Modeling of Chromium, Copper, Zinc, Arsenic and Lead Using Portable X-ray Fluorescence Spectrometer Based on Discrete Wavelet Transform
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Soil Samples, Equipment and Measurement Conditions
2.2. Spectra Processing
2.2.1. Wavelet Transform
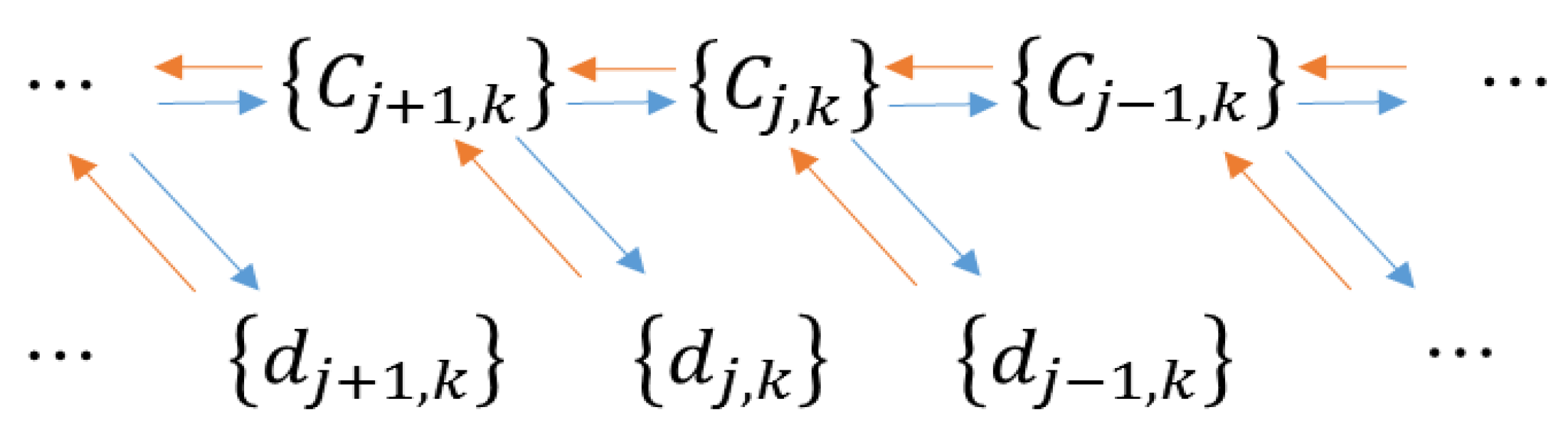
2.2.2. Mallat Algorithm
2.2.3. Wavelet Transform Processing
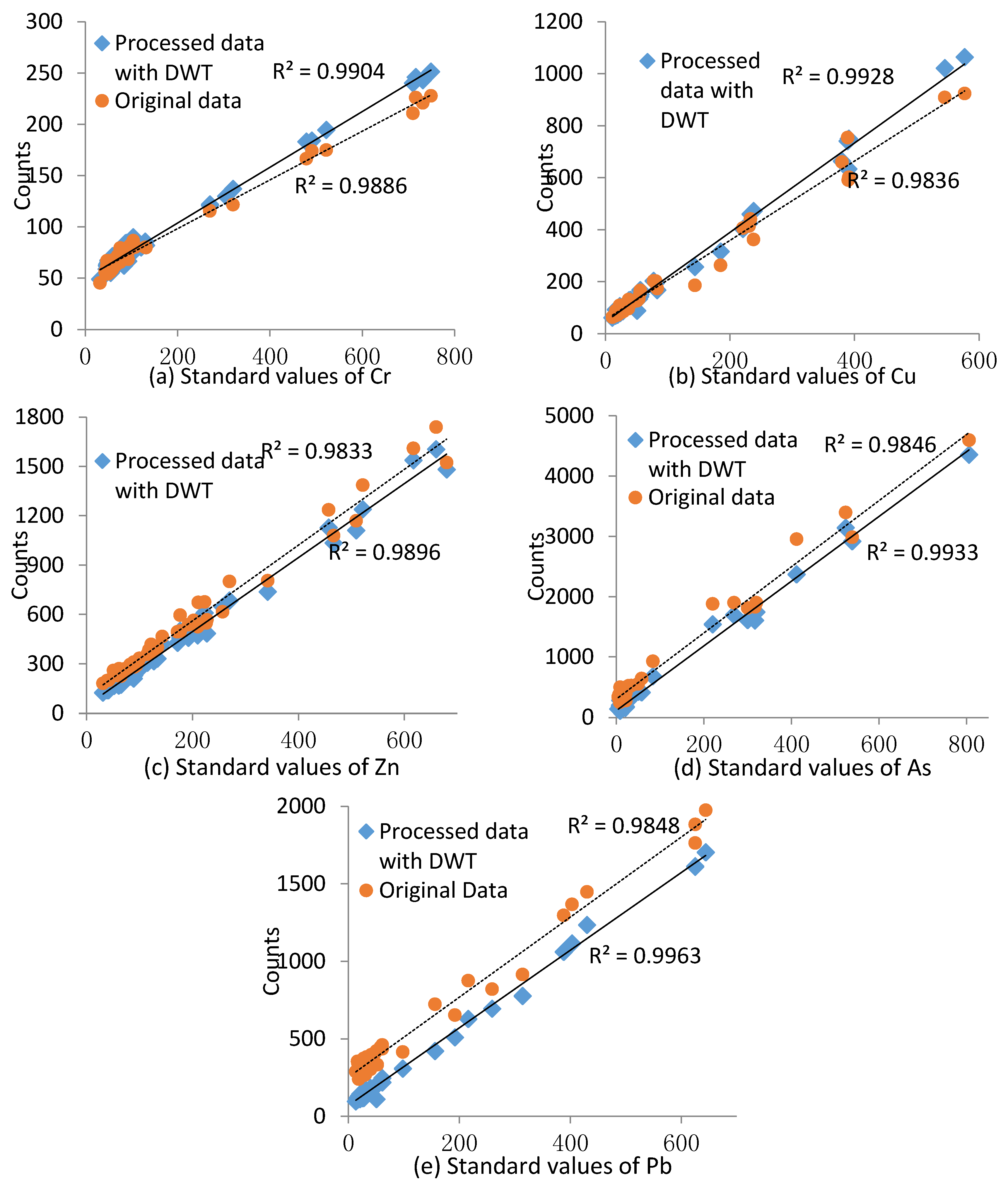
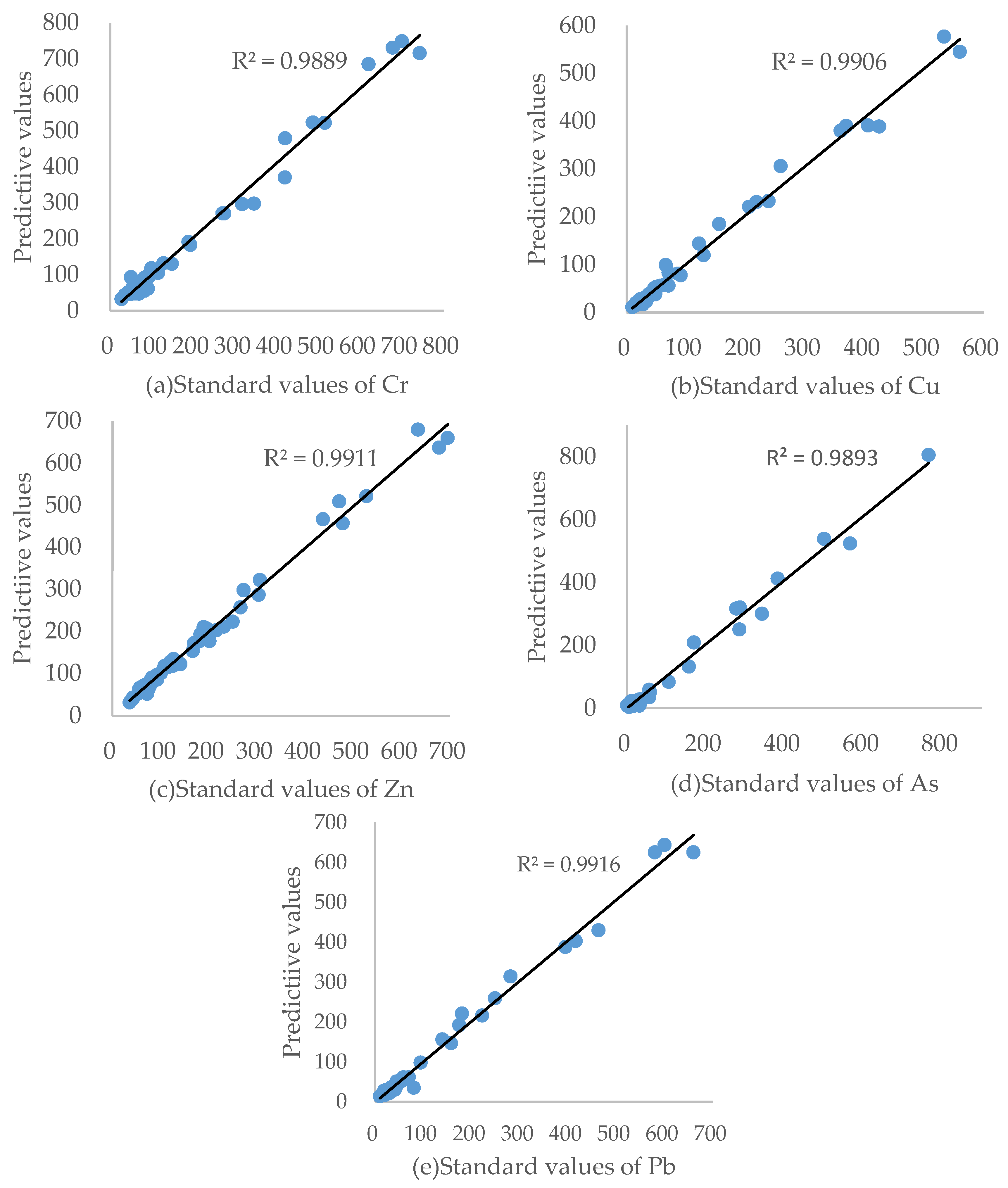
2.3. Determination and Validation of Calibration Curves
3. Results and Discussion
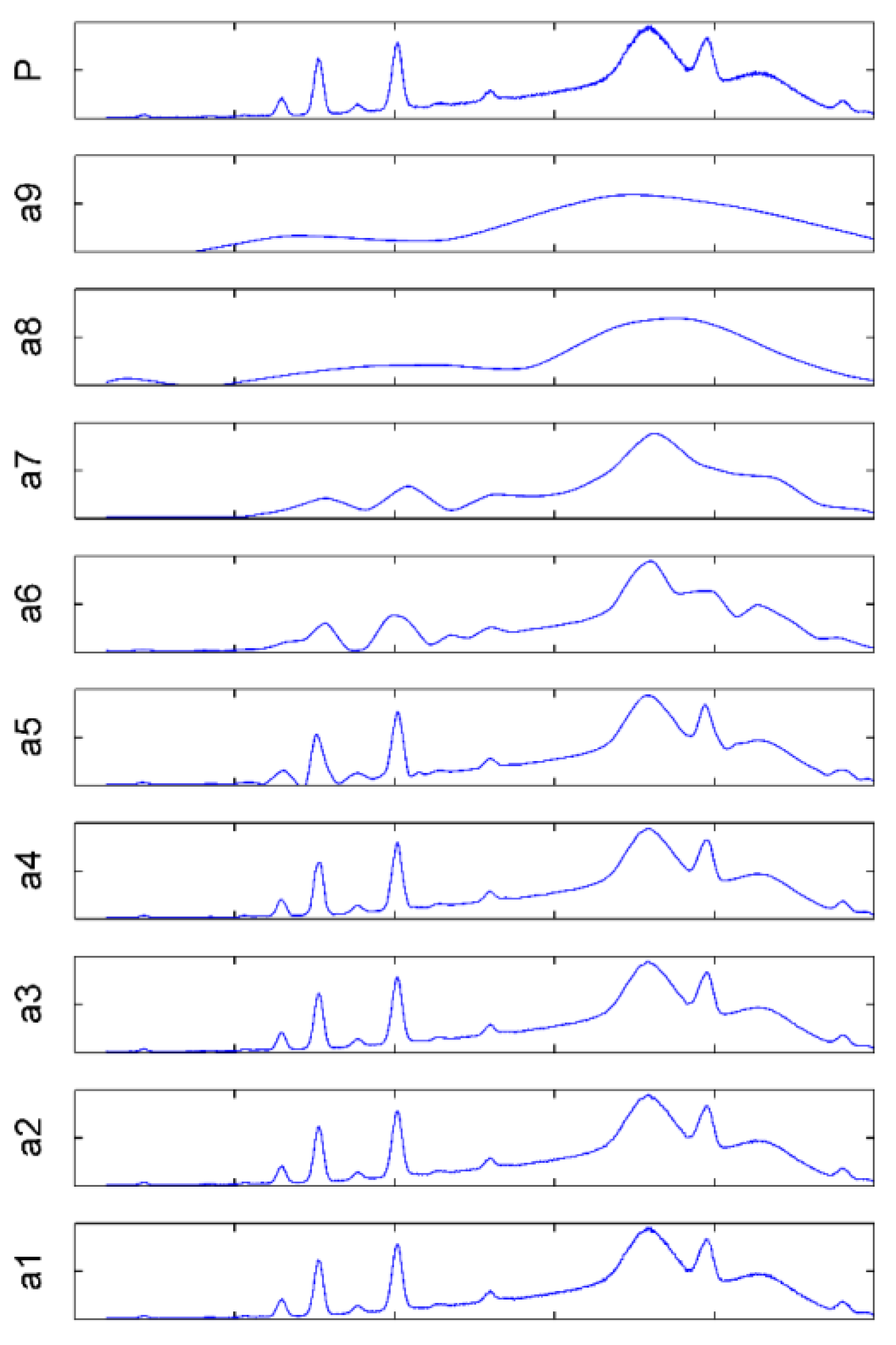
3.1. WT Processing Results
3.1.1. Evaluation Criteria of Denoising Results
3.1.2. Selection of WB
3.2. Instrument Calibration Curves
3.3. Detection Results and Detection Limits
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alloway, B.J. Sources of Heavy Metals and Metalloids in Soils. In Heavy Metals in Soils; Springer: Houten, The Netherlands, 2013; pp. 11–50. [Google Scholar]
- Cai, Q.; Long, M.L.; Zhu, M.; Zhou, Q.Z.; Zhang, L.; Liu, J. Food chain transfer of cadmium and lead to cattle in a lead-zinc smelter in Guizhou, China. Environ. Pollut. 2009, 157, 3078–3082. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Huo, X.; Li, Y.; Wu, K.; Liu, J.; Huang, J.; Zheng, G.; Xiao, Q.; Yang, H.; Wang, Y.; et al. Monitoring of lead, cadmium, chromium and nickel in placenta from an e-waste recycling town in china. Sci. Total Environ. 2010, 408, 3113–3117. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.; Sadeek, S.; Mahmoud, A.R.; Zaky, D. Comparison of aas, edxrf, icp-ms and inaa performance for determination of selected heavy metals in hfo ashes. Microchem. J. 2016, 128, 1–6. [Google Scholar] [CrossRef]
- Solek-Podwika, K.; Ciarkowska, K.; Kaleta, D. Assessment of the risk of pollution by sulfur compounds and heavy metals in soils located in the proximity of a disused for 20 years sulfur mine (SE Poland). J. Environ. Manage 2016, 180, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, A.R.; Ohde, S.; Shinjo, R.; Ganmanee, M.; Cohen, M.D. Geochemical fractions and modeling adsorption of heavy metals into contaminated river sediments in Japan and thailand determined by sequential leaching technique using ICP-MS. Arabian J. Chem. 2016. [Google Scholar] [CrossRef]
- Bull, A.; Brown, M.T.; Turner, A. Novel use of field-portable-xrf for the direct analysis of trace elements in marine macroalgae. Environ. Pollut. 2017, 220, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Paltridge, N.G.; Palmer, L.J.; Milham, P.J.; Guild, G.E.; Stangoulis, J.C.R. Energy-dispersive X-ray fluorescence analysis of zinc and iron concentration in rice and pearl millet grain. Plant Soil 2012, 361, 251–260. [Google Scholar] [CrossRef]
- Mir-Marques, A.; Martinez-Garcia, M.; Garrigues, S.; Cervera, M.L.; de la Guardia, M. Green direct determination of mineral elements in artichokes by infrared spectroscopy and X-ray fluorescence. Food Chem. 2016, 196, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Weindorf, D.C.; Zhu, Y.; McDaniel, P.; Valerio, M.; Lynn, L.; Michaelson, G.; Clark, M.; Ping, C.L. Characterizing soils via portable X-ray fluorescence spectrometer: 2. Spodic and albic horizons. Geoderma 2012, 189, 268–277. [Google Scholar] [CrossRef]
- Sharma, A.; Weindorf, D.C.; Wang, D.; Chakraborty, S. Characterizing soils via portable X-ray fluorescence spectrometer: 4. Cation exchange capacity (cec). Geoderma 2015, 239–240, 130–134. [Google Scholar] [CrossRef]
- Sharma, A.; Weindorf, D.C.; Man, T.; Aldabaa, A.A.A.; Chakraborty, S. Characterizing soils via portable X-ray fluorescence spectrometer: 3. Soil reaction (ph). Geoderma 2014, 232–234, 141–147. [Google Scholar] [CrossRef]
- Zhu, Y.; Weindorf, D.C.; Zhang, W. Characterizing soils using a portable X-ray fluorescence spectrometer: 1. Soil texture. Geoderma 2011, 167, 167–177. [Google Scholar] [CrossRef]
- Suh, J.; Lee, H.; Choi, Y. A rapid, accurate, and efficient method to map heavy metal-contaminated soils of abandoned mine sites using converted portable XRF data and gis. Int. J. Environ. Res. Public Health 2016, 13, 1191–1208. [Google Scholar] [CrossRef] [PubMed]
- Addo, M.A.; Darko, E.O.; Gordon, C.; Nyarko, B.J.B.; Gbadago, J.K. Heavy metal concentrations in road deposited dust at Ketu-South district, Ghana. Int. J. Sci. Tech. 2012, 2, 28–39. [Google Scholar]
- Weindorf, D.C.; Zhu, Y.; Chakraborty, S.; Bakr, N.; Huang, B. Use of portable X-ray fluorescence spectrometry for environmental quality assessment of peri-urban agriculture. Environ. Monit. Assess 2012, 184, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Choi, Y. Assessing statistically significant heavy-metal concentrations in abandoned mine areas via hot spot analysis of portable XRF data. Int. J. Environ. Res. Public Health 2017, 14, 654–669. [Google Scholar] [CrossRef] [PubMed]
- Jang, M. Application of portable X-ray fluorescence (pxrf) for heavy metal analysis of soils in crop fields near abandoned mine sites. Environ. Geochem. Health 2010, 32, 207–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Li, Y.; Suo, T.; Liang, J. De-noising of digital image correlation based on stationary wavelet transform. Opt. Lasers Eng. 2017, 90, 161–172. [Google Scholar] [CrossRef]
- Lark, R.M. Changes in the variance of a soil property along a transect, a comparison of a non-stationary linear mixed model and a wavelet transform. Geoderma 2016, 266, 84–97. [Google Scholar] [CrossRef] [Green Version]
- Wickerhauser, M.V. Adapted Wavelet Analysis from Theory to Software; A.K. Peters/CRC press: Ltd. Natick, MA, USA, 1994; p. 486. [Google Scholar]
- Goswami, J.C.; Chan, A.K. Fundamentals of Wavelets: Theory, Algorithms, and Applications; John Wiley & Sons: San Francisco, CA, USA, 2011; p. 359. [Google Scholar] [CrossRef]
- Araghi, A.; Baygi, M.M.; Adamowski, J.; Malard, J.; Nalley, D.; Hasheminia, S.M. Using wavelet transforms to estimate surface temperature trends and dominant periodicities in Iran based on gridded reanalysis data. Atmos. Res. 2015, 155, 52–72. [Google Scholar] [CrossRef]
- Belayneh, A.; Adamowski, J.; Khalil, B.; Quilty, J. Coupling machine learning methods with wavelet transforms and the bootstrap and boosting ensemble approaches for drought prediction. Atmos. Res. 2016, 172, 37–47. [Google Scholar] [CrossRef]
- Karthikeyan, L.; Kumar, D.N. Predictability of nonstationary time series using wavelet and emd based arma models. J. Hydr. 2013, 502, 103–119. [Google Scholar] [CrossRef]
- Bharath, R.; Srinivas, V.V. Delineation of homogeneous hydrometeorological regions using wavelet-based global fuzzy cluster analysis. Int. J. Climat. 2015, 35, 4707–4727. [Google Scholar] [CrossRef]
- Adarsh, S.; Reddy, M.J. Trend analysis of rainfall in four meteorological subdivisions of Southern India using nonparametric methods and discrete wavelet transforms. Int. J. Climat. 2015, 35, 1107–1124. [Google Scholar] [CrossRef]
- Mallat, S.G. A theory for multiresolution signal decomposition the wavelet representation. IEEE Trans. Pattern Anal. Mach. Intell. 1989, 11, 674–693. [Google Scholar] [CrossRef]
- Zimoń, M.J.; Reese, J.M.; Emerson, D.R. A novel coupling of noise reduction algorithms for particle flow simulations. J. Comput. Phys. 2016, 321, 169–190. [Google Scholar] [CrossRef]
- Yang, Z.; Ce, L.; Lian, L. Electricity price forecasting by a hybrid model, combining wavelet transform, arma and kernel-based extreme learning machine methods. Appl. Energy 2017, 190, 291–305. [Google Scholar] [CrossRef]
- Li, J.; Chang, L. A sar image compression algorithm based on mallat tower-type wavelet decomposition. Optik 2015, 126, 3982–3986. [Google Scholar] [CrossRef]
- Yajnik, A. Novel technique of oversampling the broken images using wavelet transform. Appl. Comput. Harm. Ana. 2015, 39, 357–368. [Google Scholar] [CrossRef]
- Lu, C.; Ma, L.; Yu, M.; Cui, S. Regional information entropy demons for infrared image nonrigid registration. Optik 2016, 127, 227–231. [Google Scholar]
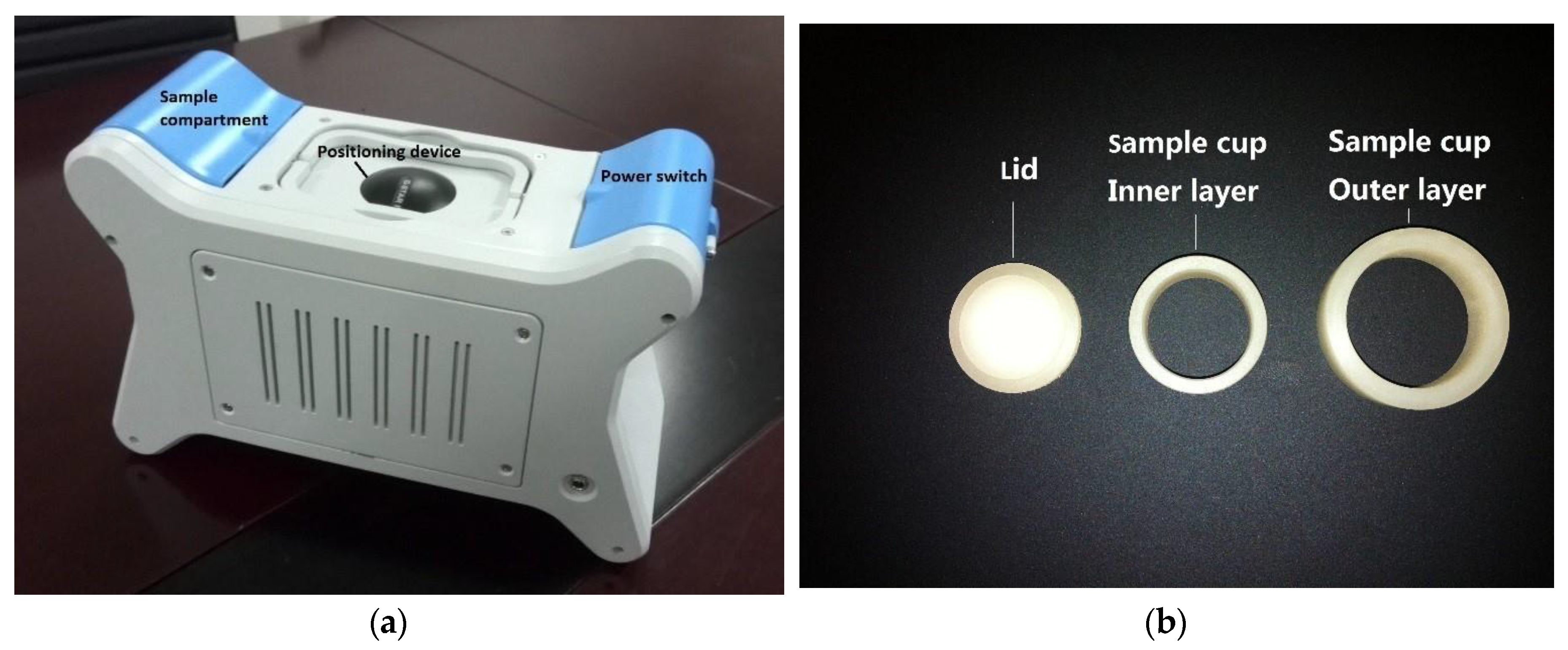
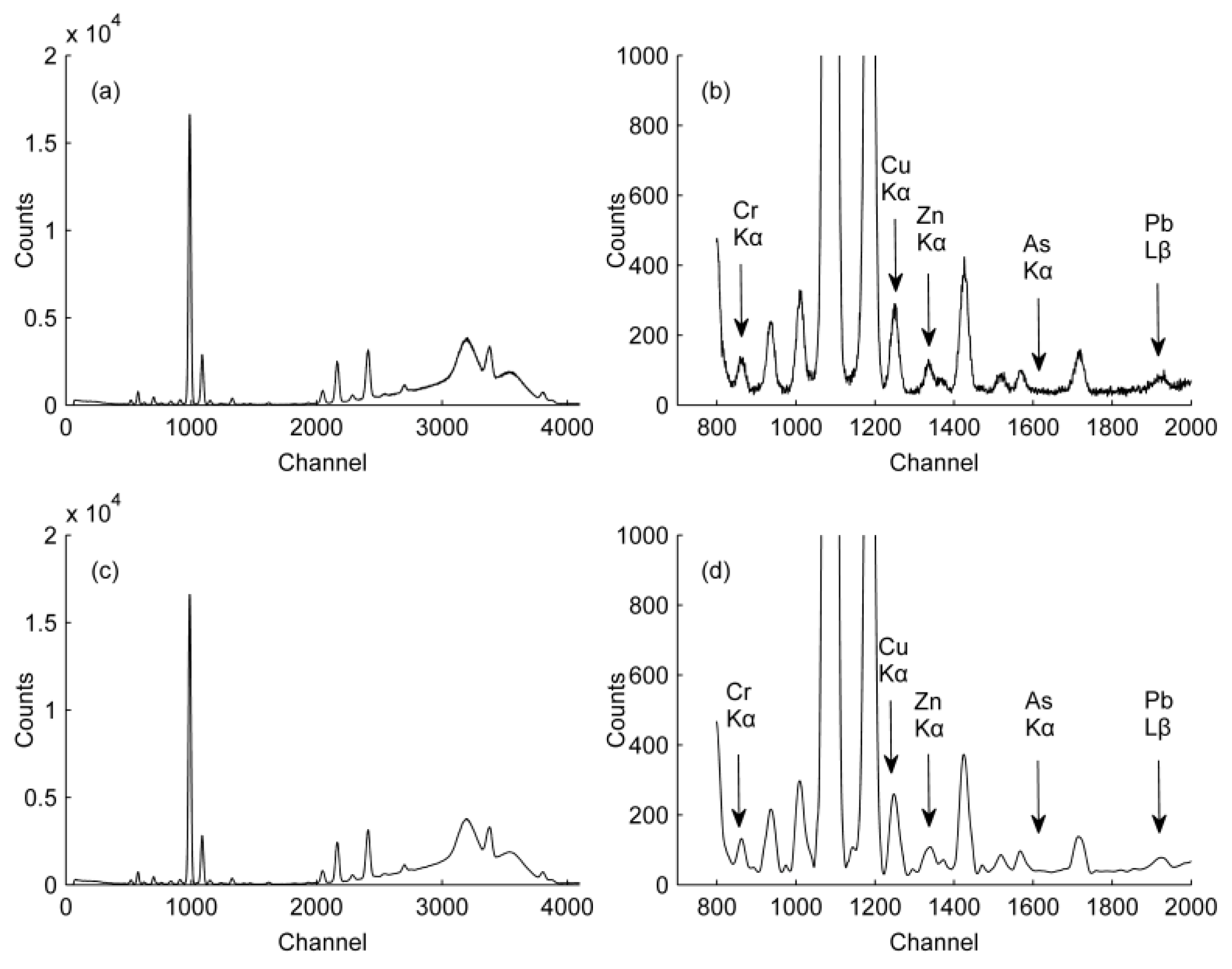
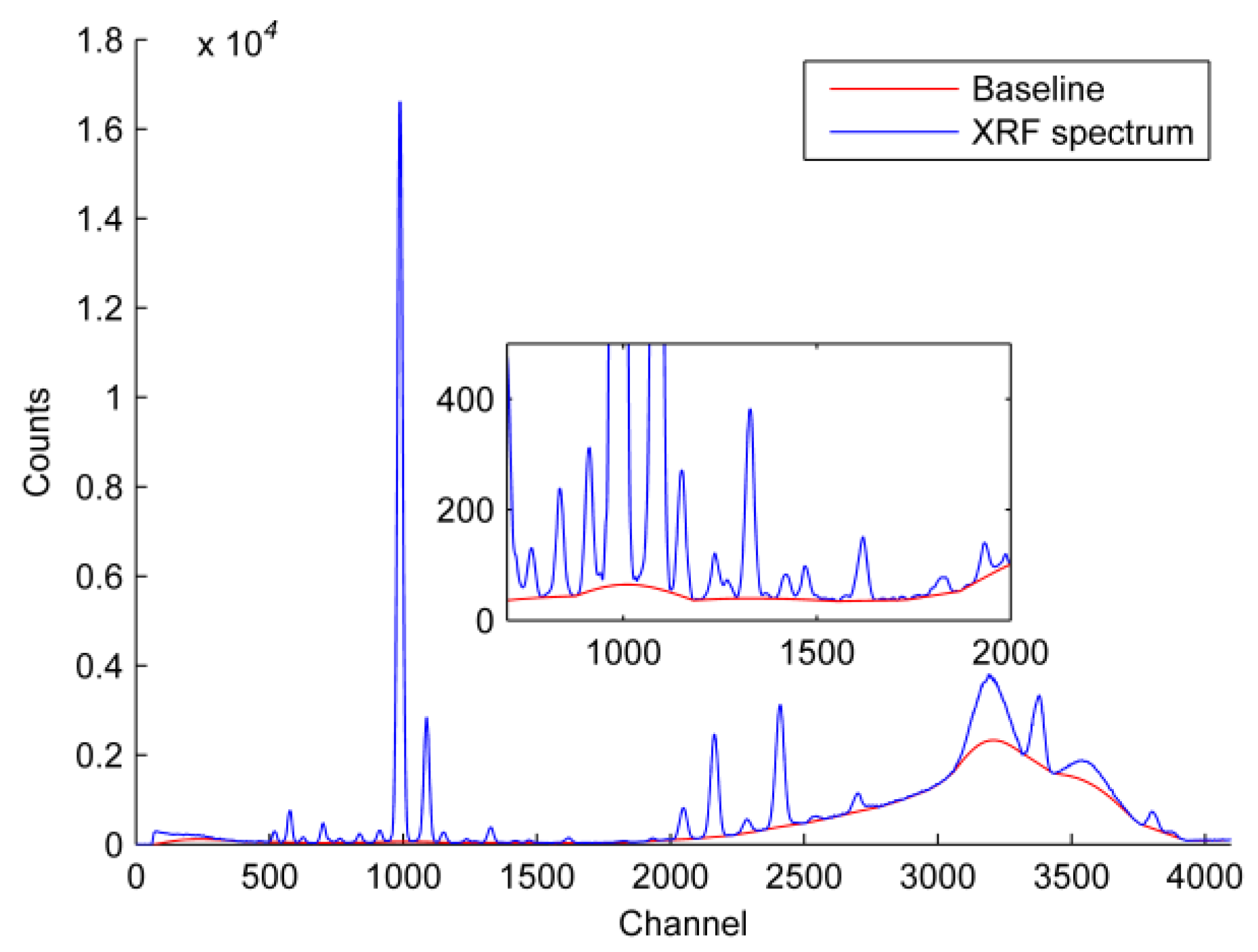
| HMs | X-ray Line for Analysis | Peak Position/keV | Corresponding Channel | Absorption Band/keV |
|---|---|---|---|---|
| Cr | Kα | 5.414 | 836 | 5.399–5.429 |
| Cu | Kα | 8.047 | 1243 | 8.032–8.062 |
| Zn | Kα | 8.638 | 1334 | 8.623–8.653 |
| As | Kα | 10.543 | 1628 | 10.528–10.598 |
| Pb | Lβ | 12.611 | 1948 | 12.595–12.625 |
| WB | SNR | MSE | H | α |
|---|---|---|---|---|
| coif2 | 103.85 | 528.59 | 0.1120 | 45.46 |
| coif3 | 110.57 | 519.53 | 0.1241 | 37.85 |
| coif4 | 110.09 | 519.36 | 0.1207 | 39.09 |
| coif5 | 98.66 | 516.30 | 0.1170 | 44.72 |
| db5 | 99.46 | 531.07 | 0.1185 | 45.05 |
| db6 | 100.62 | 524.08 | 0.1232 | 42.53 |
| db7 | 99.65 | 517.64 | 0.1140 | 45.58 |
| db8 | 111.44 | 518.96 | 0.1218 | 38.25 |
| db9 | 104.54 | 516.39 | 0.1076 | 45.93 |
| db10 | 103.59 | 520.54 | 0.1172 | 42.87 |
| sym5 | 105.45 | 520.84 | 0.1136 | 43.37 |
| sym6 | 107.66 | 519.21 | 0.1099 | 43.88 |
| sym7 | 95.79 | 517.49 | 0.1183 | 45.68 |
| sym8 | 97.53 | 519.33 | 0.1121 | 47.49 |
| Decomposition Level | SNR | MSE | H | α |
|---|---|---|---|---|
| 3 | 110.57 | 519.53 | 0.1241 | 37.85 |
| 4 | 107.46 | 603.68 | 0.1044 | 53.81 |
| 5 | 104.98 | 686.08 | 0.0584 | 111.84 |
| 6 | 97.52 | 743.46 | 0.0487 | 156.68 |
| 7 | 113.44 | 779.65 | 0.0237 | 290.31 |
| 8 | 98.06 | 798.07 | 0.0280 | 290.83 |
| 9 | 141.54 | 807.36 | 0.0313 | 182.44 |
| 10 | 92.70 | 815.65 | 0.0349 | 252.04 |
| Detection Limits | Cr | Cu | Zn | As | Pb |
|---|---|---|---|---|---|
| QDL | 11.34 | 9.33 | 7.59 | 7.25 | 11.67 |
| QNDL | 37.80 | 31.10 | 25.31 | 24.16 | 38.91 |
| WT-QDL | 2.22 | 6.13 | 3.87 | 4.52 | 5.28 |
| WT-QNDL | 7.39 | 20.43 | 12.90 | 15.08 | 17.61 |
| National level | 90 | 35 | 100 | 20 | 35 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Lu, A.; Wang, J. Modeling of Chromium, Copper, Zinc, Arsenic and Lead Using Portable X-ray Fluorescence Spectrometer Based on Discrete Wavelet Transform. Int. J. Environ. Res. Public Health 2017, 14, 1163. https://doi.org/10.3390/ijerph14101163
Li F, Lu A, Wang J. Modeling of Chromium, Copper, Zinc, Arsenic and Lead Using Portable X-ray Fluorescence Spectrometer Based on Discrete Wavelet Transform. International Journal of Environmental Research and Public Health. 2017; 14(10):1163. https://doi.org/10.3390/ijerph14101163
Chicago/Turabian StyleLi, Fang, Anxiang Lu, and Jihua Wang. 2017. "Modeling of Chromium, Copper, Zinc, Arsenic and Lead Using Portable X-ray Fluorescence Spectrometer Based on Discrete Wavelet Transform" International Journal of Environmental Research and Public Health 14, no. 10: 1163. https://doi.org/10.3390/ijerph14101163




