0.78; 7.87 | 0.017 |
| Marital status | 6.99 | 1.48; 12.50 | 0.013 |
| Social functioning (SF) | |||
| Age (year) | 0.83 | 0.65; 1.01 | <0.001 |
| DDDE (mg) | −0.02 | −0.01; −0.04 | 0.026 |
| Role emotional (RE) | |||
| Age (year) | 0.71 | 0.56; 0.87 | <0.001 |
| Mental health (MH) | |||
| Sex | −7.39 | −12.95; −1.83 | 0.009 |
| Age (year) | 0.95 | 0.82; 1.08 | <0.001 |
| Education | 4.92 | 1.09; 8.75 | 0.012 |
| Marital status | 7.38 | 1.43; 13.32 | 0.015 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamburin, S.; Federico, A.; Faccini, M.; Casari, R.; Morbioli, L.; Sartore, V.; Mirijello, A.; Addolorato, G.; Lugoboni, F. Determinants of Quality of Life in High-Dose Benzodiazepine Misusers. Int. J. Environ. Res. Public Health 2017, 14, 38. https://doi.org/10.3390/ijerph14010038
Tamburin S, Federico A, Faccini M, Casari R, Morbioli L, Sartore V, Mirijello A, Addolorato G, Lugoboni F. Determinants of Quality of Life in High-Dose Benzodiazepine Misusers. International Journal of Environmental Research and Public Health. 2017; 14(1):38. https://doi.org/10.3390/ijerph14010038
Chicago/Turabian StyleTamburin, Stefano, Angela Federico, Marco Faccini, Rebecca Casari, Laura Morbioli, Valentina Sartore, Antonio Mirijello, Giovanni Addolorato, and Fabio Lugoboni. 2017. "Determinants of Quality of Life in High-Dose Benzodiazepine Misusers" International Journal of Environmental Research and Public Health 14, no. 1: 38. https://doi.org/10.3390/ijerph14010038







