Factors Affecting Transfer of Pyrethroid Residues from Herbal Teas to Infusion and Influence of Physicochemical Properties of Pesticides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Analysis of the Four Herbal Teas and Their Infusions
2.4. Infusion Process
2.5. GC-ECD Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Extraction and SPE Conditions
3.2. Method Validation
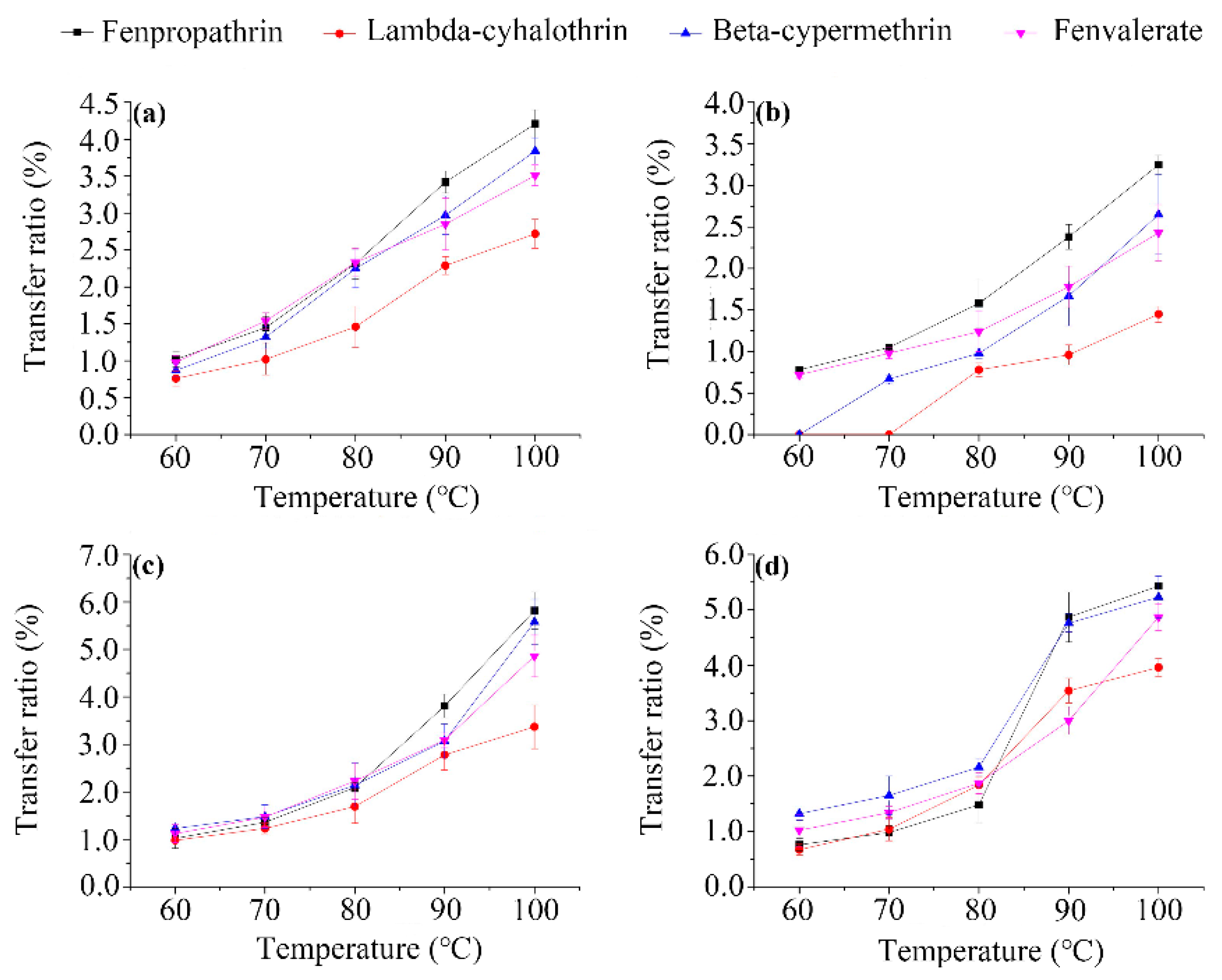
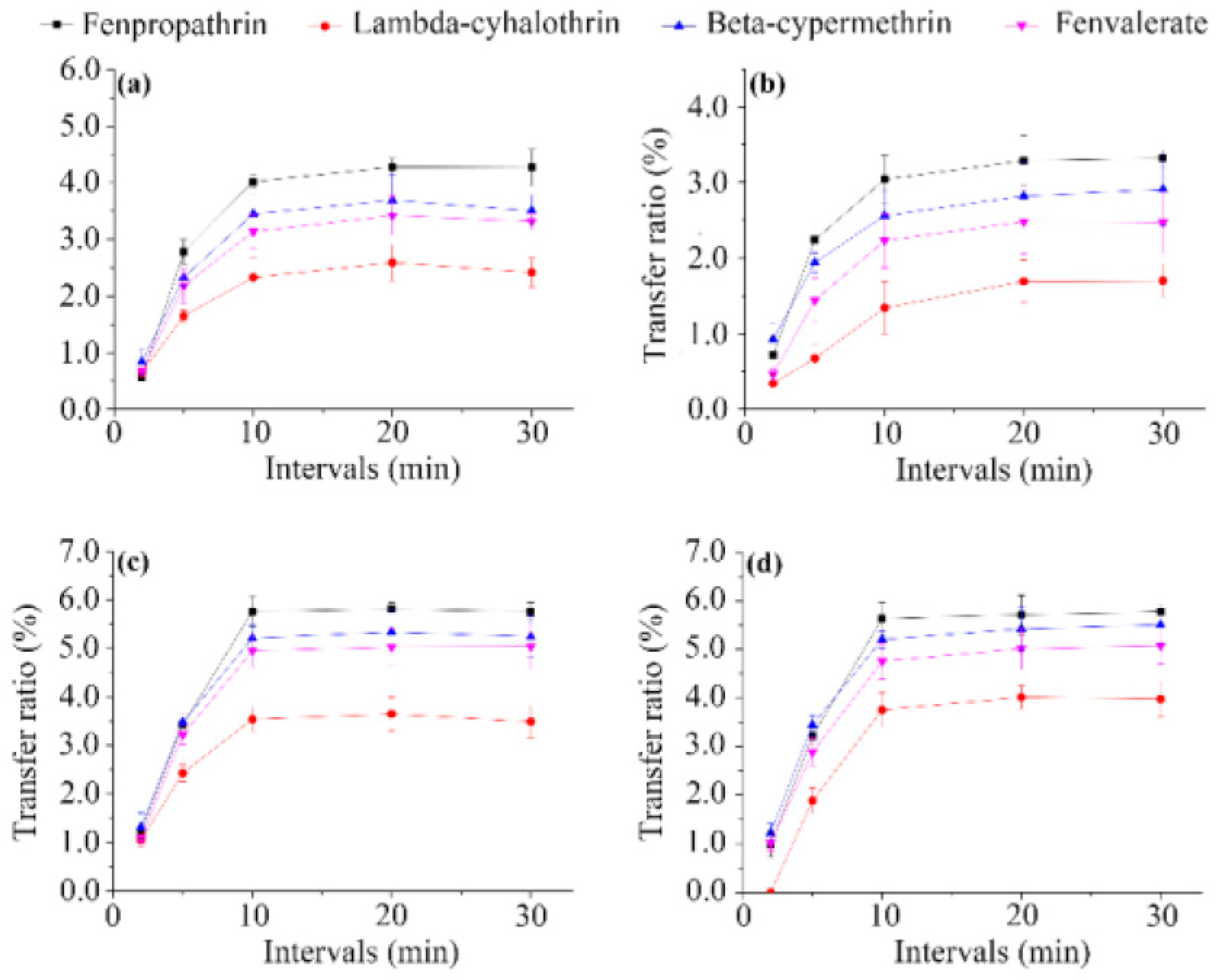
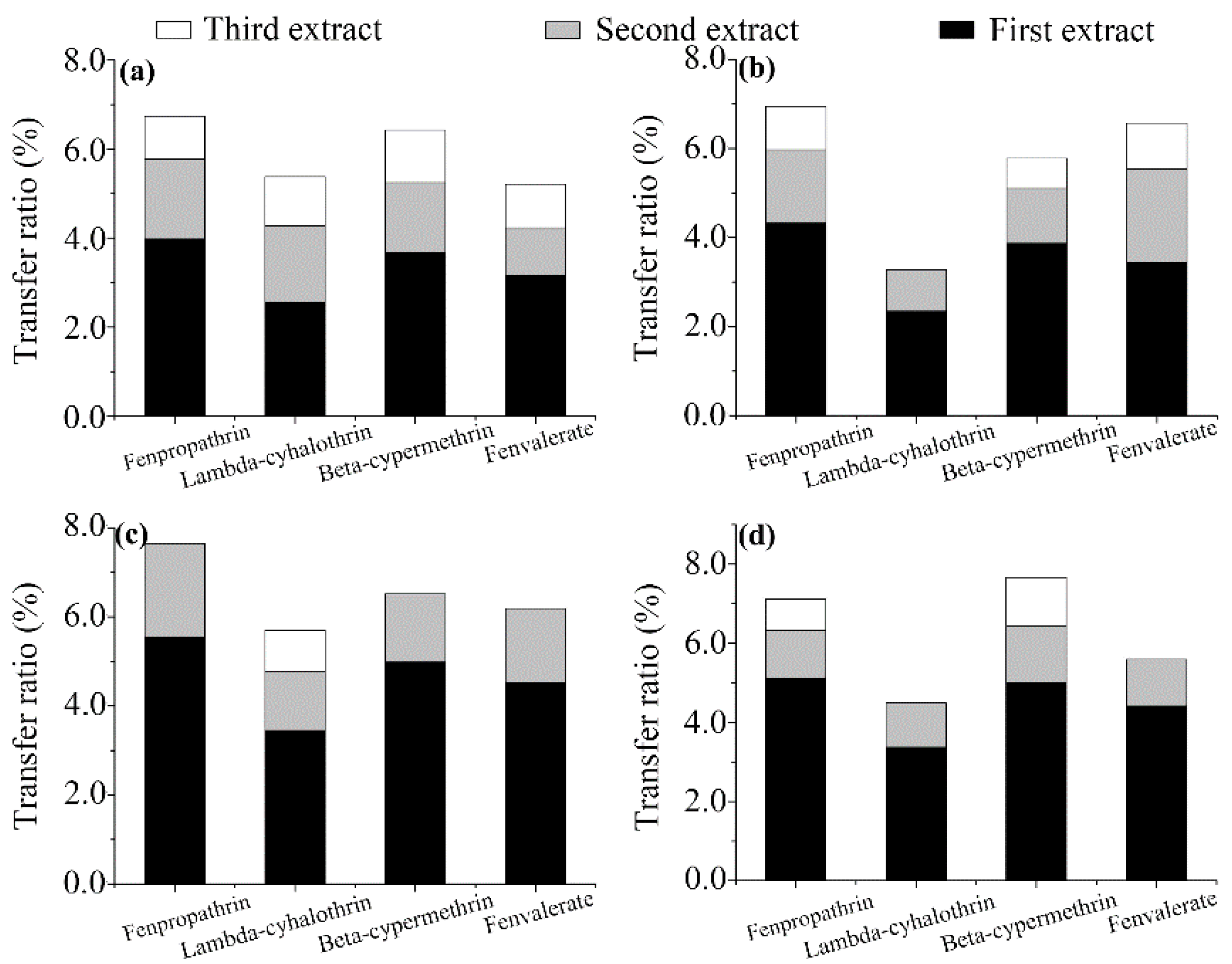
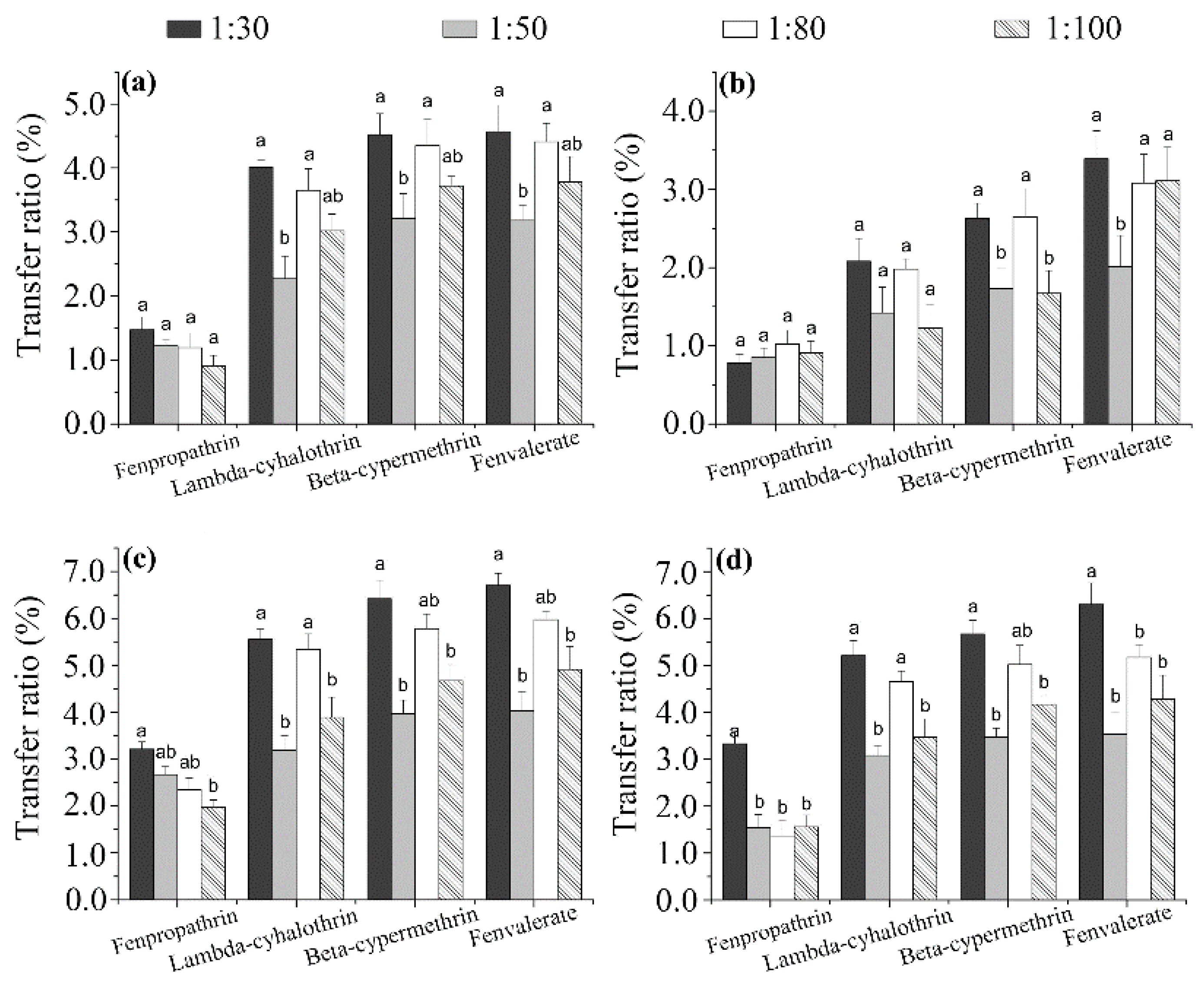
3.3. Brewing Efficiency of Pyrethroids from Tea into Infusion
3.4. Relationship between Transfer Rates and Physicochemical Properties of Pesticides
3.5. Health Risk Assessment of the Pyrethroid Residues in Infusions
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xue, J.Y.; Li, H.C.; Liu, F.M.; Xue, J.; Chen, X.C.; Zhan, J. Transfer of difenoconazole and azoxystrobin residues from chrysanthemum flower tea to its infusion. Food Addit. Contam. A 2014, 31, 666–675. [Google Scholar] [CrossRef]
- Jin, M.L.; Huang, Q.S.; Zhao, K.; Shang, P. Biological activities and potential health benefit effects of polysaccharides isolated from Lycium barbarum L. Int. J. Biol. Macromol. 2013, 54, 16–23. [Google Scholar] [CrossRef]
- Joubert, E.; de Beer, D.; Malherbe, C.J. Herbal teas—Exploring untapped potential and strengthening commercialisation. S. Afr. J. Bot. 2017, 110, 1–3. [Google Scholar] [CrossRef]
- Jennings, A.A.; Li, Z. Scope of the worldwide effort to regulate pesticide contamination in surface soils. J. Environ. Manag. 2014, 146, 420–443. [Google Scholar] [CrossRef]
- Li, Z.J.; Jennings, A. Worldwide regulations of standard values of pesticides for human health risk control: A review. Int. J. Environ. Res. Public Health 2017, 14, 826. [Google Scholar] [CrossRef]
- Greenpeace. Chinese Herbs: Elixir of Health or Pesticides Cocktail? Available online: http://www.greenpeace.org/international/Global/eastasia/publications/reports/food-agriculture/2013/chinese-herbs-pesticides-report.pdf (accessed on 4 March 2014).
- Kang, C.; Guo, L.; Zhou, T.; Zhao, D.; Kang, L.; He, Y.; Wang, S.; Zhou, L. Discussion on present situation of study on pesticide residues in chinese herbal medicines. China J. Chin. Mater. Med. 2016, 41, 155. [Google Scholar]
- Han, Y.T.; Liu, S.W.; Yang, J.; Zhong, Z.Z.; Zou, N.; Song, L.; Zhang, X.S.; Li, X.S.; Pan, C.P. Residue behavior and processing factors of eight pesticides during the production of sorghum distilled spirits. Food Control 2016, 69, 250–255. [Google Scholar] [CrossRef]
- Zhao, L.W.; Liu, F.M.; Wu, L.M.; Xue, X.F.; Hou, F. Fate of triadimefon and its metabolite triadimenol in jujube samples during jujube wine and vinegar processing. Food Control 2017, 73, 468–473. [Google Scholar] [CrossRef]
- Chen, H.; Pan, M.; Pan, R.; Zhang, M.; Liu, X.; Lu, C. Transfer rates of 19 typical pesticides and the relationship with their physicochemical property. J. Agric. Food Chem. 2015, 63, 723–730. [Google Scholar] [CrossRef]
- Zhou, L.; Luo, F.J.; Zhang, X.Z.; Jiang, Y.P.; Lou, Z.Y.; Chen, Z.M. Dissipation, transfer and safety evaluation of emamectin benzoate in tea. Food Chem. 2016, 202, 199–204. [Google Scholar] [CrossRef]
- Liao, M.; Shi, Y.H.; Cao, H.Q.; Hua, R.M.; Tang, F.; Wu, X.W.; Tang, J. Dissipation behavior of octachlorodipropyl ether residues during tea planting and brewing process. Environ. Monit. Assess. 2016, 188, 551. [Google Scholar] [CrossRef]
- Gupta, M.; Shanker, A. Fate of imidacloprid and acetamiprid residues during black tea manufacture and transfer into tea infusion. Food Addit. Contam. 2009, 26, 157–163. [Google Scholar] [CrossRef]
- Fang, Q.K.; Shi, Y.H.; Cao, H.Q.; Tong, Z.; Xiao, J.J.; Liao, M.; Wu, X.W.; Hua, R.M. Degradation dynamics and dietary risk assessments of two neonicotinoid insecticides during lonicera japonica planting, drying, and tea brewing processes. J. Agric. Food Chem. 2017, 65, 1483–1488. [Google Scholar] [CrossRef]
- Sood, C.; Jaggi, S.; Kumar, V.; Ravindranath, S.D.; Shanker, A. How manufacturing processes affect the level of pesticide residues in tea. J. Sci. Food Agric. 2004, 84, 2123–2127. [Google Scholar] [CrossRef]
- Qian, G. Research status of the pesticide residue in chinese herbal medicine. J. Anhui Agric. Sci. 2008, 23, 141. [Google Scholar]
- Ozbey, A.; Uygun, U. Behaviour of some organophosphorus pesticide residues in peppermint tea during the infusion process. Food Chem. 2007, 104, 237–241. [Google Scholar] [CrossRef]
- Ghosh, M.N.; Sharma, D. Power of tukey’s test for non-additivity. J. Roy. Statist. Soc. Ser. B 1963, 25, 213–219. [Google Scholar]
- Administration of the People’s Republic of China. GB/T2763-2014. National Food Safety Standard-Maximum Residue Limits for Pesticides in Food; Standardization Administration of the People’s Republic of China, Standards Press of China: Beijing, China, 2014. (In Chinese)
- Paramasivam, M.; Chandrasekaran, S. Persistence behaviour of deltamethrin on tea and its transfer from processed tea to infusion. Chemosphere 2014, 111, 291–295. [Google Scholar] [CrossRef]
- Martinez-Dominguez, G.; Plaza-Bolanos, P.; Romero-Gonzalez, R.; Garrido-Frenich, A. Analytical approaches for the determination of pesticide residues in nutraceutical products and related matrices by chromatographic techniques coupled to mass spectrometry. Talanta 2014, 118, 277–291. [Google Scholar] [CrossRef]
- Lee, K.G.; Lee, S.K. Monitoring and risk assessment of pesticide residues in yuza fruits (Citrus junos Sieb. Ex tanaka) and yuza tea samples produced in Korea. Food Chem. 2012, 135, 2930–2933. [Google Scholar] [CrossRef]
- Tong, H.; Tong, Y.; Xue, J.; Liu, D.; Wu, X. Multi-residual pesticide monitoring in commercial Chinese herbal medicines by gas chromatography–triple quadrupole tandem mass spectrometry. Food Anal. Methods 2014, 7, 135–145. [Google Scholar] [CrossRef]
- Jaggi, S.; Sood, C.; Kumar, V.; Ravindranath, S.D.; Shanker, A. Leaching of pesticides in tea brew. J. Agric. Food Chem. 2001, 49, 5479–5483. [Google Scholar] [CrossRef]
- Holland, P.; Hamilton, D.; Ohlin, B.; Skidmore, M. Pesticides report 31: Effects of storage and processing on pesticide residues in plant products (technical report). Pure Appl. Chem. 1994, 66, 335–356. [Google Scholar] [CrossRef]
- Cho, S.K.; Abd El-Aty, A.M.; Rahman, M.M.; Choi, J.H.; Shim, J.H. Simultaneous multi-determination and transfer of eight pesticide residues from green tea leaves to infusion using gas chromatography. Food Chem. 2014, 165, 532–539. [Google Scholar] [CrossRef]
- Wan, H.; Xia, H.; Chen, Z. Extraction of pesticide residues in tea by water during the infusion process. Food Addit. Contam. 1991, 8, 497–500. [Google Scholar] [CrossRef]
- Peixoto, R.R.A.; Devesa, V.; Velez, D.; Cervera, M.L.; Cadore, S. Study of the factors influencing the bioaccessibility of 10 elements from chocolate drink powder. J. Food Compos. Anal. 2016, 48, 41–47. [Google Scholar] [CrossRef]
- Samaniego-Sanchez, C.; Inurreta-Salinas, Y.; Quesada-Granados, J.J.; Blanca-Herrera, R.; Villalon-Mir, M.; de la Serrana, H.L.G.; Martinez, M.C.L. The influence of domestic culinary processes on the trolox equivalent antioxidant capacity of green tea infusions. J. Food Compos. Anal. 2011, 24, 79–86. [Google Scholar] [CrossRef]
- Lin, D.H.; Zhu, L.Z.; Luo, L. Factors affecting transfer of polycyclic aromatic hydrocarbons from made tea to tea infusion. J. Agric. Food Chem. 2006, 54, 4350–4354. [Google Scholar] [CrossRef]
- Tomlin, C.D.S.E. The Pesticide Manual, 11th ed.; BCPC: Hampshire, UK, 1997. [Google Scholar]
- Chen, L.; Chen, J.F.; Guo, Y.; Li, J.R.; Yang, Y.Q.; Xu, L.J.; Fu, F.F. Study on the simultaneous determination of seven benzoylurea pesticides in oolong tea and their leaching characteristics during infusing process by HPLC-ms/ms. Food Chem. 2014, 143, 405–410. [Google Scholar] [CrossRef]
- Chen, H.P.; Pan, M.L.; Liu, X.; Lu, C.Y. Evaluation of transfer rates of multiple pesticides from green tea into infusion using water as pressurized liquid extraction solvent and ultra-performance liquid chromatography tandem mass spectrometry. Food Chem. 2017, 216, 1–9. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, M.; Shanker, A. Fenvalerate residue level and dissipation in tea and in its infusion. Food Addit. Contam. 2008, 25, 97–104. [Google Scholar] [CrossRef]
- Li, Z.; Jennings, A.A. Implied maximum dose analysis of standard values of 25 pesticides based on major human exposure pathways. AIMS Public Health 2017, 4, 383–398. [Google Scholar] [CrossRef]

| Pesticide | Matrix | Fortified Level (mg·mL−1) | Average Recovery ± SD a (RSD b) | |||
|---|---|---|---|---|---|---|
| Wolfberry | Licorice | Honeysuckle | Chrysanthemum | |||
| Fenpropathrin | Herb tea | 0.5 | 96.5 ± 7.4 (5.3) | 85.1 ± 6.2 (4.3) | 90.3 ± 3.9 (5.4) | 84.5 ± 3.7 (5.6) |
| 0.05 | 94.2 ± 3.9 (4.4) | 94.5 ± 4.8 (8.1) | 96.3 ± 4.1 (5.0) | 93.3 ± 4.6 (7.7) | ||
| 0.01 | 101.6 ± 6.3 (6.7) | 93.5 ± 6.0 (10.2) | 98.4 ± 4.4 (7.3) | 89.4 ± 4.0 (9.5) | ||
| Tea infusion | 0.2 | 101.9 ± 4.2 (5.0) | 90.3 ± 3.9 (4.3) | 85.9 ± 5.8 (4.3) | 91.2 ± 9.0 (7.0) | |
| 0.02 | 90.9 ± 7.1 (8.3) | 92.4 ± 8.0 (6.3) | 89.8 ± 7.7 (4.4) | 80.9 ± 7.1 (8.5) | ||
| 0.002 | 112.4 ± 5.3 (3.0) | 102.4 ± 4.1 (9.3) | 94.5 ± 6.0 (7.2) | 102.6 ± 5.0 (3.2) | ||
| Lambda-cyhalothrin | Herb tea | 0.5 | 96.2 ± 4.2 (9.2) | 110.5 ± 4.7 (6.2) | 85.3 ± 9.7 (5.2) | 98.3 ± 9.5 (6.6) |
| 0.05 | 84.5 ± 5.6 (8.3) | 85.9 ± 7.2 (8.3) | 90.2 ± 11.8 (7.5) | 107.9 ± 8.3 (8.7) | ||
| 0.01 | 95.1 ± 8.2 (9.1) | 92.8 ± 6.2 (9.1) | 94.5 ± 9.7 (9.1) | 102.4 ± 10.1 (6.9) | ||
| Tea infusion | 0.2 | 109.5 ± 8.9 (5.4) | 89.5 ± 6.1 (7.3) | 87.2 ± 3.6 (5.0) | 90.0 ± 7.0 (5.4) | |
| 0.02 | 103.8 ± 7.9 (10.5) | 103.8 ± 7.3 (8.3) | 98.2 ± 4.6 (6.9) | 93.6 ± 9.5 (10.5) | ||
| 0.002 | 110.4 ± 3.9 (8.4) | 102.4 ± 6.6 (9.6) | 89.4 ± 5.7 (9.4) | 101.4 ± 6.2 (8.34) | ||
| Beta-cypermethrin | Herb tea | 0.5 | 89.3 ± 9.2 (5.7) | 82.0 ± 8.5 (5.7) | 86.3 ± 6.9 (5.3) | 88.4 ± 4.5 (6.4) |
| 0.05 | 85.2 ± 3.7 (6.2) | 80.2 ± 3.2 (6.3) | 94.7 ± 3.9 (6.6) | 90.5 ± 4.1 (1.2) | ||
| 0.01 | 92.7 ± 9.8 (6.2) | 91.9 ± 5.5 (9.3) | 89.6 ± 4.9 (6.8) | 93.6 ± 6.9 (9.9) | ||
| Tea infusion | 0.2 | 115.4 ± 3.6 (5.6) | 81.4 ± 4.7 (4.4) | 87.4 ± 8.3 (5.7) | 95.4 ± 9.9 (5.6) | |
| 0.02 | 101.5 ± 4.1 (5.2) | 91.5 ± 7.6 (6.8) | 93.6 ± 6.9 (6.3) | 81.5 ± 7.9 (11.0) | ||
| 0.002 | 88.4 ± 3.8 (5.9) | 98.4 ± 5.8 (7.6) | 91.2 ± 9.0 (9.3) | 89.6 ±4.9 (5.9) | ||
| Fenvalerate | Herb tea | 0.5 | 90.1 ± 4.0 (6.1) | 102.8 ± 9.6 (10.1) | 94.2 ± 3.9 (5.3) | 89.8 ± 9.5 (6.5) |
| 0.05 | 96.2 ± 3.7 (2.3) | 96.2 ± 8.4 (7.3) | 102.4 ± 7.9 (8.4) | 95.7 ± 9.3 (8.5) | ||
| 0.01 | 103.5 ± 3.5 (7.6) | 84.3 ± 4.7 (5.6) | 97.2 ± 3.5 (9.3) | 98.3 ± 3.7 (9.1) | ||
| Tea infusion | 0.2 | 89.2 ± 5.8 (6.4) | 80.2 ± 3.8 (9.4) | 94.3 ± 4.9 (4.2) | 87.3 ± 8.6 (6.4) | |
| 0.02 | 88.6 ± 6.9 (5.2) | 98.5 ± 7.9 (7.2) | 96.2 ± 6.9 (5.5) | 98.3 ± 7.8 (5.2) | ||
| 0.002 | 113.5 ± 7.7 (7.1) | 91.4 ± 4.7 (8.4) | 95.4 ± 5.8 (7.3) | 103.5 ± 6.7 (7.1) | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, J.-J.; Li, Y.; Fang, Q.-K.; Shi, Y.-H.; Liao, M.; Wu, X.-W.; Hua, R.-M.; Cao, H.-Q. Factors Affecting Transfer of Pyrethroid Residues from Herbal Teas to Infusion and Influence of Physicochemical Properties of Pesticides. Int. J. Environ. Res. Public Health 2017, 14, 1157. https://doi.org/10.3390/ijerph14101157
Xiao J-J, Li Y, Fang Q-K, Shi Y-H, Liao M, Wu X-W, Hua R-M, Cao H-Q. Factors Affecting Transfer of Pyrethroid Residues from Herbal Teas to Infusion and Influence of Physicochemical Properties of Pesticides. International Journal of Environmental Research and Public Health. 2017; 14(10):1157. https://doi.org/10.3390/ijerph14101157
Chicago/Turabian StyleXiao, Jin-Jing, Yang Li, Qing-Kui Fang, Yan-Hong Shi, Min Liao, Xiang-Wei Wu, Ri-Mao Hua, and Hai-Qun Cao. 2017. "Factors Affecting Transfer of Pyrethroid Residues from Herbal Teas to Infusion and Influence of Physicochemical Properties of Pesticides" International Journal of Environmental Research and Public Health 14, no. 10: 1157. https://doi.org/10.3390/ijerph14101157





