Joint Toxicity of Different Heavy Metal Mixtures after a Short-Term Oral Repeated-Administration in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Experimental Design and Preparation
2.2.2. Morris Water Maze Test
2.2.3. Organ/Body Weight Coefficients, and Hematological, Biochemical, Electrolyte and Thyroxine Parameter Analysis
2.2.4. Histopathological Examination
2.2.5. Western Blot Analysis
2.2.6. Statistical Analysis
3. Results
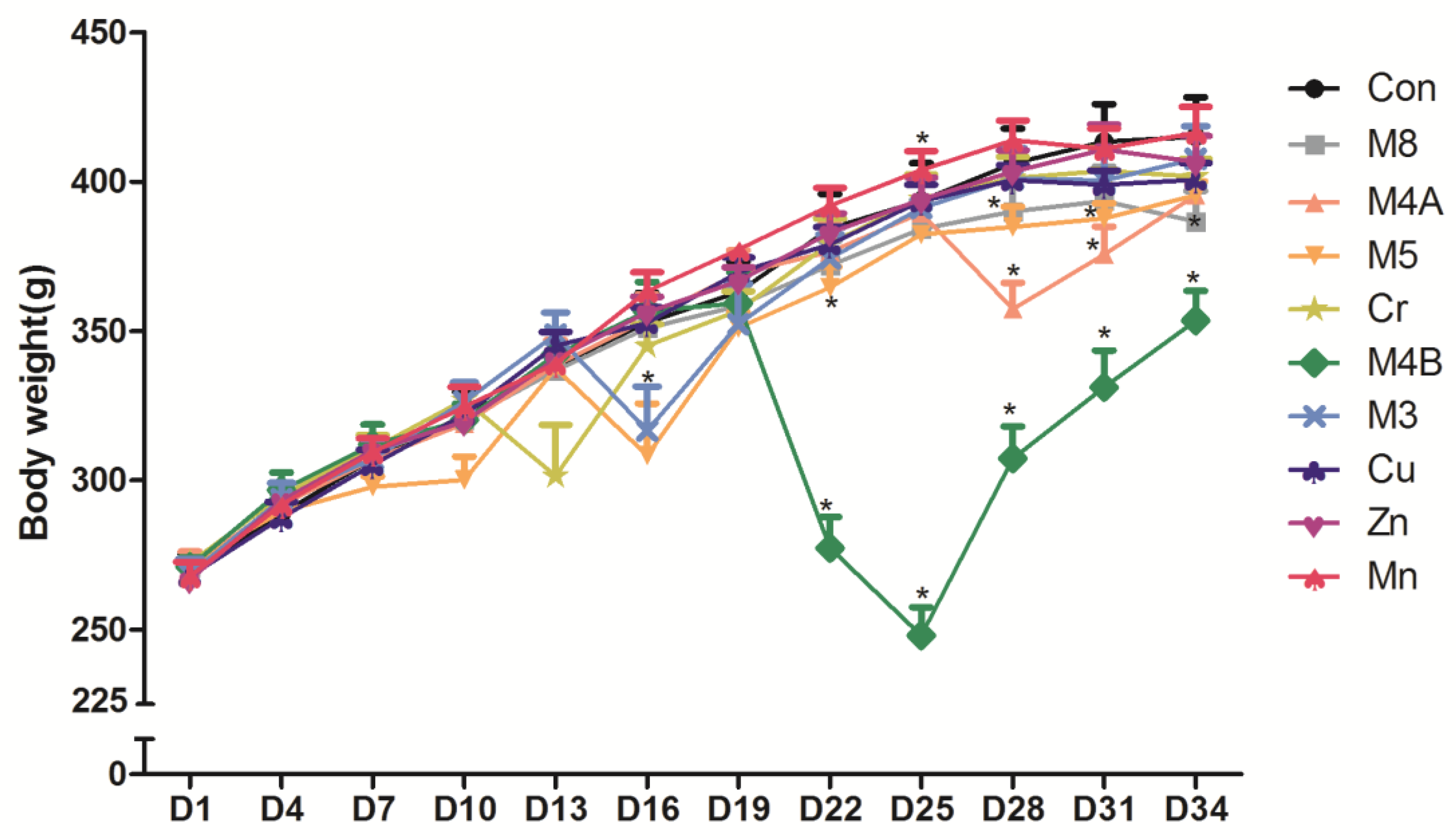
3.1. The Body Weight Change
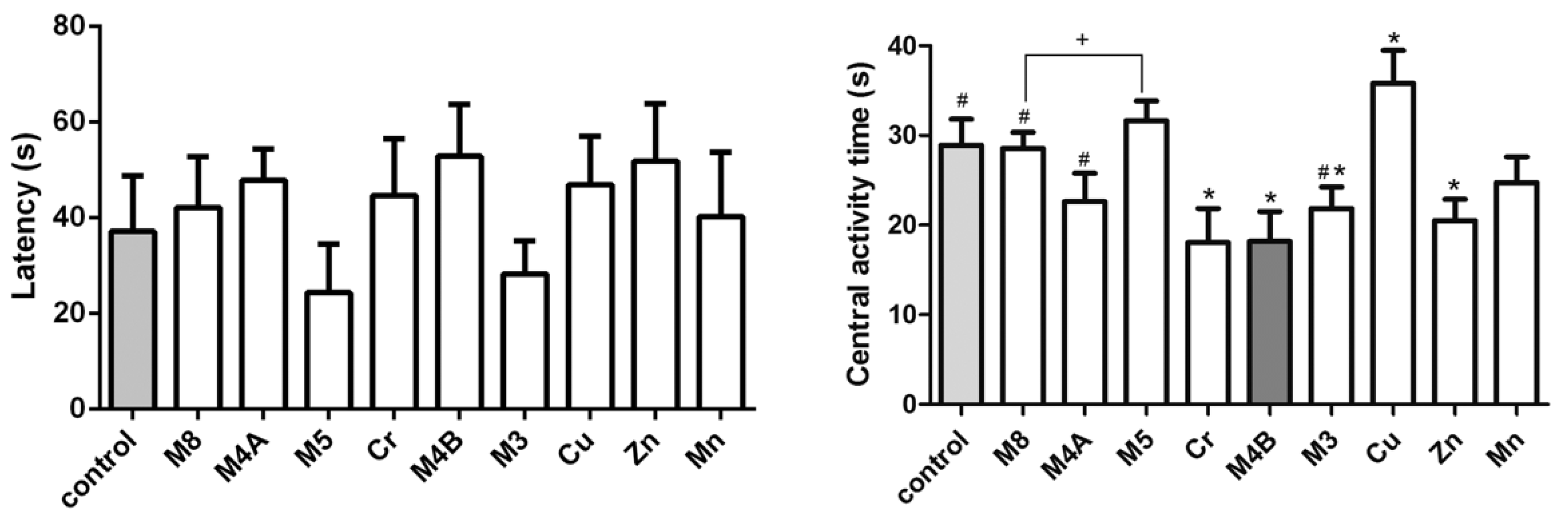
3.2. Morris Water Maze Test
3.3. Organ/Body Weight Coefficients, Hematological, Biochemical, Electrolyte and Thyroxine Parameter Analysis
3.3.1. Organ/body Weight Coefficients
3.3.2. Hematological Analysis
3.3.3. Biochemical, Electrolyte and Thyroxine Analysis
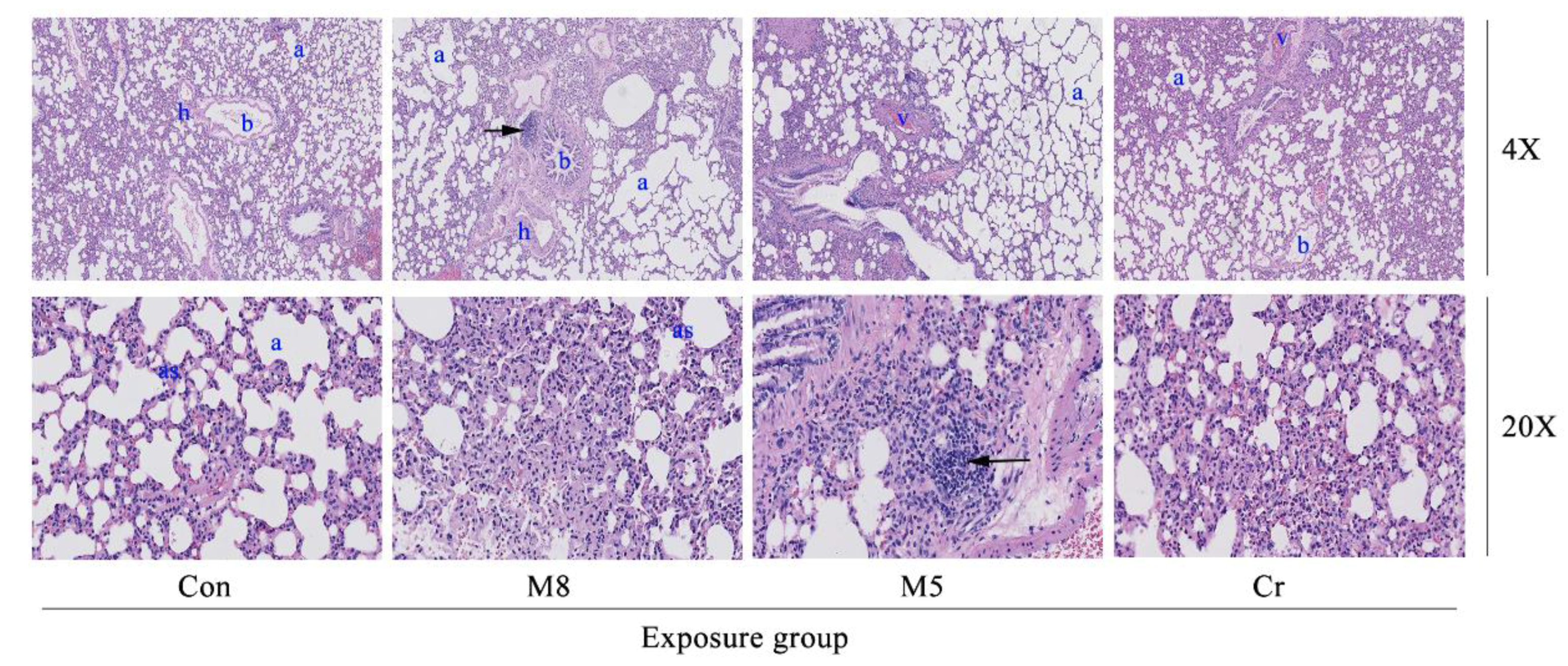
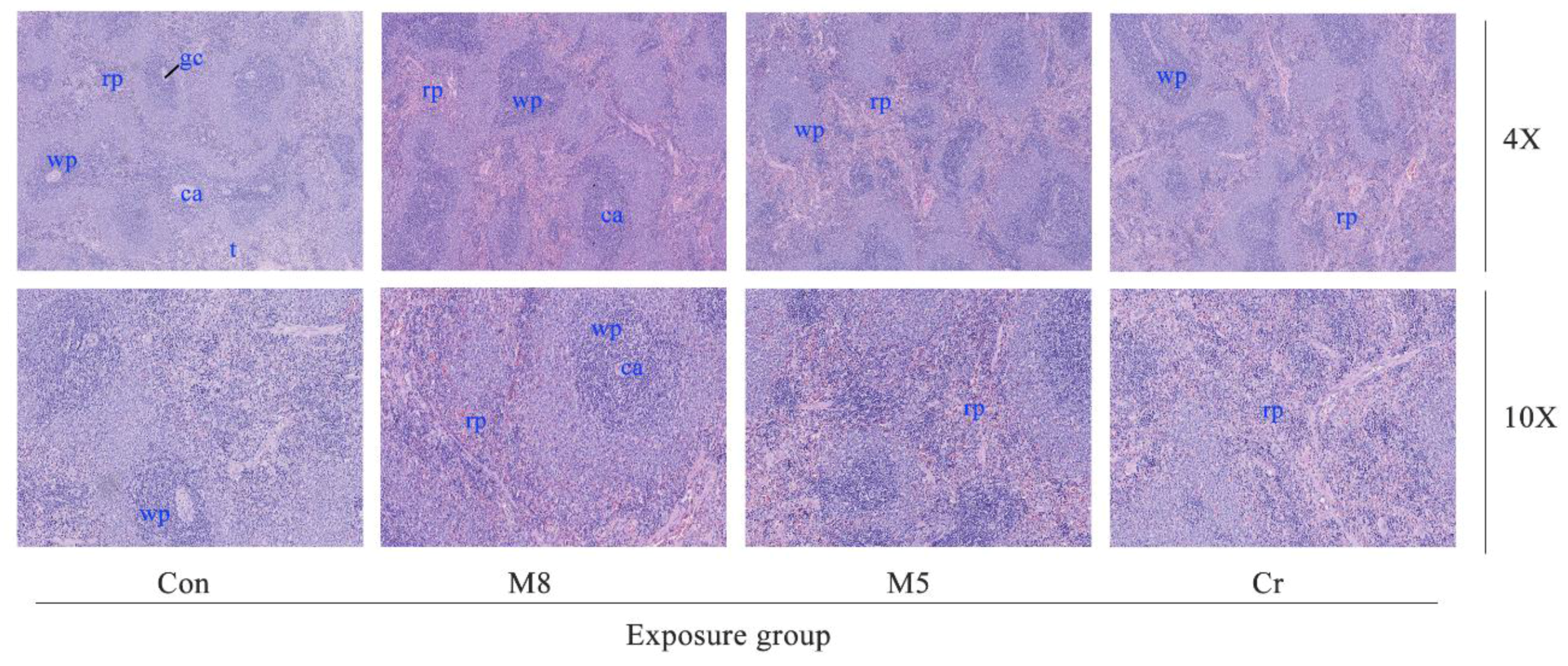
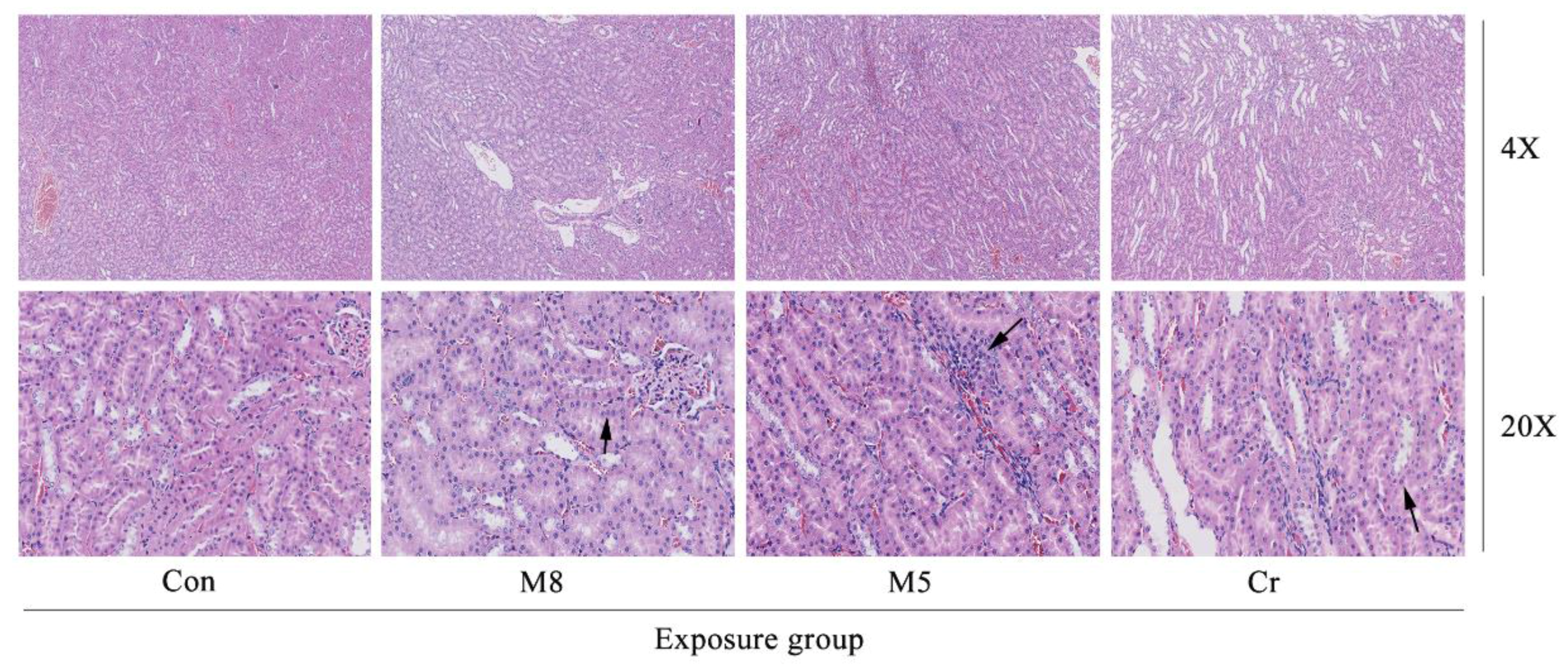
3.4. Histopathological Examination
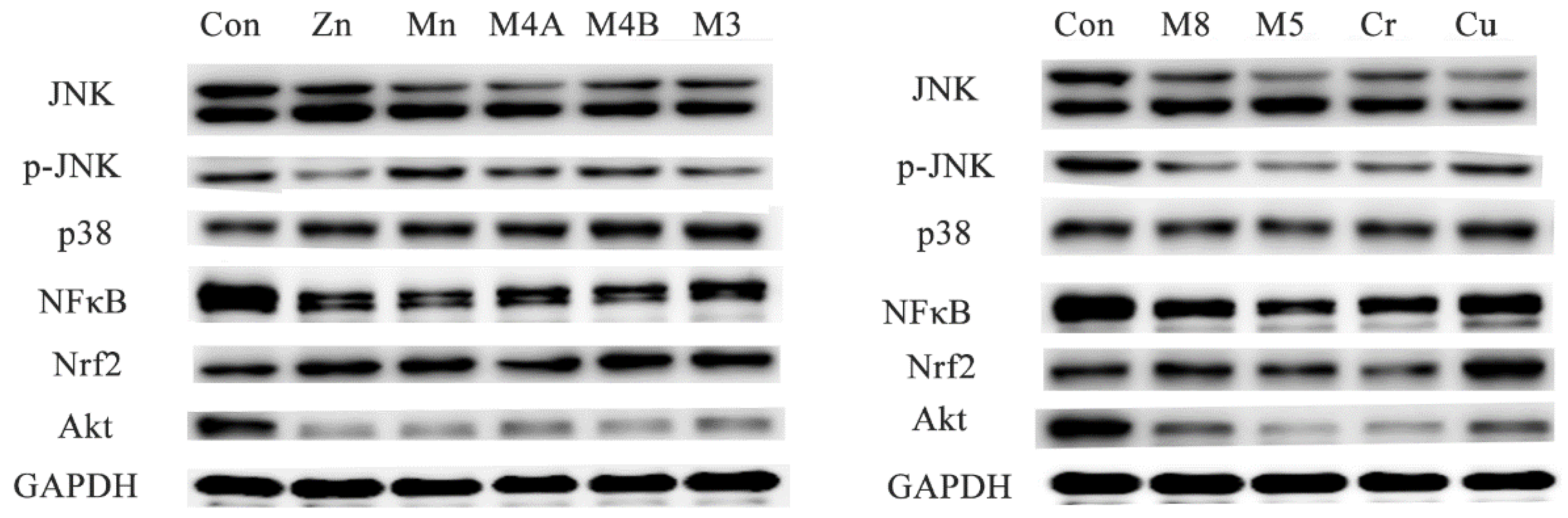
3.5. Western Blot Analysis
4. Discussion
4.1. Systemic Toxicities
4.2. Hematological, Hepatic, Renal and Spleen Toxicities
4.3. Neurotoxicity
5. Concussions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cheremisinoff, N.P. Agency for Toxic Substances and Disease Registry (Atsdr); John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 83–93. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metals toxicity and the environment. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar]
- Millichap, J. Lead Neurotoxicity. Pediatr. Neurol. Briefs 1995, 9, 42. [Google Scholar] [CrossRef]
- Faroon, O.; Ashizawa, A.; Wright, S.; Tucker, P.; Jenkins, K.; Ingerman, L.; Rudisill, C. Toxicological Profile for Cadmium; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2012.
- Tchounwou, P.B.; Ishaque, A.B.; Schneider, J. Cytotoxicity and transcriptional activation of stress genes in human liver carcinoma cells (HepG2) exposed to cadmium chloride. Mol. Cell. Biochem. 2001, 222, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Patlolla, A.K.; Barnes, C.; Yedjou, C.; Velma, V.R.; Tchounwou, P.B. Oxidative stress, DNA damage, and antioxidant enzyme activity induced by hexavalent chromium in Sprague-Dawley rats. Environ. Toxicol. 2009, 24, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Magaye, R.R.; Xia, Y.; Zou, B.; Shi, H.; Yu, H.; Liu, K.; Lin, X.; Jin, X.; Cui, Y.; Wu, A. Acute toxicity of nickel nanoparticles in rats after intravenous injection. Int. J. Nanomed. 2014, 9, 1393–1420. [Google Scholar]
- Piao, F.; Yokoyama, K.; Ma, N.; Yamauchi, T. Subacute toxic effects of zinc on various tissues and organs of rats. Toxicol. Lett. 2003, 145, 28–35. [Google Scholar] [CrossRef]
- Williams, M.; Todd, G.D.; Roney, N.; Crawford, J.; Coles, C.; Mcclure, P.R.; Garey, J.D.; Zaccaria, K.; Citra, M. Relevance to Public Health—Toxicological Profile for Manganese—NCBI Bookshelf; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2012.
- Duffus, J.H. “Heavy metals” a meaningless term? (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 793–807. [Google Scholar] [CrossRef]
- Norwood, W.P.; Borgmann, U.; Dixon, D.G.; Wallace, A. Effects of metal mixtures on aquatic biota: A review of observations and methods. Hum. Ecol. Risk Assess. Int. J. 2003, 9, 795–811. [Google Scholar] [CrossRef]
- Kortenkamp, A. Ten years of mixing cocktails: A review of combination effects of endocrine-disrupting chemicals. Environ. Health Perspect. 2007, 115, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Mauderly, J.L.; Samet, J.M. Is there evidence for synergy among air pollutants in causing health effects? Environ. Health Perspect. 2009, 117, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pandya, C.D.; Pillai, P.P.; Gupta, S.S. Lead and cadmium co-exposure mediated toxic insults on hepatic steroid metabolism and antioxidant system of adult male rats. Biol. Trace Elem. Res. 2010, 134, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Goyer, R.A. Toxic and essential metal interactions. Ann. Rev. Nutr. 2003, 17, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Damian, E.C.; Obinna, O.M.; Ndidi, O.C. Predictive modelling of heavy metal-metal interactions in environmental setting: Laboratory simulative approach. Environ. Ecol. Res. 2014, 2, 248–252. [Google Scholar]
- Prato, E.; Biandolino, F. Combined toxicity of mercury, copper and cadmium on embryogenesis and early larval stages of the. Environ. Technol. 2007, 28, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, Y.; Wang, Y.; Wang, Y. Assessment of toxic interactions of heavy metals in multi-component mixtures using sea urchin embryo-larval bioassay. Toxicol. Vitr. 2011, 25, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cobbina, S.J.; Mao, G.; Xu, H.; Zhang, Z.; Yang, L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res. 2016, 23, 8244–8259. [Google Scholar] [CrossRef] [PubMed]
- Lin, X. Study on the Joint Toxicity of Common Heavy Metal Pollutants in Aquatic Products. Ph.D. Thesis, Ningbo University, Ningbo, China, 2015. [Google Scholar]
- Lin, X.; Gu, Y.; Zhou, Q.; Mao, G.; Ma, D.; Zhao, J.; Zou, B. Study: Joint toxicity of heavy metal mixtures related to proportions of fish consumption. J. Ningbo Univ. 2016, 29, 22–27. [Google Scholar]
- Lin, X.; Gu, Y.; Zhou, Q.; Mao, G.; Zou, B.; Zhao, J. Combined toxicity of heavy metal mixtures in liver cells. J. Appl. Toxicol. JAT 2016, 36, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- López, A.M.; Prieto, M.F.; Miranda, M.; Castillo, C.; Hernández, J.; Luis, B.J. Interactions between toxic (As, Cd, Hg and Pb) and nutritional essential (Ca, Co, Cr, Cu, Fe, Mn, Mo, Ni, Se, Zn) elements in the tissues of cattle from NW Spain. Biometals 2004, 17, 389–397. [Google Scholar]
- Eslami, S.; Moghaddam, A.H.; Jafari, N.; Nabavi, S.F.; Nabavi, S.M.; Ebrahimzadeh, M.A. Trace element level in different tissues of Rutilus frisii kutum collected from Tajan river, Iran. Biol. Trace Elem. Res. 2011, 143, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.S.; Yu, S.; Zhu, Y.G.; Li, X.D. Trace metal contamination in urban soils of China. Sci. Total Environ. 2012, 421–422, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Plessl, C.; Otachi, E.O.; Korner, W.; Avenant-Oldewage, A.; Jirsa, F. Fish as bioindicators for trace element pollution from two contrasting lakes in the eastern rift valley, Kenya: Spatial and temporal aspects. Environ. Sci. Pollut. Res. Int. 2017, 24, 19767–19776. [Google Scholar] [CrossRef] [PubMed]
- Loubières, Y.; De, L.A.; Bernier, M.; Vieillard-Baron, A.; Schmitt, J.M.; Page, B.; Jardin, F. Acute, fatal, oral chromic acid poisoning. J. Toxicol. Clin. Toxicol. 1999, 37, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Ren, A.; Gao, Y. Effects of single and combinative pollutions of lead, cadmium and chromium on the germination of Brassica chinensis L. Chin. J. Ecol. 2000, 19, 19–22. [Google Scholar]
- Eder, K.; Kralik, A.; Kirchgessner, M. The effect of manganese supply on thyroid hormone metabolism in the offspring of manganese-depleted dams. Biol. Trace Elem. Res. 1996, 55, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T. Hexavalent chromium toxicity in pituitary and thyroid glands. Pak. J. Zool. 2008, 40, 91. [Google Scholar]
- Brzoska, M.M.; Moniuszko-Jakoniuk, J. Interactions between cadmium and zinc in the organism. Food Chem. Toxicol. 2001, 39, 967–980. [Google Scholar] [CrossRef]
- Cobbina, S.J.; Chen, Y.; Zhou, Z.; Wu, X.; Feng, W.; Wang, W.; Mao, G.; Xu, H.; Zhang, Z.; Wu, X. Low concentration toxic metal mixture interactions: Effects on essential and non-essential metals in brain, liver, and kidneys of mice on sub-chronic exposure. Chemosphere 2015, 132, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Hantson, P.; Caenegem, O.V.; Decordier, I.; Haufroid, V.; Lison, D. Hexavalent chromium ingestion: Biological markers of nephrotoxicity and genotoxicity. Clin. Toxicol. 2008, 43, 111–112. [Google Scholar] [CrossRef]
- Bornhorst, J.; Meyer, S.; Weber, T.; Böker, C.; Marschall, T.; Mangerich, A.; Beneke, S.; Bürkle, A.; Schwerdtle, T. Molecular mechanisms of Mn induced neurotoxicity: RONS generation, genotoxicity, and DNA-damage response. Mol. Nutr. Food Res. 2013, 57, 1255–1269. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.D.; Chen, W.D.; Yu, D.; Forman, B.M.; Huang, W. The g-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor kappa light-chain enhancer of activated b cells (NF-κB) in mice. Hepatology 2011, 54, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Heckscher, E.S.; Fetter, R.D.; Marek, K.W.; Albin, S.D.; Davis, G.W. NF-κB, Ikappab, and IRAK control glutamate receptor density at the drosophila NMJ. Neuron 2007, 55, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Meffert, M.K.; Chang, J.M.; Wiltgen, B.J.; Fanselow, M.S.; Baltimore, D. NF-κB functions in synaptic signaling and behavior. Nat. Neurosci. 2003, 6, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Levenson, J.M.; Choi, S.; Lee, S.Y.; Cao, Y.A.; Ahn, H.J.; Worley, K.C.; Pizzi, M.; Liou, H.C.; Sweatt, J.D. A bioinformatics analysis of memory consolidation reveals involvement of the transcription factor c-rel. J. Neurosci. 2004, 24, 3933–3943. [Google Scholar] [CrossRef] [PubMed]
- Horwood, J.M.; Dufour, F.; Laroche, S.; Davis, S. Signalling mechanisms mediated by the phosphoinositide 3-kinase/Akt cascade in synaptic plasticity and memory in the rat. Eur. J. Neurosci. 2006, 23, 3375–3384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yu, J.; Yang, T.; Xu, D.; Chi, Z.; Xia, Y.; Xu, Z. A novel c-Jun N-terminal kinase (JNK) signaling complex involved in neuronal migration during brain development. J. Biol. Chem. 2016, 291, 11466–11475. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Das, M.; Reilly, J.; Davis, R.J. JNK regulates FoxO-dependent autophagy in neurons. Genes Dev. 2011, 25, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Kanninen, K.; Heikkinen, R.; Malm, T.; Rolova, T.; Kuhmonen, S.; Leinonen, H.; Yla-Herttuala, S.; Tanila, H.; Levonen, A.L.; Koistinaho, M.; et al. Intrahippocampal injection of a lentiviral vector expressing Nrf2 improves spatial learning in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2009, 106, 16505–16510. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, T.; Pu, J.; Guo, J.; Chen, Z.; Wang, Y.; Jia, G. Multi-element distribution profile in sprague-dawley rats: Effects of intratracheal instillation of Cr(VI) and Zn intervention. Toxicol. Lett. 2014, 226, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.I.; Lin, H.; Zhang, Q.R.; Bai, W.B.; University, S. Ameliorative effect of cyanidin-3-glucoside on heavy metal-induced oxidative stress in the liver of SD rats. Chin. J. Ecol. 2016, 5, 1358–1363. [Google Scholar]
- Lyon, M.F. Problems in Extrapolation of Animal Data to Humans; Springer: New York, NY, USA, 1983; pp. 289–305. [Google Scholar]

| Group | Compound Dose (mg/Kg Body Weight) | |||||||
|---|---|---|---|---|---|---|---|---|
| (CH3COO)2Pb·3H2O | CdCl2·2.5H2O | CH3HgCl | NiCl2·6H2O | K2Cr2O7 | CuSO4·5H2O | ZnSO4·7H2O | MnCl2·4H2O | |
| Con | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M8 | 0.087 | 0.193 | 0.009 | 0.874 | 1.681 | 8.831 | 33.840 | 4.484 |
| M5 | 0.087 | 0.193 | 0.009 | 0.874 | 1.681 | 0 | 0 | 0 |
| M4A | 0.087 | 0.193 | 0.009 | 0.874 | 0 | 0 | 0 | 0 |
| M4B | 0 | 0 | 0 | 0 | 1.681 | 8.831 | 33.840 | 4.484 |
| M3 | 0 | 0 | 0 | 0 | 0 | 8.831 | 33.840 | 4.484 |
| Cr | 0 | 0 | 0 | 0 | 1.681 | 0 | 0 | 0 |
| Cu | 0 | 0 | 0 | 0 | 0 | 8.831 | 0 | 0 |
| Zn | 0 | 0 | 0 | 0 | 0 | 0 | 33.840 | 0 |
| Mn | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4.484 |
| Group | Organ/Body Weight Coefficients (% Weight) | ||||||
|---|---|---|---|---|---|---|---|
| Heart | Liver | Spleen | Lung | Kidney | Brain | Testis | |
| Con | 0.38 ± 0.01 | 3.56 ± 0.08 | 0.18 ± 0.01 | 0.41 ± 0.01 | 0.75 ± 0.01 | 0.48 ± 0.02 | 0.70 ± 0.02 |
| M8 | 0.39 ± 0.03 | 3.49 ± 0.05 | 0.19 ± 0.01 | 0.46 ± 0.01 * | 0.77 ± 0.04 | 0.50 ± 0.02 | 0.75 ± 0.05 |
| M4A | 0.40 ± 0.02 | 3.46 ± 0.09 | 0.19 ± 0.01 | 0.44 ± 0.02 | 0.74 ± 0.03 | 0.49 ± 0.02 | 0.73 ± 0.05 |
| M5 | 0.37 ± 0.01 | 3.64 ± 0.05 | 0.19 ± 0.01 | 0.44 ± 0.01 | 0.79 ± 0.01 | 0.50 ± 0.01 | 0.76 ± 0.03 |
| Cr | 0.41 ± 0.02 | 3.61 ± 0.09 | 0.21 ± 0.01 * | 0.45 ± 0.01 | 0.76 ± 0.02 | 0.48 ± 0.01 | 0.78 ± 0.03 |
| M4B | 0.41 ± 0.02 | 3.62 ± 0.09 | 0.22 ± 0.01 * | 0.52 ± 0.02 * | 0.82 ± 0.01 * | 0.56 ± 0.02 * | 0.87 ± 0.03 * |
| M3 | 0.38 ± 0.01 | 3.51 ± 0.06 | 0.19 ± 0.01 | 0.47 ± 0.01 * | 0.75 ± 0.02 | 0.46 ± 0.02 | 0.74 ± 0.02 |
| Cu | 0.38 ± 0.02 | 3.54 ± 0.07 | 0.19 ± 0.01 | 0.44 ± 0.01 | 0.75 ± 0.02 | 0.48 ± 0.01 | 0.77 ± 0.02 |
| Zn | 0.41 ± 0.02 | 3.68 ± 0.06 | 0.18 ± 0.01 | 0.43 ± 0.02 | 0.78 ± 0.02 | 0.47 ± 0.01 | 0.72 ± 0.03 |
| Mn | 0.39 ± 0.02 | 3.49 ± 0.06 | 0.18 ± 0.01 | 0.43 ± 0.01 | 0.75 ± 0.03 | 0.47 ± 0.01 | 0.71 ± 0.01 |
| Group | WBC | NE% | LY% | BASO% | RBC | Hb | MCV | MCHC | PLT | MPV |
|---|---|---|---|---|---|---|---|---|---|---|
| Con | 8.30 ± 0.71 | 17.03 ± 1.96 | 76.43 ± 2.18 | 0.10 ± 0.03 | 7.53± 0.10 | 142.50± 1.03 | 60.97± 0.54 | 311.00± 1.21 | 759.50± 111.26 | 7.07± 0.16 |
| M8 | 7.53 ± 0.60 | 17.82 ± 2.04 | 76.23 ± 2.47 | 0.02 ± 0.02 * | 7.79 ± 0.24 | 145.17 ± 3.01 | 60.80 ± 1.05 | 307.33 ± 2.06 | 728.83 ± 120.80 | 7.23 ± 0.10 |
| M5 | 9.10 ± 1.38 | 13.65 ± 0.62 | 81.65 ± 0.82 | 0.02 ± 0.02 * | 7.89 ± 0.10 | 146.00 ± 1.10 | 59.33 ± 0.55 | 312.00 ± 1.57 | 891.00 ± 36.62 | 6.93 ± 0.14 |
| Cr | 8.68 ± 0.81 | 17.12 ± 4.63 | 76.48 ± 6.13 | 0.10 ± 0.03 | 7.57 ± 0.12 | 141.17 ± 1.58 | 61.17 ± 0.71 | 305.67 ± 1.09 * | 853.67 ± 76.48 | 6.83 ± 0.13 |
| Cu | 8.85 ± 0.64 | 17.25 ± 2.45 | 76.00 ± 2.83 | 0.07 ± 0.03 | 8.08 ± 0.18 * | 148.50 ± 2.53 | 58.88 ± 0.55 * | 312.17 ± 1.85 | 903.33 ± 13.39 * | 6.77 ± 0.19 |
| Zn | 9.18 ± 0.89 | 14.52 ± 1.84 | 78.78 ± 2.10 | 0.08 ± 0.02 | 8.04 ± 0.16 * | 148.17 ± 2.27 | 59.35 ± 0.37 * | 311.00 ± 2.22 | 860.83 ± 41.34 | 6.80 ± 0.21 |
| Mn | 9.52 ± 0.98 | 14.17 ± 1.42 | 80.13 ± 1.90 | 0.10 ± 0.00 | 7.91 ± 0.14 | 149.17 ± 2.63 * | 60.65 ±0.92 | 310.83 ± 1.38 | 901.17 ± 43.06 | 6.80 ± 0.22 |
| M4A | 8.68 ± 0.96 | 17.27 ± 2.59 | 76.28 ± 2.46 | 0.07 ± 0.02 | 7.64 ± 0.13 | 143.67 ± 1.71 | 60.30 ± 0.58 | 311.50 ± 1.41 | 848.33 ± 53.73 | 6.82 ± 0.19 |
| M4B | 8.43 ± 1.24 | 31.84 ± 3.57 * | 44.60 ± 11.74 * | 0.08 ± 0.02 | 7.58 ± 0.08 | 142.67 ± 1.23 | 60.73 ± 0.44 | 309.67 ± 1.43 | 903.83 ± 53.06 * | 6.50 ± 0.18 |
| M3 | 8.17 ± 0.35 | 15.57 ± 1.70 | 77.63 ± 1.36 | 0.08 ± 0.02 | 7.81 ± 0.19 | 144.50 ± 3.38 | 59.07 ± 0.28 | 312.67 ± 2.22 | 723.83 ± 100.47 | 6.55 ± 0.21 |
| Group | Liver profile | Renal profile | Lipid profile | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TBIL | TP | ALB | GLOB | ALT | AST | ALP | UR/Cr | URCA | CHOL | TG | HDLC | LDLC | |||
| Con | 0.27 + 0.20 | 51.38 + 2.70 | 34.58 + 0.30 | 16.80 + 2.80 | 70.00 + 3.31 | 110.67 + 7.23 | 173.50 + 11.51 | 0.26 + 0.02 | 140.17 + 15.53 | 1.27 + 0.10 | 0.93 + 0.13 | 0.67 + 0.05 | 0.23 + 0.02 | ||
| M8 | 0.10 + 0.31 | 52.94 + 0.58 | 34.00 + 0.47 | 18.94 + 0.15 | 75.20 + 5.42 | 159.00 + 38.07 | 188.00 + 26.98 | 0.29 + 0.03 | 120.20 + 11.81 | 1.08 + 0.06 | 0.86 + 0.19 | 0.60 + 0.04 | 0.21 + 0.01 | ||
| M5 | 0.15 + 0.14 | 55.68 + 0.49 | 35.57 + 0.51 | 20.12 + 0.22 | 90.33 + 17.63 | 222.50 + 69.25 | 226.50 + 21.54 | 0.30 + 0.01 | 133.17 + 17.55 | 1.23 + 0.05 | 1.02 + 0.11 | 0.64 + 0.02 | 0.22 + 0.01 | ||
| Cr | 0.65 + 0.27 | 54.17 + 0.85 | 33.85 + 0.47 | 20.32 + 0.44 | 67.00 + 4.87 | 107.67 + 14.06 | 198.17 + 20.24 | 0.28 + 0.02 | 95.33 + 9.42 * | 1.30 + 0.07 | 0.87 + 0.11 | 0.66 + 0.04 | 0.22 + 0.01 | ||
| Cu | 0.20 + 0.28 | 57.68 + 0.60 * | 35.68 + 0.36 | 22.00 + 0.37 | 71.00 + 1.61 | 118.50 + 11.04 | 232.00 + 23.68 | 0.30 + 0.01 | 88.17 + 6.14 * | 1.20 + 0.04 | 0.92 + 0.09 | 0.63 + 0.02 | 0.23 + 0.01 | ||
| Zn | 0.05 + 0.23 | 56.65 + 1.16 | 35.10 + 0.51 | 21.55 + 0.77 | 66.50 + 3.09 | 119.17 + 16.25 | 175.50 + 12.24 | 0.30 + 0.02 | 80.83 + 13.37 * | 1.10 + 0.06 | 1.11 + 0.17 | 0.59 + 0.03 | 0.21 + 0.02 | ||
| Mn | 0.30 + 0.28 | 57.32 + 1.53 * | 35.57 + 0.91 | 21.75 + 0.69 | 69.67 + 2.35 | 115.83 + 13.47 | 195.00 + 21.41 | 0.28 + 0.02 | 122.00 + 16.29 | 1.14 + 0.19 | 1.03 + 0.08 | 0.65 + 0.03 | 0.19 + 0.04 | ||
| M4A | 0.32 + 0.20 | 53.63 + 1.45 | 34.00 + 0.50 | 19.63 + 1.31 | 71.67 + 3.87 | 140.67 + 25.29 | 158.00 + 11.17 | 0.27 + 0.02 | 102.50 + 14.57 | 1.26 + 0.06 | 0.96 + 0.13 | 0.64 + 0.04 | 0.22 + 0.01 | ||
| M4B | 1.06 + 0.37 * | 54.70 + 1.36 | 35.04 + 0.51 | 19.66 + 1.23 | 79.80 + 2.94 | 113.20 + 19.07 | 242.00 + 18.17 * | 0.31 + 0.02 * | 121.60 + 15.62 | 1.49 + 0.053 | 0.62 + 0.08 | 0.81 + 0.03 * | 0.26 + 0.01 * | ||
| M3 | 0.27 + 0.25 | 53.55 + 1.12 | 34.37 + 0.30 | 19.18 + 1.09 | 63.33 + 3.88 | 143.00 + 32.73 | 152.67 + 15.17 | 0.28 + 0.02 | 114.67 + 18.99 | 1.07 + 0.04 | 0.94 + 0.04 | 0.55 + 0.02 | 0.22 + 0.01 | ||
| Group | Electrolyte Parameters | Thyroxine Parameters | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Na | Ca | Mg | PHOS | Fe | FT3 | FT4 | T3 | T4 | |
| Con | 139.47 ± 0.61 | 2.64 ± 0.02 | 1.12 ± 0.04 | 2.59 ± 0.08 | 36.17 ± 1.14 | 2.07 ± 0.01 | 12.31 ± 0.48 | 0.61 ± 0.03 | 56.73 ± 2.05 |
| M8 | 139.16 ± 0.14 | 2.72 ± 0.03 | 1.16 ± 0.02 | 2.63 ± 0.12 | 35.80 ± 0.97 | 2.04 ± 0.15 | 12.41 ± 0.92 | 0.65 ± 0.04 | 57.02 ± 3.87 |
| M5 | 140.05 ± 0.36 | 2.79 ± 0.04 | 1.21 ± 0.04 | 2.77 ± 0.10 | 34.83 ± 1.33 | 2.10 ± 0.10 | 10.75 ± 0.56 | 0.69 ± 0.03 | 48.71 ± 0.10 |
| Cr | 140.37 ± 0.38 | 2.71 ± 0.04 | 1.08 ± 0.03 | 2.70 ± 0.08 | 36.50 ± 3.42 | 1.94 ± 0.06 | 12.03 ± 0.70 | 0.58 ± 0.02 | 54.76 ± 3.32 |
| Cu | 141.07 ± 0.22 | 2.78 ± 0.03 | 1.12 ± 0.02 | 2.68 ± 0.05 | 32.67 ± 1.36 | 2.10 ± 0.07 | 11.57 ± 0.73 | 0.63 ± 0.04 | 55.04 ± 3.37 |
| Zn | 140.73 ± 0.47 | 2.80 ± 0.05 | 1.17 ± 0.04 | 2.63 ± 0.09 | 36.83 ± 2.18 | 1.89 ± 0.07 | 10.61 ± 0.35 | 0.60 ± 0.02 | 58.592 ± 4.56 |
| Mn | 124.70 ± 15.01 | 2.74 ± 0.04 | 1.19 ± 0.05 | 2.78 ± 0.05 | 35.83 ± 1.45 | 1.85 ± 0.07 | 11.93 ± 0.70 | 0.55 ± 0.02 | 56.43 ± 5.29 |
| M4A | 140.10 ± 0.59 | 2.69 ± 0.05 | 1.12 ± 0.03 | 2.67 ± 0.021 | 36.17 ± 1.40 | 1.97 ± 0.18 | 13.04 ± 1.03 | 0.62 ± 0.03 | 56.42 ± 3.28 |
| M4B | 141.32 ± 0.61 | 2.76 ± 0.02 * | 1.21 ± 0.06 | 2.73 ± 0.08 * | 45.00 ± 3.44 | 1.80 ± 0.08 | 13.64 ± 0.95 | 0.62 ± 0.04 | 70.67 ± 6.26 * |
| M3 | 139.60 ± 0.90 | 2.73 ± 0.03 | 1.15 ± 0.03 | 2.68 ± 0.04 | 37.83 ± 1.42 | 1.99 ± 0.03 | 11.90 ± 1.05 | 0.63 ± 0.03 | 51.78 ± 4.41 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, H.; Li, Z.; Fiati Kenston, S.S.; Shi, H.; Wang, Y.; Song, X.; Gu, Y.; Barber, T.; Aldinger, J.; Zou, B.; et al. Joint Toxicity of Different Heavy Metal Mixtures after a Short-Term Oral Repeated-Administration in Rats. Int. J. Environ. Res. Public Health 2017, 14, 1164. https://doi.org/10.3390/ijerph14101164
Su H, Li Z, Fiati Kenston SS, Shi H, Wang Y, Song X, Gu Y, Barber T, Aldinger J, Zou B, et al. Joint Toxicity of Different Heavy Metal Mixtures after a Short-Term Oral Repeated-Administration in Rats. International Journal of Environmental Research and Public Health. 2017; 14(10):1164. https://doi.org/10.3390/ijerph14101164
Chicago/Turabian StyleSu, Hong, Zhou Li, Samuel Selorm Fiati Kenston, Hongbo Shi, Yafei Wang, Xin Song, Yuanliang Gu, Tabatha Barber, Joni Aldinger, Baobo Zou, and et al. 2017. "Joint Toxicity of Different Heavy Metal Mixtures after a Short-Term Oral Repeated-Administration in Rats" International Journal of Environmental Research and Public Health 14, no. 10: 1164. https://doi.org/10.3390/ijerph14101164





