Occurrence, Ecological and Human Health Risks, and Seasonal Variations of Phenolic Compounds in Surface Water and Sediment of a Potential Polluted River Basin in China
Abstract
:1. Introduction
2. Materials and Methods
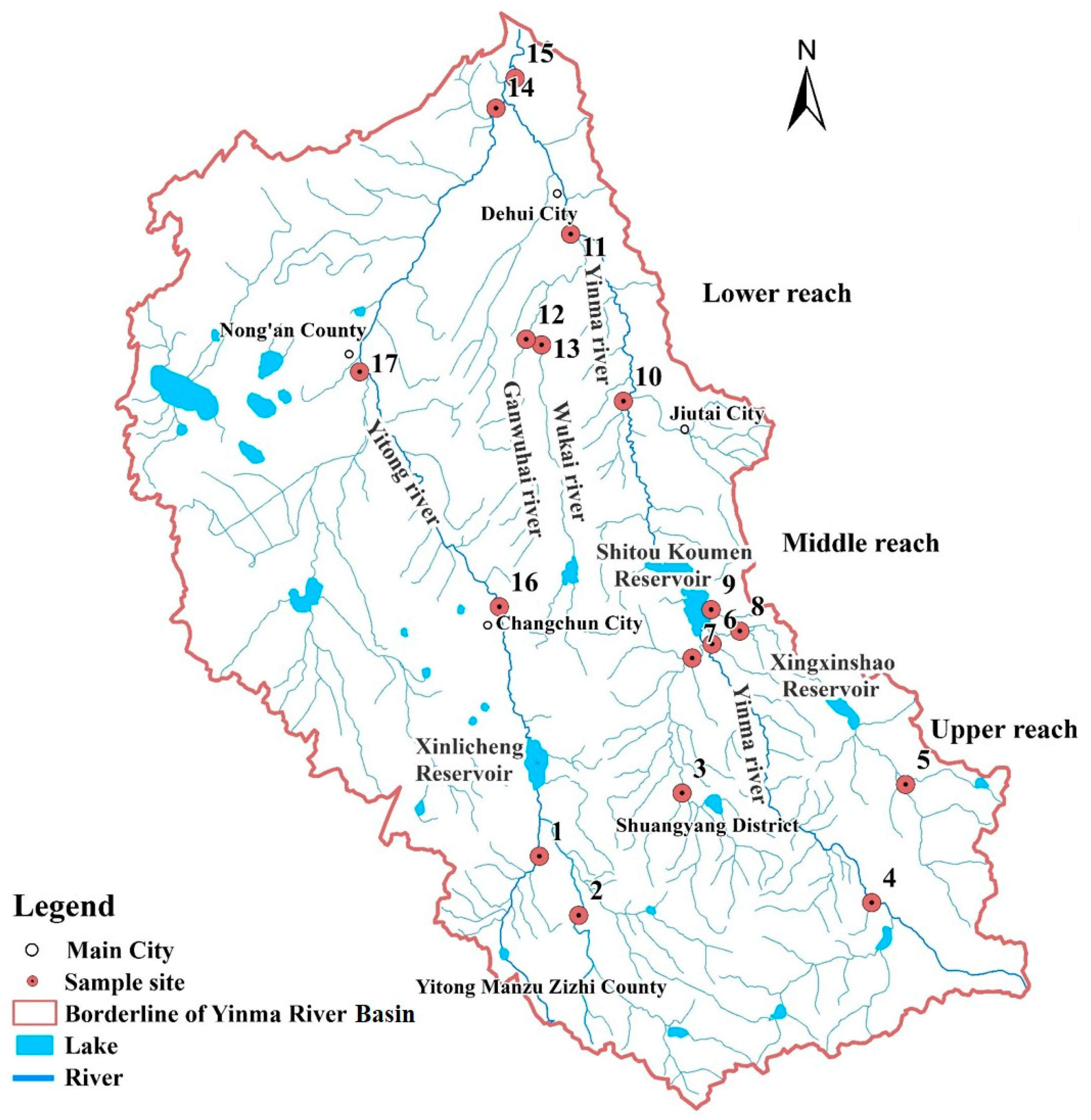
2.1. Study Area, Sampling, and Sample Pre-Treatment
2.2. Extraction Methods
2.2.1. Water
2.2.2. Sediment
2.2.3. Derivatization of the Phenolic Compounds
2.3. Quantification Methods
2.4. Quality Control
2.5. Risk Assessment
2.5.1. Ecological Risk Assessment
2.5.2. Human Health Risk Assessment
3. Results
3.1. Occurrence of the Five Phenolic Compounds in Water and Sediment
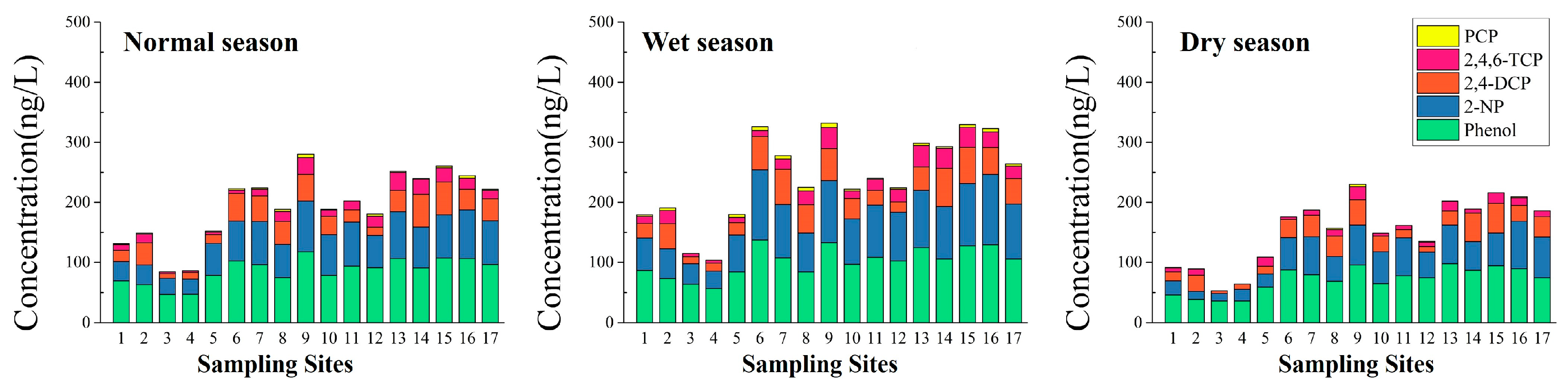
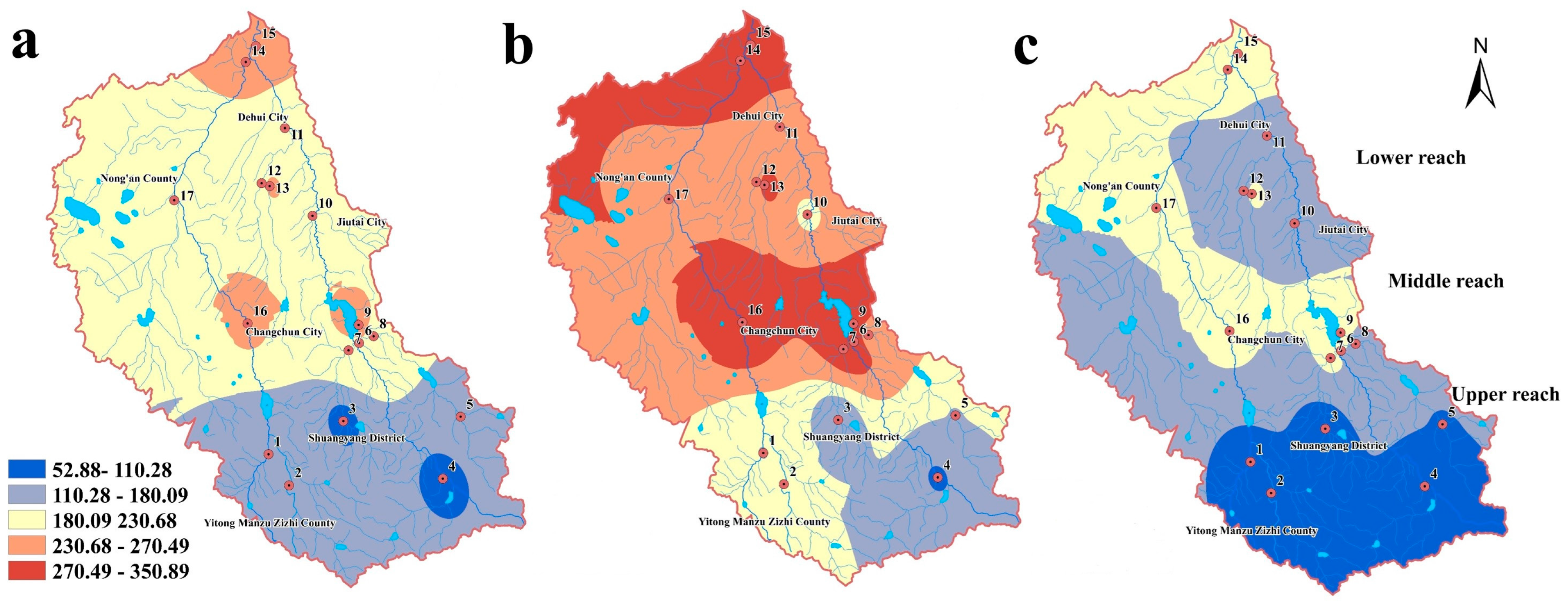
3.1.1. Spatial-Seasonal Variations of the Five Phenolic Compounds in Water
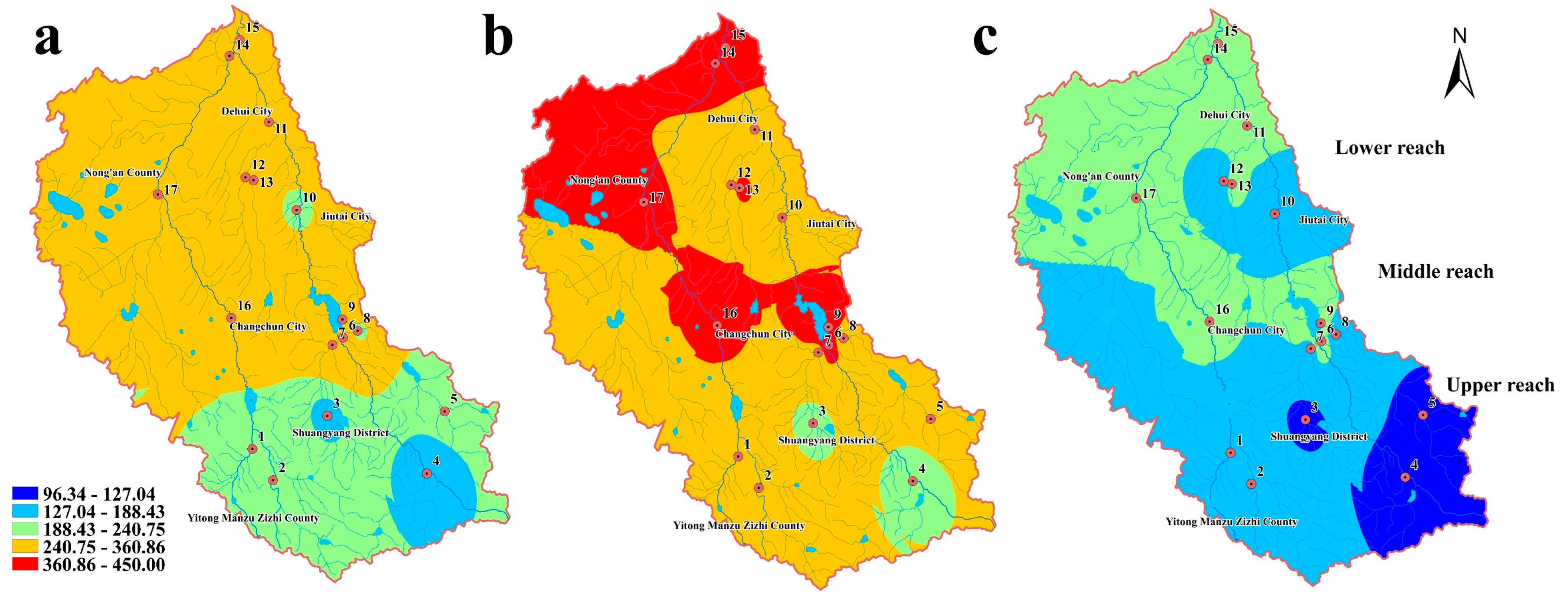
3.1.2. Spatial-Seasonal Variations of the Five Phenolic Compounds in Sediment
3.2. Risk Assessment
3.2.1. Ecological Risk Assessment
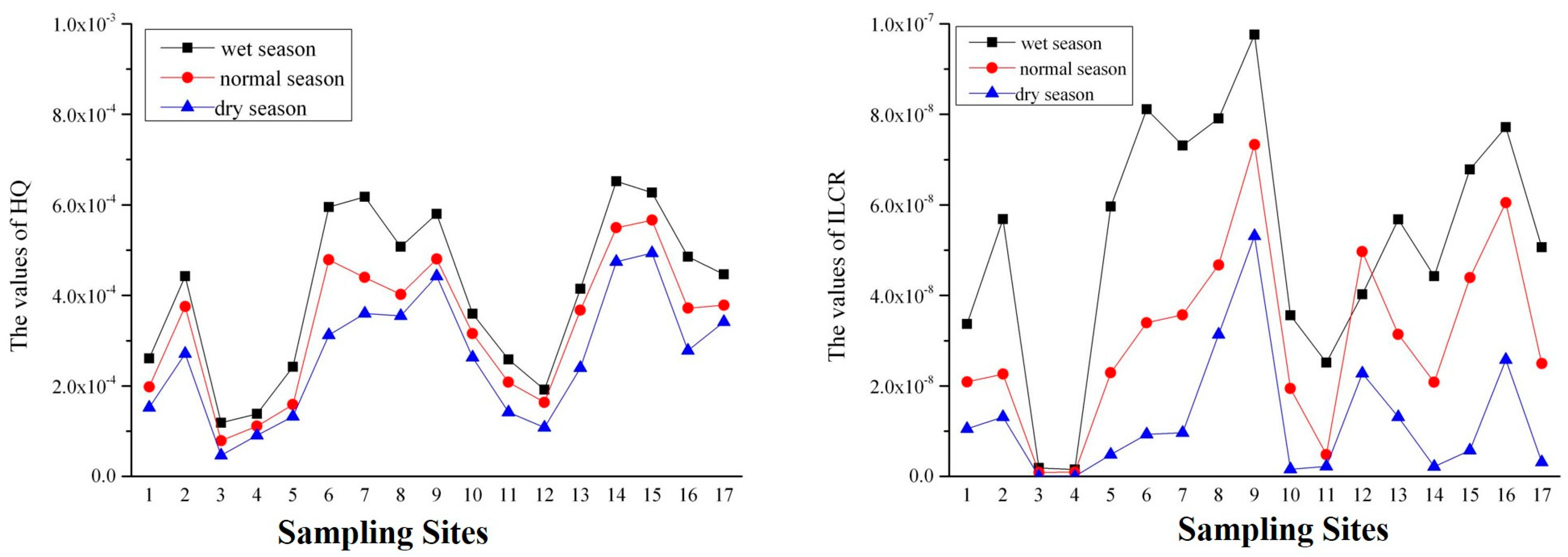
3.2.2. Human Health Risk Assessment
4. Discussion
4.1. Occurrence of the Five Phenolic Compounds in Water and Sediment
4.2. Risk Assessment
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, M.; Tang, R.; Fu, G.; Xu, B.; Zhu, P.; Qiao, S.; Chen, X.; Xu, B.; Qin, Y.; Lu, C.; et al. Association of exposure to phenols and idiopathic male infertility. J. Hazard. Mater. 2013, 250, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Santana, C.M.; Ferrera, Z.S.; Padron, M.E.T.; Rodrigues, J.J.S. Methodologies for the extraction of phenolic compounds from environmental samples: New approaches. Molecules 2009, 14, 298–320. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Wang, D.; Xu, X. Phenol removal efficiencies of sewage treatment processes and ecological risks associated with phenols ineffluents. J. Hazard. Mater. 2012, 217, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Zgoła-Grześkowiak, A.; Grześkowiak, T.; Rydlichowski, R.; Łukaszewski, Z. Determination of nonylphenol and short-chained nonylphenol ethoxylates in drain water from an agricultural area. Chemosphere 2009, 75, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Schummer, C.; Groff, C.; Al Chami, J.; Jaber, F.; Millet, M. Analysis of phenols and nitrophenols in rainwater collected simultaneously on an urban and rural site in east of France. Sci. Total Environ. 2009, 407, 5637–5643. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, D.; Oh, J.-R.; Je, J.-G. Seasonal and spatial distribution of nonylphenol in Shihwa Lake, Korea. Chemosphere 2004, 56, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Mussmann, P.; Levsen, K.; Radeek, W. Gas chromatographic determination of phenols in aqueous samples after solid phase extraction. Fresenius J. Anal. Chem. 1994, 348, 654–659. [Google Scholar] [CrossRef]
- Walker, K.; Vallero, D.A.; Lewis, R.G. Factors influencing the distribution of lindane and other hexachlorocyclohexanes in the environment. Environ. Sci. Technol. 1999, 33, 4373–4378. [Google Scholar] [CrossRef]
- Mielke, H.W.; Wang, G.; Gonzales, C.R.; Le, B.; Quach, V.N.; Mielke, P.W. PAH and metal mixtures in New Orleans soilsand sediments. Sci. Total Environ. 2001, 281, 217–227. [Google Scholar] [CrossRef]
- Michalowicz, J.; Duda, W. Phenols-sources and toxicity. Pol. J. Environ. Stud. 2007, 16, 347–362. [Google Scholar]
- Marianna, C. Sources and transformations of chlorophenols in the natural environment. Sci. Total Environ. 2004, 322, 21–39. [Google Scholar] [CrossRef]
- Gough, M. Human exposures from dioxin in soil—A meeting report. J. Toxicol. Environ. Health 1991, 32, 205–235. [Google Scholar] [CrossRef] [PubMed]
- Everaert, G.; De Laender, F.; Goethals, P.L.; Janssen, C.R. Multidecadal field data support intimate links between phytoplankton dynamics and PCB concentrations in marine sediments and biota. Sci. Total Environ. 2015, 49, 8704–8711. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhang, Y.; Zhao, X.; Zhou, C.; Guo, C.; Luo, Y.; Meng, W.; Zou, G.; Xu, J. Polybrominated diphenylethers (PBDEs) and polychlorinated biphenyls (PCBs) in sediments of Liaohe River: Levels, spatial and temporal distribution, possible sources, and inventory. Environ. Sci. Pollut. Res. 2015, 22, 4256–4264. [Google Scholar] [CrossRef] [PubMed]
- Khairy, M.A. Assessment of priority phenolic compounds in sediments from an extremely polluted coastal wetland (Lake Maryut, Egypt). Environ. Monit. Assess. 2013, 185, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.M.; Vega, A.B.; Frenich, A.G.; González, F.E.; Liebanas, F.A. Determination of fifteen priority phenolic compounds in environmental samples from Andalusia (Spain) by liquid chromatography-mass spectrometry. Anal. Bioanal. Chem. 2004, 379, 125–130. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Volume I: Human health evaluation manual (Part A). In EPA 540/1-86/060 Risk Assessment Guidance for Superfund; US Environmental Protection Agency: Washington, DC, USA, 1986. [Google Scholar]
- United States Environmental Protection Agency (USEPA). Environmental Science Center PBT Profile. 16 June 2013. Available online: http://www.pbtprofiler.net/Results.asp (accessed on 22 September 2017).
- Li, B.; Liu, R.; Gao, H.; Tan, R.; Zeng, P.; Song, Y. Spatial distribution and ecological risk assessment of phthalic acidesters and phenols in surface sediment from urban rivers in Northeast China. Environ. Pollut. 2016, 219, 409–415. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency (USEPA). Volume III—Part A, Process for conducting probabilistic risk assessment. In EPA 540-R-02-002 Risk Assessment Guidance for Superfund; US Environmental Protection Agency: Washington, DC, USA, 2001. [Google Scholar]
- United States Environmental Protection Agency (USEPA). Integrated Risk Information System (IRIS). 16 June 2013. Available online: http://www.epa.gov/IRIS/ (accessed on 22 September 2017).
- United States Naval Facilities Engineering Command. Available online: http://id.loc.gov/authorities/names/n79125717 (accessed on 26 September 2017).
- Butterworth, B.E.; Bogdanffy, M.S. A comprehensive approach for integration of toxicityand cancer risk assessments. Regul. Toxicol. Pharmacol. 1999, 29, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Giesy, J.P.; Jin, X.; Wang, J. Tiered probabilistic assessment of organohalogen compounds in the Han River and Danjiangkou Reservoir, central China. Sci. Total Environ. 2017, 586, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Barrico, M.L.; Nabais, C.; Martins, M.J.; Freitas, H. Sources of phenolic compounds in two catchments of southern Portugal—Effect of season, land use and soil type. Chemosphere 2006, 65, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Etchegoyen, M.A.; Ronco, A.E.; Almada, P.; Abelando, M.; Marino, D.J. Occurrence and fate of pesticides in the Argentine stretch of the Paraguay-Paraná basin. Environ. Monit. Assess. 2017, 189, 63. [Google Scholar] [CrossRef] [PubMed]


| Water (ng/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal Season | Wet Season | Dry Season | |||||||
| Range | Mean | CV% | Range | Mean | CV% | Range | Mean | CV% | |
| Phenol | 46.75–117.85 | 86.33 | 24.3 | 56.73–137.35 | 101.68 | 23.9 | 35.72–97.85 | 71.04 | 29.9 |
| 2-NP | 25.44–84.32 | 59.75 | 32.5 | 28.77–116.98 | 78.86 | 33.7 | 12.74–79.24 | 46.32 | 45.6 |
| 2,4-DCP | 7.59–54.83 | 31.71 | 47.3 | 11.45–63.78 | 38.3 | 44.7 | 4.37–49.38 | 25.84 | 53.0 |
| 2,4,6-TCP | 2.73–29.98 | 14.68 | 58.2 | 4.62–35.66 | 19.89 | 51.6 | ND-21.88 | 9.31 | 63.9 |
| PCP | ND-5.47 | 2.16 | 72.9 | ND-7.34 | 3.86 | 55.7 | ND-3.92 | 0.79 | 139.7 |
| Total | 84.07–280.04 | 194.64 | 29.9 | 103.67–331.95 | 242.6 | 29.9 | 52.83–230.03 | 153.29 | 35.6 |
| Phenol | 63.90–159.47 | 108.31 | 22.42 | 74.50–184.63 | 127.76 | 23.1 | 32.60–132.46 | 85.32 | 31.5 |
| 2-NP | 56.17–106.63 | 83.24 | 16.8 | 67.89–150.40 | 108.41 | 23.3 | 34.53–75.41 | 56.57 | 24.3 |
| 2,4-DCP | 15.75–58.06 | 34.22 | 40.7 | 20.52–85.52 | 56.46 | 38.0 | 10.63–29.18 | 18.48 | 27.3 |
| 2,4,6-TCP | 6.63–32.77 | 18.95 | 42.8 | 9.62–52.63 | 28.74 | 46.7 | 3.32–23.88 | 11.53 | 48.3 |
| PCP | ND-6.24 | 3.23 | 60.1 | 0.40–9.43 | 5.47 | 50.8 | ND-3.65 | 1.66 | 74.0 |
| Total | 149.49–348.60 | 247.95 | 20.4 | 188.14–448.09 | 326.83 | 22.7 | 96.30–250.00 | 173.57 | 26.54 |
| Water | Sediment | |||||
|---|---|---|---|---|---|---|
| RQ | Normal Season | Wet Season | Dry Season | Normal Season | Wet Season | Dry Season |
| Phenol | 0.033 | 0.038 | 0.027 | 1.985 | 2.298 | 1.649 |
| 2-NP | 0.030 | 0.042 | 0.028 | 1.145 | 1.614 | 0.809 |
| 2,4-DCP | 0.069 | 0.081 | 0.063 | 1.392 | 2.051 | 0.700 |
| 2,4,6-TCP | 0.088 | 0.105 | 0.064 | 0.532 | 0.854 | 0.387 |
| PCP | 0.094 | 0.126 | 0.068 | 0.215 | 0.325 | 0.126 |
| total | 0.315 | 0.392 | 0.250 | 5.269 | 7.142 | 3.671 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Zhang, J.; Sun, C. Occurrence, Ecological and Human Health Risks, and Seasonal Variations of Phenolic Compounds in Surface Water and Sediment of a Potential Polluted River Basin in China. Int. J. Environ. Res. Public Health 2017, 14, 1140. https://doi.org/10.3390/ijerph14101140
Zhou M, Zhang J, Sun C. Occurrence, Ecological and Human Health Risks, and Seasonal Variations of Phenolic Compounds in Surface Water and Sediment of a Potential Polluted River Basin in China. International Journal of Environmental Research and Public Health. 2017; 14(10):1140. https://doi.org/10.3390/ijerph14101140
Chicago/Turabian StyleZhou, Mo, Jiquan Zhang, and Caiyun Sun. 2017. "Occurrence, Ecological and Human Health Risks, and Seasonal Variations of Phenolic Compounds in Surface Water and Sediment of a Potential Polluted River Basin in China" International Journal of Environmental Research and Public Health 14, no. 10: 1140. https://doi.org/10.3390/ijerph14101140





