How Fencing Affects the Soil Quality and Plant Biomass in the Grassland of the Loess Plateau
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design and Measurements
2.3. Statistical Analysis
3. Results
3.1. Plant Characteristics’ Response to Restoration Time
3.2. Soil Properties Response to Restoration Time
3.3. Soil Bulk Density, Carbon, Nitrogen, and Phosporus Stocks Response to Restoration Time
3.4. The Relations between Soil Properties Studied and Plant Biomass across the Succession
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schönbach, P.; Wan, H.; Gierus, M.; Bai, Y.; Müller, K.; Lin, L.; Susenbeth, A.; Taube, F. Grassland responses to grazing: Effects of grazing intensity and management system in an Inner Mongolian steppe ecosystem. Plant Soil 2011, 340, 103–115. [Google Scholar] [CrossRef]
- Deng, L.; Zhang, Z.; Shangguan, Z. Long-term fencing effects on plant diversity and soil properties in China. Soil Tillage Res. 2014, 137, 7–15. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Kreyling, O.; Steffens, M.; Schoenbach, P.; Wan, H.; Gierus, M.; Taube, F.; Kölbl, A.; Kögel-Knabner, I. Short-term degradation of semiarid grasslands—Results from a controlled-grazing experiment in Northern China. J. Plant Nutr. Soil Sci. 2012, 175, 434–442. [Google Scholar] [CrossRef]
- Hilker, T.; Natsagdorj, E.; Waring, R.H.; Lyapustin, A.; Wang, Y. Satellite observed widespread decline in Mongolian grasslands largely due to overgrazing. Glob. Chang. Biol. 2014, 20, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Z.; Wang, Z.; Wu, X.; Li, X.; Hu, J.; Shi, H.; Guo, F.; Zhang, Y.; Hou, X. Pathways of Leymus chinensis individual aboveground biomass decline in natural semiarid grassland induced by overgrazing: A study at the plant functional trait scale. PLoS ONE 2015, 10, e0124443. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Sweeney, S.; Shangguan, Z.P. Grassland responses to grazing disturbance: Plant diversity changes with grazing intensity in a desert steppe. Grass Forage Sci. 2014, 69, 524–533. [Google Scholar] [CrossRef]
- Dlamini, P.; Chivenge, P.; Chaplot, V. Overgrazing decreases soil organic carbon stocks the most under dry climates and low soil pH: A meta-analysis shows. Agric. Ecosyst. Environ. 2016, 221, 258–269. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, W. Vegetation and soil property response of short-time fencing in temperate desert of the Hexi Corridor, northwestern China. CATENA 2015, 133, 43–51. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, X.; Tang, Y.; Gu, S.; Zhou, L. Alpine grassland degradation and its control in the source region of the Yangtze and Yellow Rivers, China. Grassl. Sci. 2005, 51, 191–203. [Google Scholar] [CrossRef]
- Wu, G.-L.; Liu, Z.-H.; Zhang, L.; Chen, J.-M.; Hu, T.-M. Long-term fencing improved soil properties and soil organic carbon storage in an alpine swamp meadow of western China. Plant Soil 2010, 332, 331–337. [Google Scholar] [CrossRef]
- Xiong, D.; Shi, P.; Zhang, X.; Zou, C.B. Effects of grazing exclusion on carbon sequestration and plant diversity in grasslands of China—A meta-analysis. Ecol. Eng. 2016, 94, 647–655. [Google Scholar] [CrossRef]
- Zeng, Q.; An, S.; Liu, Y. Soil bacterial community response to vegetation succession after fencing in the grassland of China. Sci. Total Environ. 2017, 609, 2–10. [Google Scholar] [CrossRef] [PubMed]
- U.S. Soil Conservation Service. Soil Taxonomy: A Basic System of Soil Classification for Making and Insterpreting Soil Surveys; USDA Soil Conservation Service: Washington, DC, USA, 1999. [Google Scholar]
- Shi, X.; Yu, D.; Xu, S.; Warner, E.; Wang, H.; Sun, W.; Zhao, Y.; Gong, Z. Cross-reference for relating Genetic Soil Classification of China with WRB at different scales. Geoderma 2010, 155, 344–350. [Google Scholar] [CrossRef]
- Zeng, Q.C.; Li, X.; Dong, Y.H.; An, S.S.; Darboux, F. Soil and plant components ecological stoichiometry in four steppe communities in the Loess Plateau of China. CATENA 2016, 147, 481–488. [Google Scholar] [CrossRef]
- Tscherko, D.; Hammesfahr, U.; Marx, M.-C.; Kandeler, E. Shifts in rhizosphere microbial communities and enzyme activity of Poa alpina across an alpine chronosequence. Soil Biol. Biochem. 2004, 36, 1685–1698. [Google Scholar] [CrossRef]
- Jiao, J.Y.; Zhang, Z.G.; Bai, W.J.; Jia, Y.F.; Wang, N. Assessing the ecological success of restoration by afforestation on the Chinese Loess Plateau. Restor. Ecol. 2012, 20, 240–249. [Google Scholar] [CrossRef]
- Blake, G.; Hartge, K. Particle density. In Methods of Soil Analysis: Part 1—Physical and Mineralogical Methods; 1986; pp. 377–382. [Google Scholar]
- Zeng, Q.; Dong, Y.; An, S. Bacterial community responses to soils along a latitudinal and vegetation gradient on the Loess Plateau, China. PLoS ONE 2016, 11, e0152894. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Xue, Z.; Xiang, Y.; Darboux, F.; An, S. Soil organic carbon sequestration in relation to revegetation on the Loess Plateau, China. Plant Soil 2015, 397, 31–42. [Google Scholar] [CrossRef]
- Ellert, B.; Bettany, J. Calculation of organic matter and nutrients stored in soils under contrasting management regimes. Can. J. Soil Sci. 1995, 75, 529–538. [Google Scholar] [CrossRef]
- Jiao, F.; Wen, Z.-M.; An, S.-S. Changes in soil properties across a chronosequence of vegetation restoration on the Loess Plateau of China. CATENA 2011, 86, 110–116. [Google Scholar] [CrossRef]
- Van Hall, R.; Cammeraat, L.; Keesstra, S.; Zorn, M. Impact of secondary vegetation succession on soil quality in a humid Mediterranean landscape. CATENA 2016, 149, 836–843. [Google Scholar] [CrossRef]
- Martens, D.A.; Reedy, T.E.; Lewis, D.T. Soil organic carbon content and composition of 130-year crop, pasture and forest land-use managements. Glob. Chang. Biol. 2004, 10, 65–78. [Google Scholar] [CrossRef]
- Zhang, K.; Dang, H.; Tan, S.; Cheng, X.; Zhang, Q. Change in soil organic carbon following the “Grain-for-Green” programme in China. Land Degrad. Dev. 2010, 21, 13–23. [Google Scholar] [CrossRef]
- Yuan, Z.-Q.; Yu, K.-L.; Guan, X.-K.; Fang, C.; Li, M.; Shi, X.-Y.; Li, F.-M. Medicago sativa improves soil carbon sequestration following revegetation of degraded arable land in a semi-arid environment on the Loess Plateau, China. Agric. Ecosyst. Environ. 2016, 232, 93–100. [Google Scholar] [CrossRef]
- Graetz, R.; Tongway, D.J. Influence of grazing management on vegetation, soil structure and nutrient distribution and the infiltration of applied rainfall in a semi-arid chenopod shrubland. Austral Ecol. 1986, 11, 347–360. [Google Scholar] [CrossRef]
- Villamil, M.B.; Amiotti, N.M.; Peinemann, N. Soil degradation related to overgrazing in the semi-arid southern Caldenal area of Argentina. Soil Sci. 2001, 166, 441–452. [Google Scholar] [CrossRef]
- Li, Y.; Shao, M. Change of soil physical properties under long-term natural vegetation restoration in the Loess Plateau of China. J. Arid Environ. 2006, 64, 77–96. [Google Scholar] [CrossRef]
- Macdonald, C.A.; Crawley, M.J.; Wright, D.J.; Kuczynski, J.; Robinson, L.; Knight, R.; Al-Soud, W.A.; Sorensen, S.J.; Deng, Y.; Zhou, J.; et al. Identifying qualitative effects of different grazing types on below-ground communities and function in a long-term field experiment. Environ. Microbiol. 2015, 17, 841–854. [Google Scholar] [CrossRef] [PubMed]
- McNally, S.R.; Laughlin, D.C.; Rutledge, S.; Dodd, M.B.; Six, J.; Schipper, L.A. Root carbon inputs under moderately diverse sward and conventional ryegrass-clover pasture: Implications for soil carbon sequestration. Plant Soil 2015, 392, 289–299. [Google Scholar] [CrossRef]
- Berg, B.; Mcclaugherty, C. Plant Litter. Decomposition, Humus Formation, Carbon Sequestration; Springer Science & Business Media: Berlin, Germany, 2014. [Google Scholar]
- Maeght, J.-L.; Gonkhamdee, S.; Clément, C.; Isarangkool Na Ayutthaya, S.; Stokes, A.; Pierret, A. Seasonal patterns of fine root production and turnover in a mature rubber tree (Hevea Brasiliensis Müll. Arg.) stand-differentiation with soil depth and implications for soil carbon stocks. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Boyce, C.K. The evolutionary history of roots and leaves. In Vascular Transport in Plants; Elsevier Academic Press: Cambridge, MA, USA, 2005; pp. 479–500. [Google Scholar]
- Lobet, G.; Hachez, C.; Chaumont, F.; Javaux, M.; Draye, X. Plant Roots. The Hidden Half. In Root Water Uptake and Water Flow in the Soil-Root Domain; CRC Press Book: Boca Raton, FL, USA, 2013. [Google Scholar]
- Rasse, D.P.; Rumpel, C.; Dignac, M.-F. Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 2005, 269, 341–356. [Google Scholar] [CrossRef]
- Cong, W.F.; Ruijven, J.; Mommer, L.; De Deyn, G.B.; Berendse, F.; Hoffland, E. Plant species richness promotes soil carbon and nitrogen stocks in grasslands without legumes. J. Ecol. 2014, 102, 1163–1170. [Google Scholar] [CrossRef]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- An, S.S.; Huang, Y.M.; Zheng, F.L.; Yang, J.G. Aggregate characteristics during natural revegetation on the Loess Plateau. Pedosphere 2008, 18, 809–816. [Google Scholar] [CrossRef]
- Yuan, Z.-Q.; Yu, K.-L.; Wang, B.-X.; Zhang, W.-Y.; Zhang, X.-L.; Siddique, K.H.; Stefanova, K.; Turner, N.C.; Li, F.-M. Cutting improves the productivity of lucerne-rich stands used in the revegetation of degraded arable land in a semi-arid environment. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Abiven, S.; Menasseri, S.; Angers, D.; Leterme, P. Dynamics of aggregate stability and biological binding agents during decomposition of organic materials. Eur. J. Soil Sci. 2007, 58, 239–247. [Google Scholar] [CrossRef]
- Chaplot, V.; Cooper, M. Soil aggregate stability to predict organic carbon outputs from soils. Geoderma 2015, 243, 205–213. [Google Scholar] [CrossRef]
- Erktan, A.; Cécillon, L.; Graf, F.; Roumet, C.; Legout, C.; Rey, F. Increase in soil aggregate stability along a Mediterranean successional gradient in severely eroded gully bed ecosystems: Combined effects of soil, root traits and plant community characteristics. Plant Soil 2016, 398, 121–137. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Mahamood, M.; Zhang, S.; Huang, S.; Liang, W. Effect of long-term combined application of organic and inorganic fertilizers on soil nematode communities within aggregates. Sci. Rep. 2016, 6, 31118. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Peng, X.; Peth, S.; Xiao, T. Effects of vegetation restoration on soil aggregate microstructure quantified with synchrotron-based micro-computed tomography. Soil Tillage Res. 2012, 124, 17–23. [Google Scholar] [CrossRef]
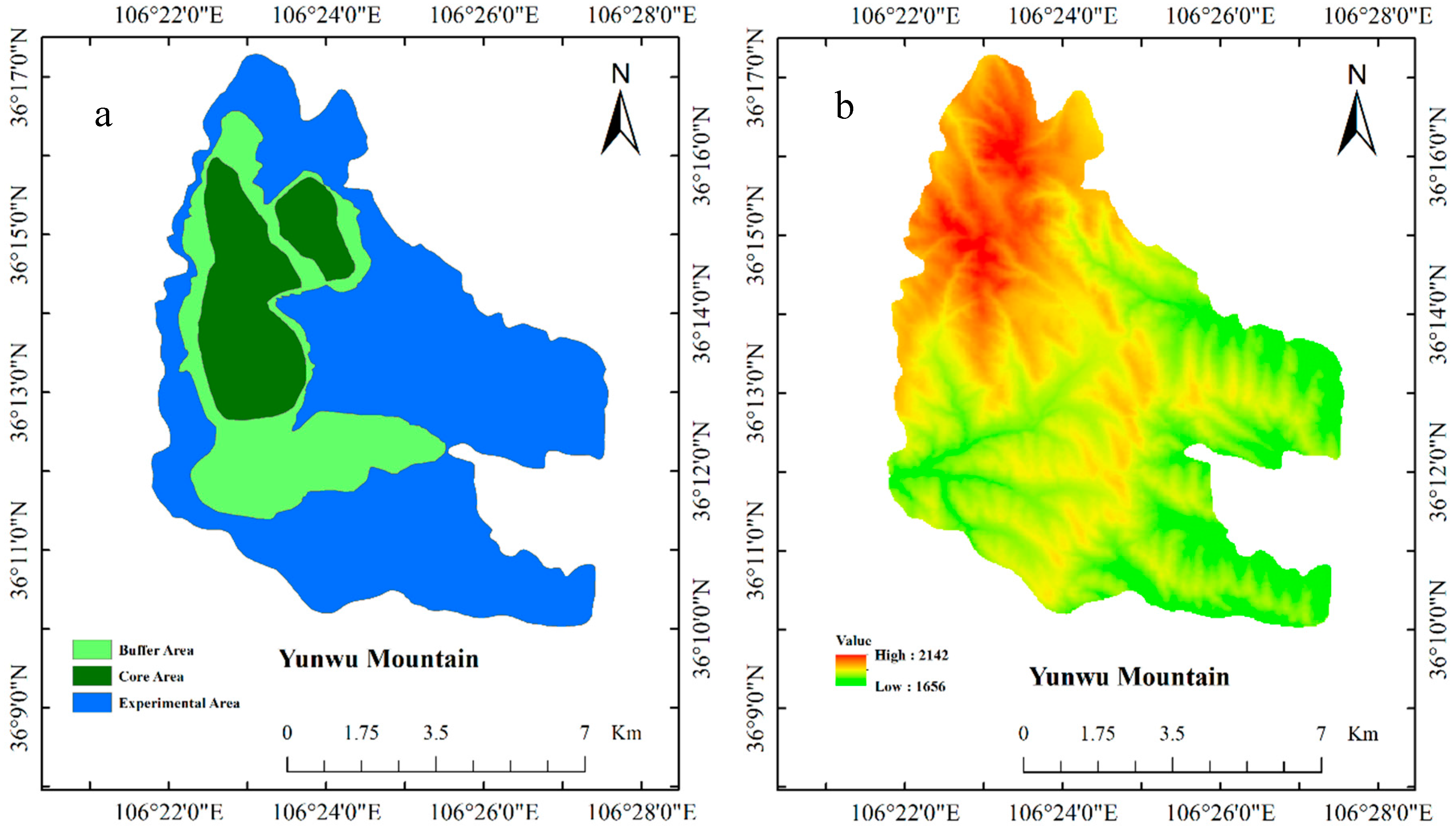
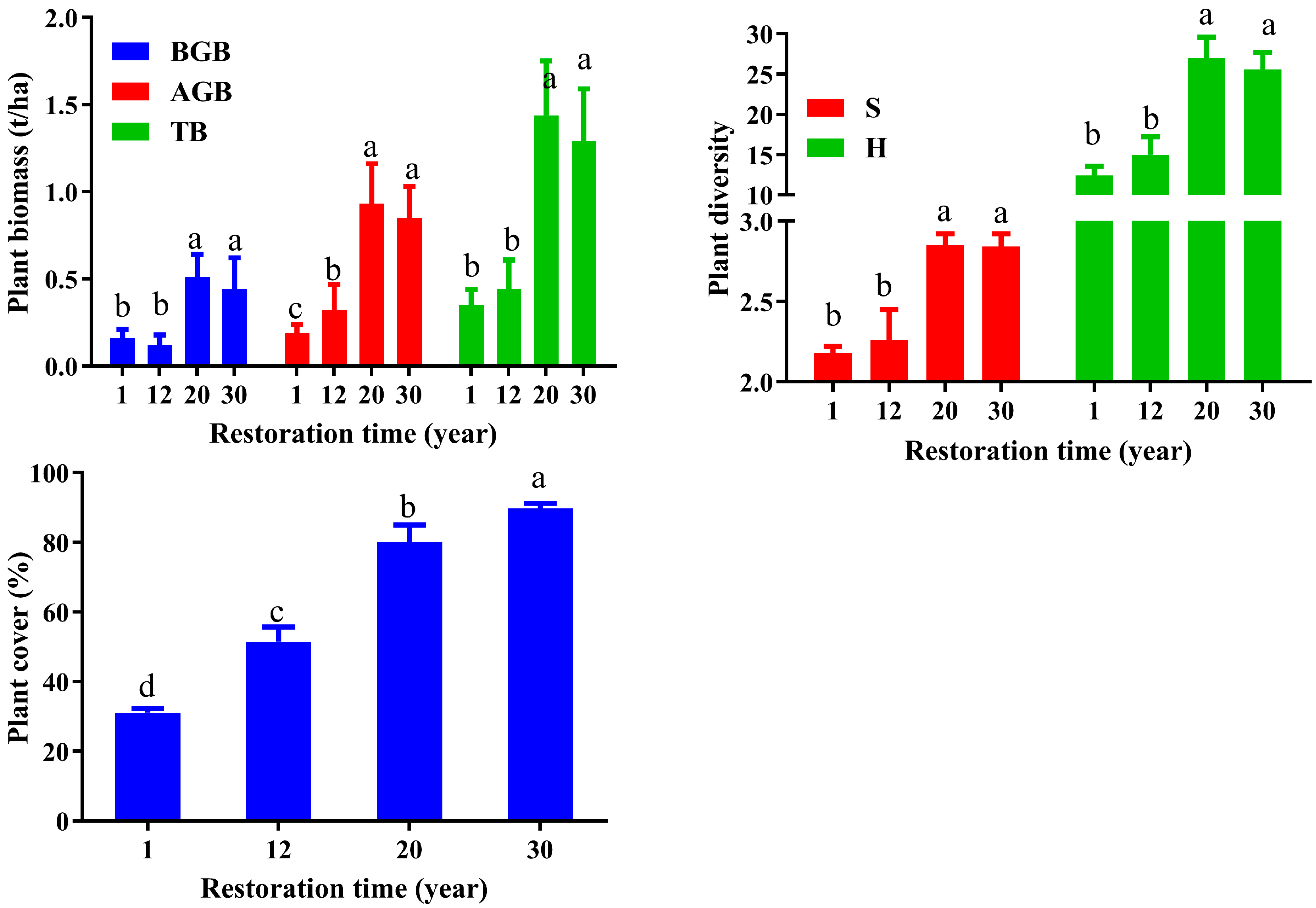
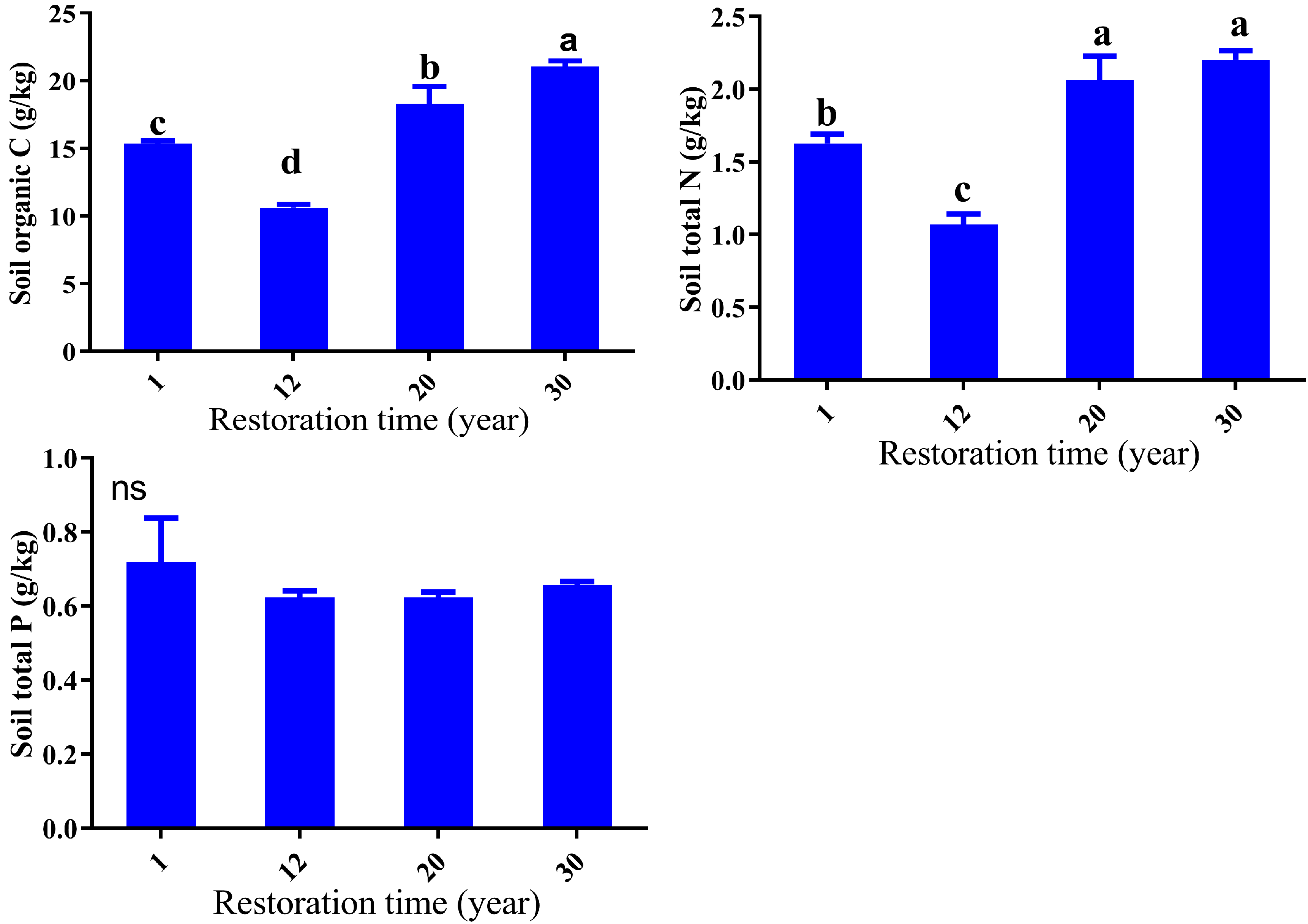
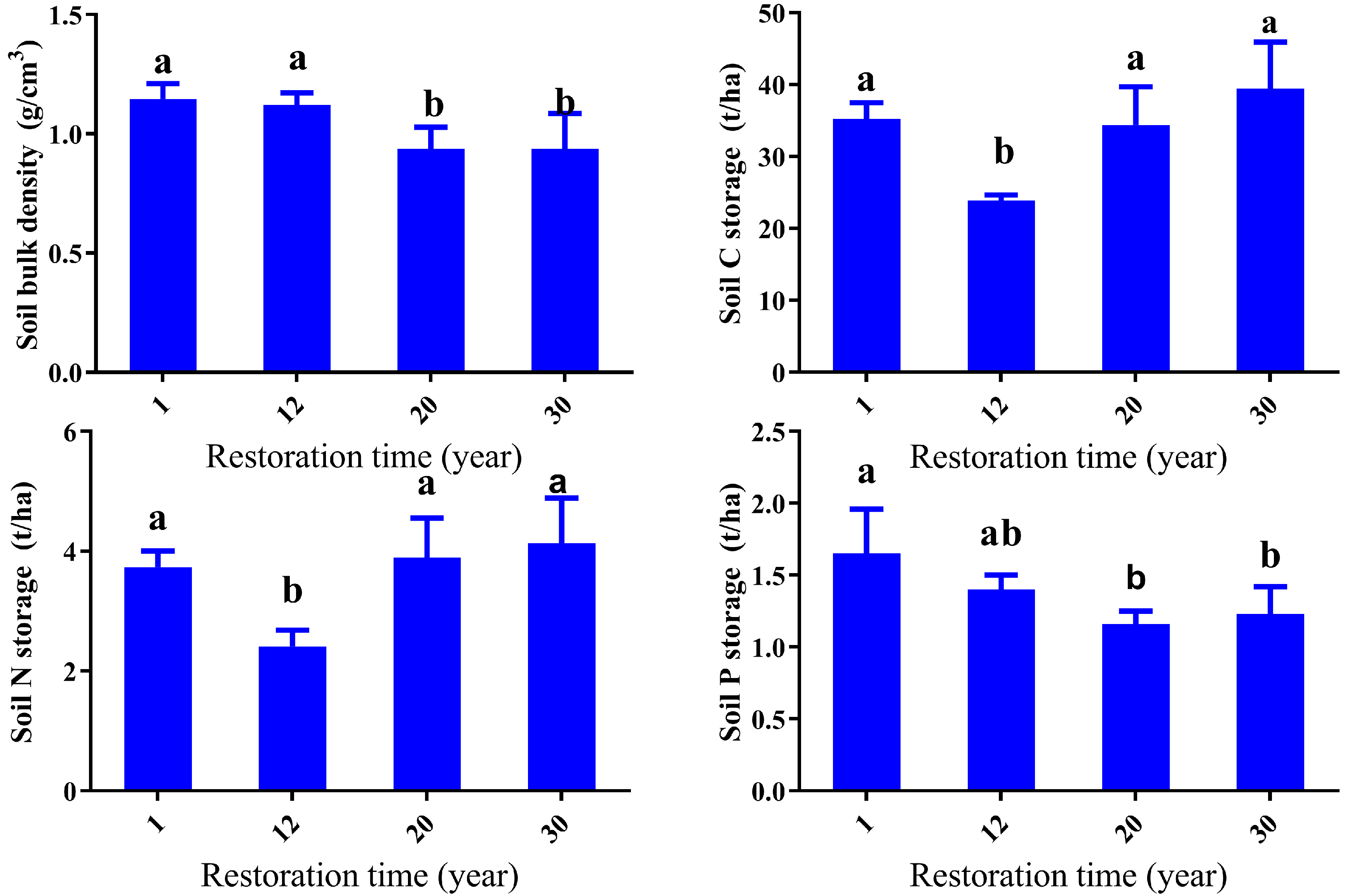
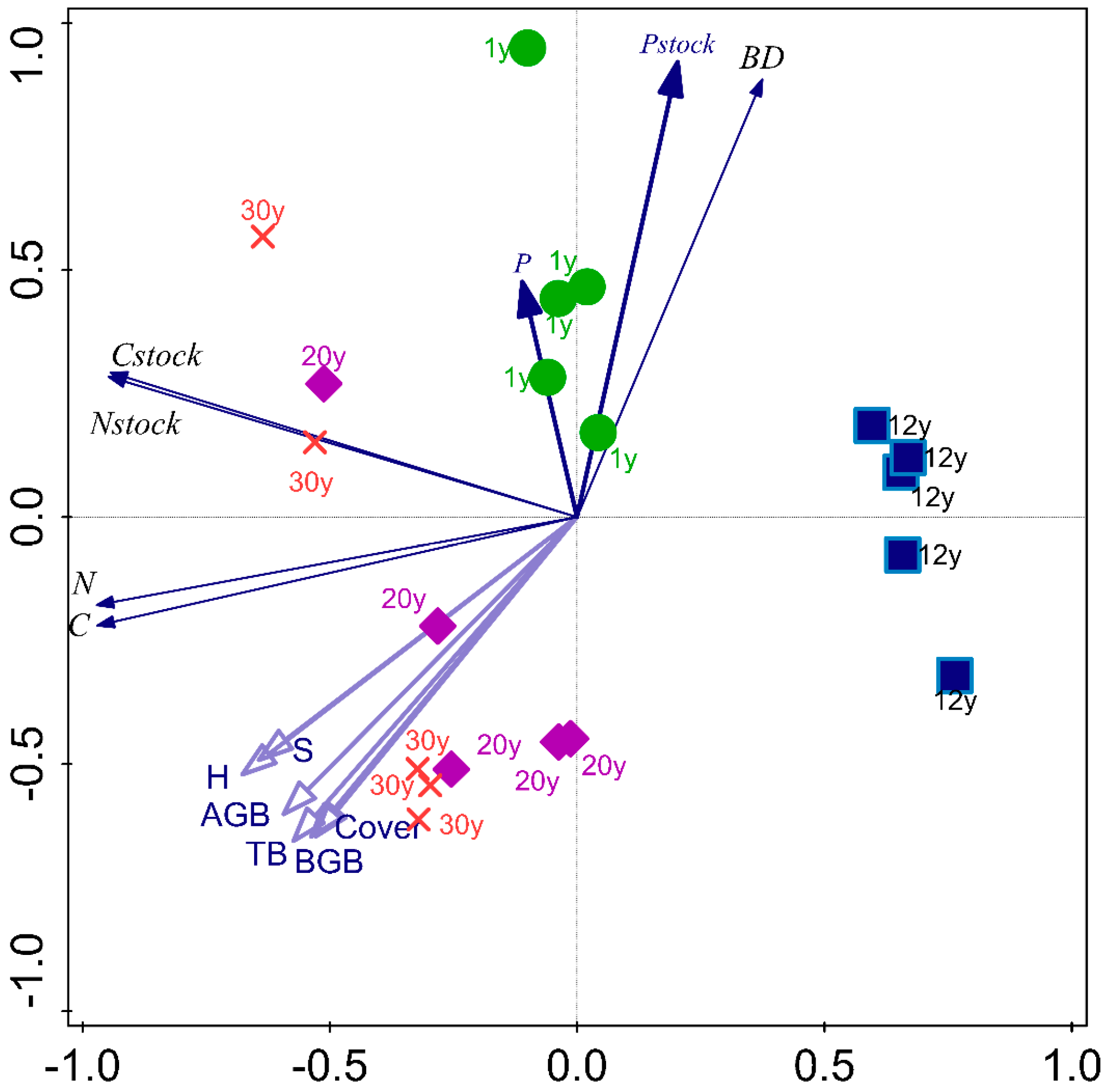
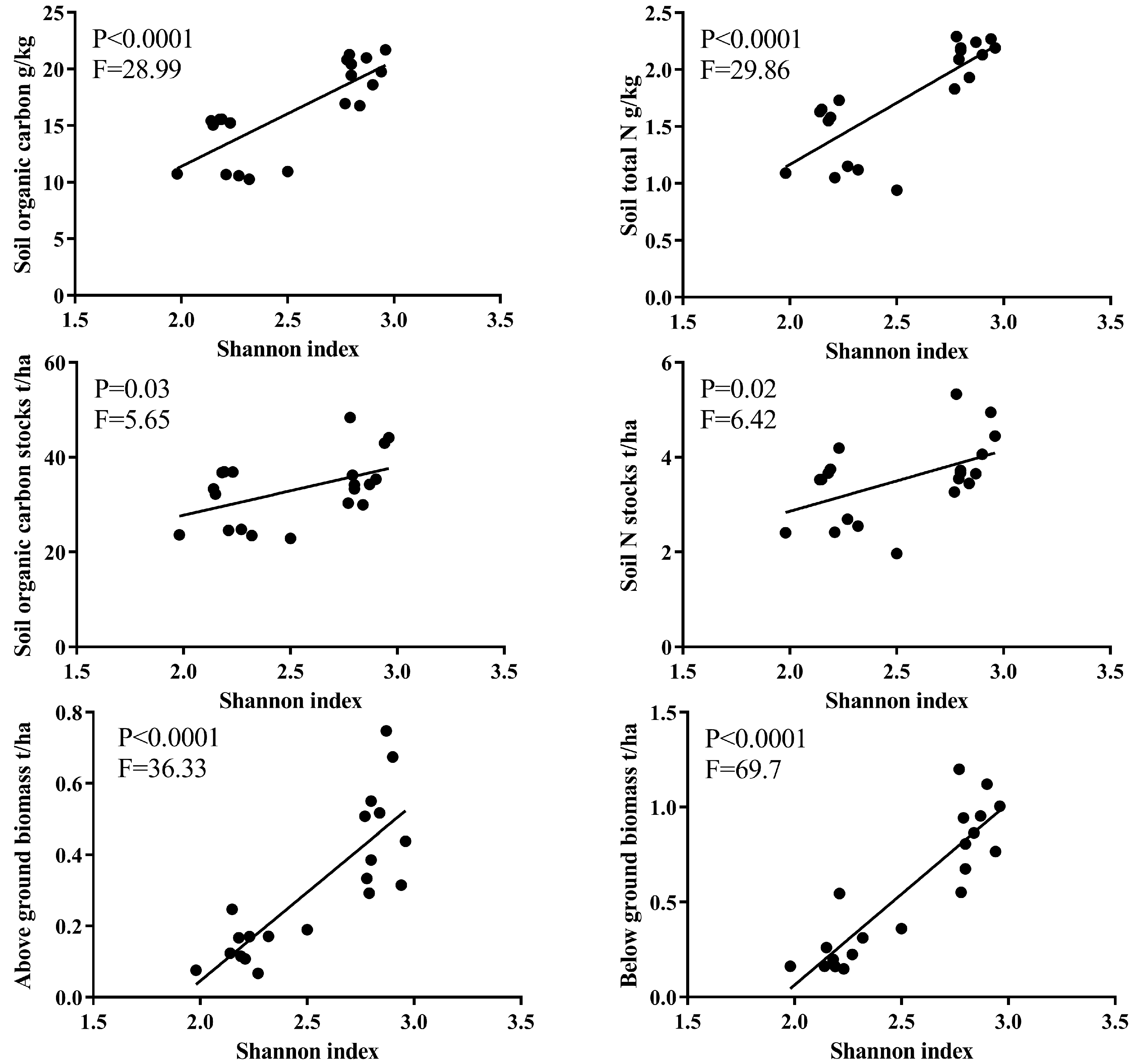
| Ages | Altitude/m | Longitude | Latitude | Dominant Species |
|---|---|---|---|---|
| 1a | 1762–1774 | 106°24′15.61′′ | 36°10′2.01′′ | Stipa bungeana; Artemisia scoparia; Potentilla acaulis; Heteropappus altaicus |
| 12a | 1778–1795 | 106°24′48.46′′ | 36°10′28.05′′ | Thymus mongolicus; Leymus secalinus; Artemisia scoparia; Stipa bungeana |
| 20a | 2077–2104 | 106°23′20.87′′ | 36°16′32.46′′ | Heteropappus altaicus; Cyperaceae; Thymus mongolicus; Artemisia vestita |
| 30a | 2083–2115 | 106°23′15.76′′ | 36°16′2.14′′ | Artemisia vestita; Stipa przewalskii; Heteropappus altaicus |
| Soil Properties | BGB | AGB | TB | Cover | S | H |
|---|---|---|---|---|---|---|
| BD | −0.786 ** | −0.776 ** | −0.811 ** | −0.752 ** | −0.656 ** | −0.696 ** |
| SOC | 0.697 ** | 0.669 ** | 0.708 ** | 0.697 ** | 0.741 ** | 0.785 ** |
| TN | 0.736 ** | 0.661 ** | 0.717 ** | 0.685 ** | 0.758 ** | 0.790 ** |
| TP | −0.16 | −0.275 | −0.241 | −0.341 | −0.267 | −0.252 |
| C-stocks | 0.318 | 0.289 | 0.314 | 0.331 | 0.467 * | 0.489 * |
| N-stocks | 0.382 | 0.307 | 0.349 | 0.346 | 0.506 * | 0.513 * |
| P-stocks | −0.632 ** | −0.696 ** | −0.698 ** | −0.726 ** | −0.609 ** | −0.631 ** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Q.; Liu, Y.; Xiao, L.; Huang, Y. How Fencing Affects the Soil Quality and Plant Biomass in the Grassland of the Loess Plateau. Int. J. Environ. Res. Public Health 2017, 14, 1117. https://doi.org/10.3390/ijerph14101117
Zeng Q, Liu Y, Xiao L, Huang Y. How Fencing Affects the Soil Quality and Plant Biomass in the Grassland of the Loess Plateau. International Journal of Environmental Research and Public Health. 2017; 14(10):1117. https://doi.org/10.3390/ijerph14101117
Chicago/Turabian StyleZeng, Quanchao, Yang Liu, Li Xiao, and Yimei Huang. 2017. "How Fencing Affects the Soil Quality and Plant Biomass in the Grassland of the Loess Plateau" International Journal of Environmental Research and Public Health 14, no. 10: 1117. https://doi.org/10.3390/ijerph14101117





