Analysis of Transmission and Control of Tuberculosis in Mainland China, 2005–2016, Based on the Age-Structure Mathematical Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Model Formulation
2.3. Theoretical Results of Model (2)
- Model (2) has the following positively invariant set:
- Making use of the next generation matrix (see [29]), we obtained the basic reproduction number of Model (2) as follows:
- This model has a disease-free equilibrium , whereand the endemic equilibrium , which is determined by the following equations
- If , the disease-free equilibrium is globally asymptotically stable.
3. Numerical Simulations and Sensitivity Analysis
3.1. Parameter Estimation
3.2. Numerical Simulations from 2005 to 2016
3.3. Uncertainty and Sensitivity Analysis of
3.4. Feasibility Assessment of Reaching WHO End TB Strategy
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
Appendix A.1. Proof of the Positively Invariant Set
Appendix A.2. Proof of the Existence of Positive Steady State
Appendix A.3. Proof of the Global Stability of P0
References
- Blower, S.M.; Daley, C.L. Problems and solutions for the stop tb partnership. Lancet Infect. Dis. 2002, 2, 374–376. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report. 2013. Available online: http://www.who.int/tb/publications/global_report/en/ (accessed on 13 August 2017).
- Wang, Y. The fifth national tuberculosis epidemiological survey in 2010. Chin. J. Autituberc. 2012, 8, 485–508. [Google Scholar]
- Smith, J.P.; Strauss, S.; Zhao, Y.H. Healthy Aging in China. J. Econ. Ageing 2014, 4, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Houben, M.G.; Wu, C.Y.; Rhines, A.S.; Denholm, J.T.; Gomez, G.B.; Hippner, P. Feasibility of achieving the 2025 WHO global tuberculosis target in South Africa, China, and India: A combined analysis of 11 mathematical models. Lancet Glob. Health 2016, 4, e806–e815. [Google Scholar] [CrossRef]
- Li, M.T.; Sun, G.Q.; Wu, Y.F.; Zhang, J.; Jin, Z. Transmission dynamics of a multi-group brucellosis model with mixed cross infection in public farm. Appl. Math. Comput. 2014, 237, 582–594. [Google Scholar] [CrossRef]
- Waaler, H.T.; Gese, A.; Anderson, S. The use of mathematical models in the study of the epidemiology of tuberculosis. Am. J. Public Health 1962, 52, 1002–1013. [Google Scholar] [CrossRef]
- Li, M.T.; Sun, G.Q.; Zhang, J.; Jin, Z.; Sun, X.D.; Wang, Y.M.; Huang, B.X.; Zheng, Y.H. Transmission dynamics and control for a brucellosis model in hinggan league of inner mongolia, China. Math. Biosci. Eng. 2014, 5, 1115–1137. [Google Scholar]
- Li, M.T.; Jin, Z.; Sun, G.Q.; Zhang, J. Modeling direct and indirect disease transmission using multi-group model. J. Math. Anal. Appl. 2014, 446, 1292–1309. [Google Scholar] [CrossRef]
- Blower, S.M.; McLean, A.R.; Porco, T.C. The intrinsic transmission dynamics of tuberculosis epidemics. Nat. Med. 1995, 8, 815–821. [Google Scholar] [CrossRef]
- Blower, S.M.; Small, P.M.; Hopewell, P.C. Control strategies for tuberculosis epidemics: New models for old problems. Science 1996, 273, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Dye, C.; Watt, C.J.; Bleed, D. Low access to a high-cure therapy: A challenge for international tuberculosis control. Bull. World Health Organ. 2002, 80, 437–444. [Google Scholar] [PubMed]
- Jia, Z.W.; Tang, G.Y.; Jin, Z. Modeling the impact of immigration on the epidemiology of tuberculosis. Theor. Popul. Biol. 2008, 73, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Bhunu, C.P.; Garira, W.; Mukandavire, Z.; Zimba, M. Tuberculosis transmission model with chemoprophylaxis and treatment. Bull. Math. Biol. 2008, 70, 1163–1191. [Google Scholar]
- Bowong, S.; Kurths, J. Modeling and analysis of the transmission dynamics of tuberculosis without and with seasonality. Nonlinear Dyn. 2012, 67, 2027–2051. [Google Scholar] [CrossRef]
- Cao, H.; Zhou, Y.C. The discrete age-structured SEIT model with application to tuberculosis transmission in China. Math. Comput. Model. 2012, 55, 385–395. [Google Scholar] [CrossRef]
- Feng, Z.L.; Castillo, C.C.; Capurro, A.F. A model for tuberculosis with exogenous reinfection. Theor. Popul. Biol. 2000, 57, 235–247. [Google Scholar]
- Kar, T.K.; Mondal, P.K. Global dynamics of a tuberculosis epidemic model and the influence of backward bifurcation. J. Math. Model. Algor. 2012, 11, 433–459. [Google Scholar] [CrossRef]
- Liu, L.J.; Zhao, X.Q.; Zhou, Y.C. A tuberculosis model with seasonality. Bull. Math. Biol. 2010, 72, 931–952. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Shi, X.Y.; Cheng, H.D. Modelling and stability analysis for a tuberculosis model with healthy education and treatment. Comput. Appl. Math. 2013, 32, 245–260. [Google Scholar] [CrossRef]
- Whang, S.; Choi, S.; Jung, E. A dynamic model for tuberculosis transmission and optimal treatment strategies in South Korea. J. Theor. Biol. 2011, 270, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Okuonghae, D.; Omosigho, S.E. Analysis of a mathematical model for tuberculosis: What could be done to increase case detection. J. Theor. Biol. 2011, 269, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Mccluskey, C.C.; Driessche, P.V. Global analysis of two tuberculosis models. J. Dyn. Diff. Equ. 2004, 16, 139–166. [Google Scholar] [CrossRef]
- Huynh, G.H.; Klein, D.J.; Chin, D.P.; Wagner, B.G.; Eckhoff, P.A.; Liu, R.Z.; Wang, L.X. Tuberculosis control strategies to reach the 2035 global targets in China: The role of changing demographics and reactivation dsease. BMC Med. 2015, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Garnett, G.P. Mathematical modelling of the epidemiology of tuberculosis. Modeling Parasite Transformation and Control 673. Adv. Exp. Med. Biol. 2010, 673, 127–140. [Google Scholar] [PubMed]
- World Health Organization. Tuberculosis. 2017. Available online: http://www.who.int/mediacentre/factsheets/fs104/en/ (accessed on 13 August 2017).
- Lowrie, D.B. Tuberculosis vaccine research in China. Emerg. Microbes Infect. 2012, 1, e7. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.; Donnelly, C.A.; Cauchemez, S.; Hanage, W.P.; Van Kerkhove, M.D.; Hollingsworth, T.D.; Griffin, J.; Baggaley, R.F.; Jenkins, H.E.; Lyons, E.J.; et al. Pandemic Potential of a Strain of Influenza A (H1N1): Early Findings. Science 2009, 324, 1557–1561. [Google Scholar] [CrossRef] [PubMed]
- Van den Driessche, P.; Watmough, J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 2002, 180, 29–48. [Google Scholar] [CrossRef]
- Millet, J.P.; Shaw, E.; Orcau, A.; Casals, M.; Miro, J.M.; Cayla, J.A. The Barcelona Tuberculosis Recurrence Working Group’ Tuberculosis Recurrence after Completion Treatment in a European City: Reinfection or Relapse? PLoS ONE 2013, 8, e64898. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.D.; Liu, J.J.; Chin, D.P. Progress in tuberculosis control and the evolving public-health system in china. Lancet 2007, 369, 691–696. [Google Scholar] [CrossRef]
- China Population Statistic Yearbook. 2017. Available online: http://www.stats.gov.cn/tjsj/ndsj/ (accessed on 13 August 2017).
- National Scientific Data Sharing Platform for Population and Health. 2017. Available online: http://www.ncmi.cn/info/69/1544 (accessed on 13 August 2017).
- Devore, J.L. Probability and Statistics for Engineering and the Sciences, 8th ed.; Cengage Learning: Boston, MA, USA, 2011; pp. 508–510. [Google Scholar]
- Blower, S.M.; Dowlatabadi, H. Sensitivity and uncertainty analysis of complex models of disease transmission: An HIV model as an example. Int. Stat. Rev. 1994, 62, 229–243. [Google Scholar] [CrossRef]
- Sanchez, M.A.; Blower, S.M. Uncertainty and sensitivity analysis of the basic reproductive rate: Tuberculosis as an example. Am. J. Epidemiol. 1997, 145, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO End TB Strategy. 2014. Available online: http://www.who.int/tb/post2015$_$strategy/en/ (accessed on 13 August 2017).
- Ziv, E.; Daley, C.L.; Blower, S.M. Early therapy for latent tuberculosis infection. Am. J. Epidemiol. 2001, 153, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Ziv, E.; Daley, C.L.; Blower, S.M. Potential public health impact of new tuberculosis vaccines. Emerg. Infect. Dis. 2004, 10, 1529–1535. [Google Scholar]
- LaSalle, J.P. The Stability of Dynamical Systems, Regional Conference Series in Applied Mathematics; SIAM: Philadelphia, PA, USA, 1976. [Google Scholar]
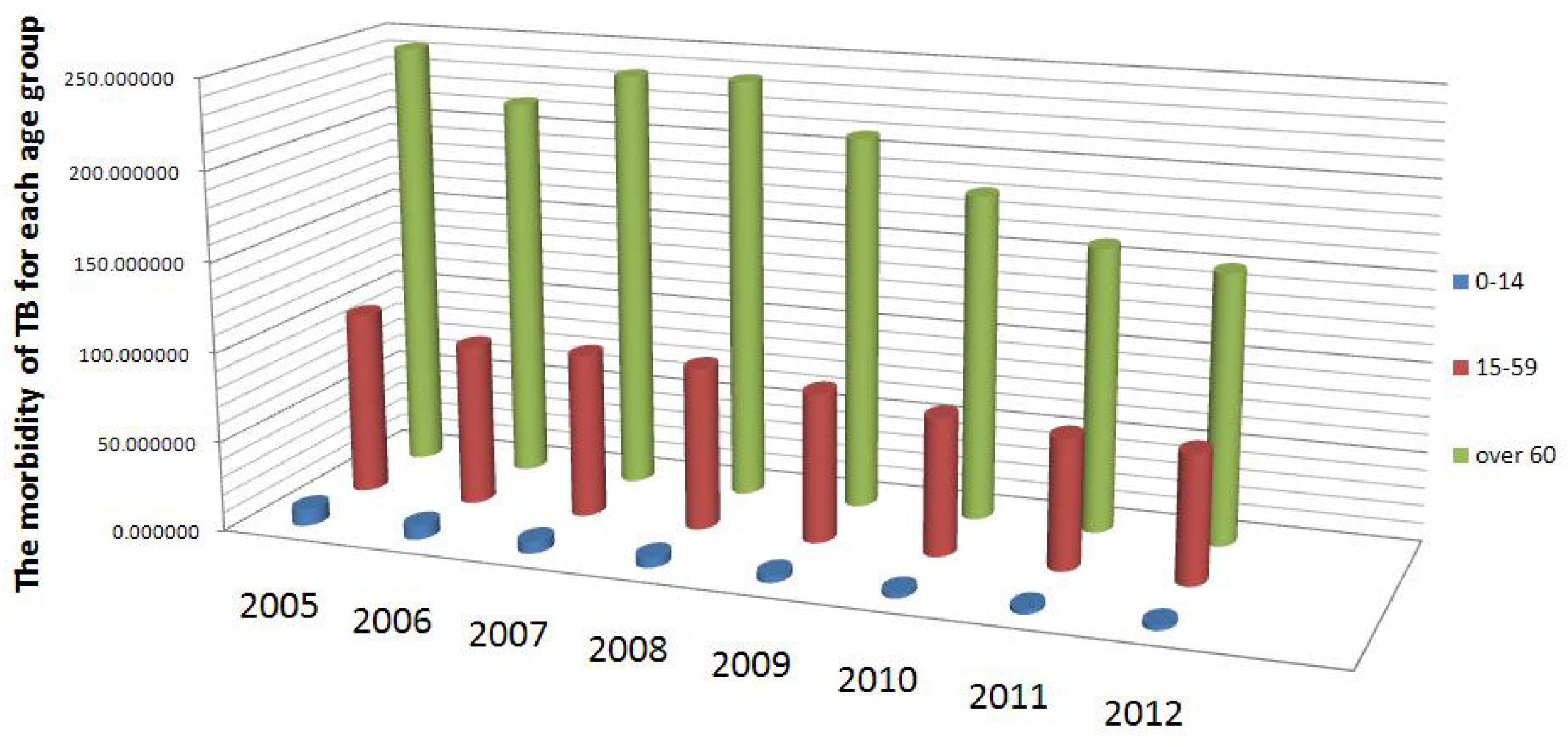

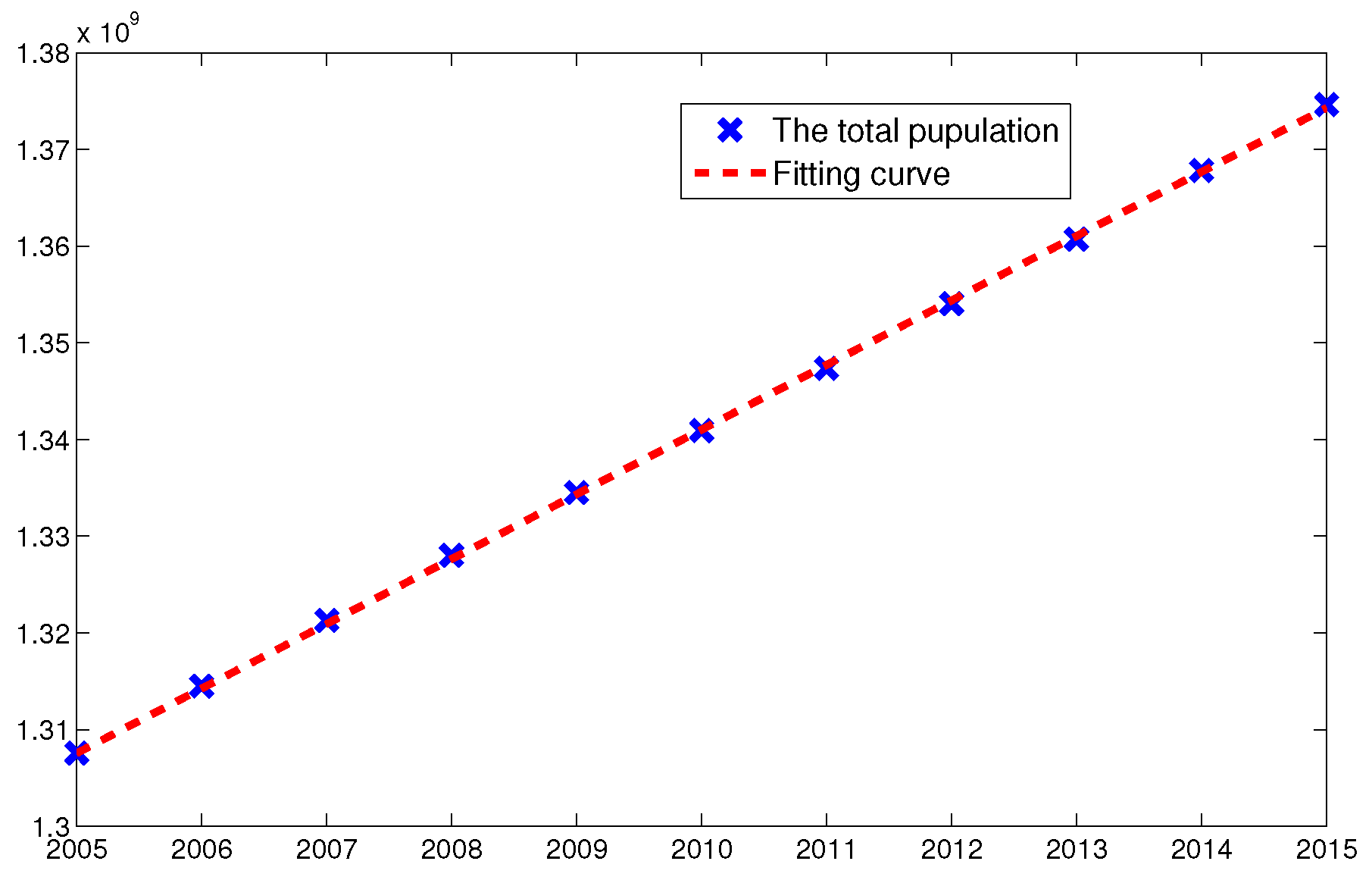
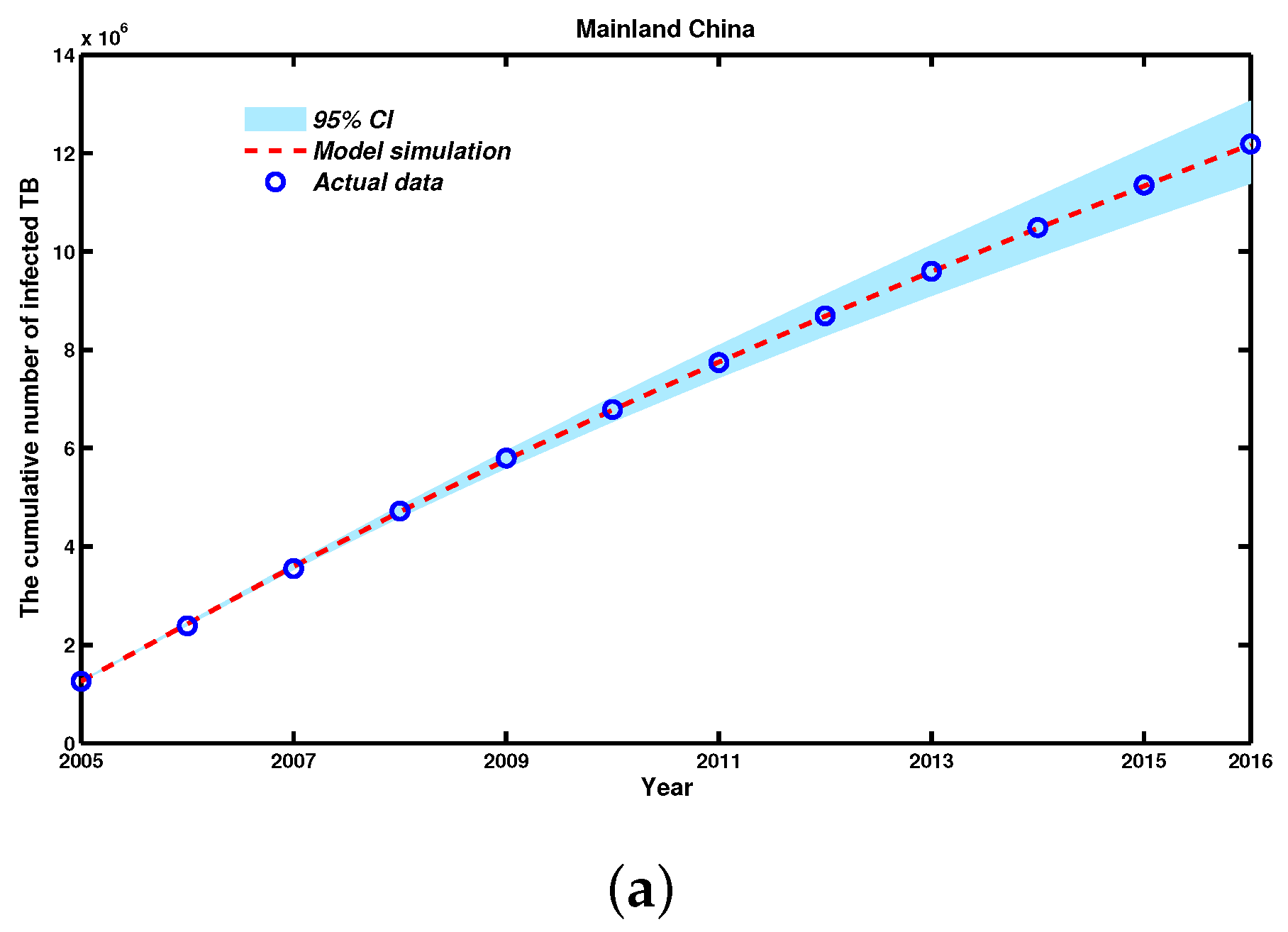
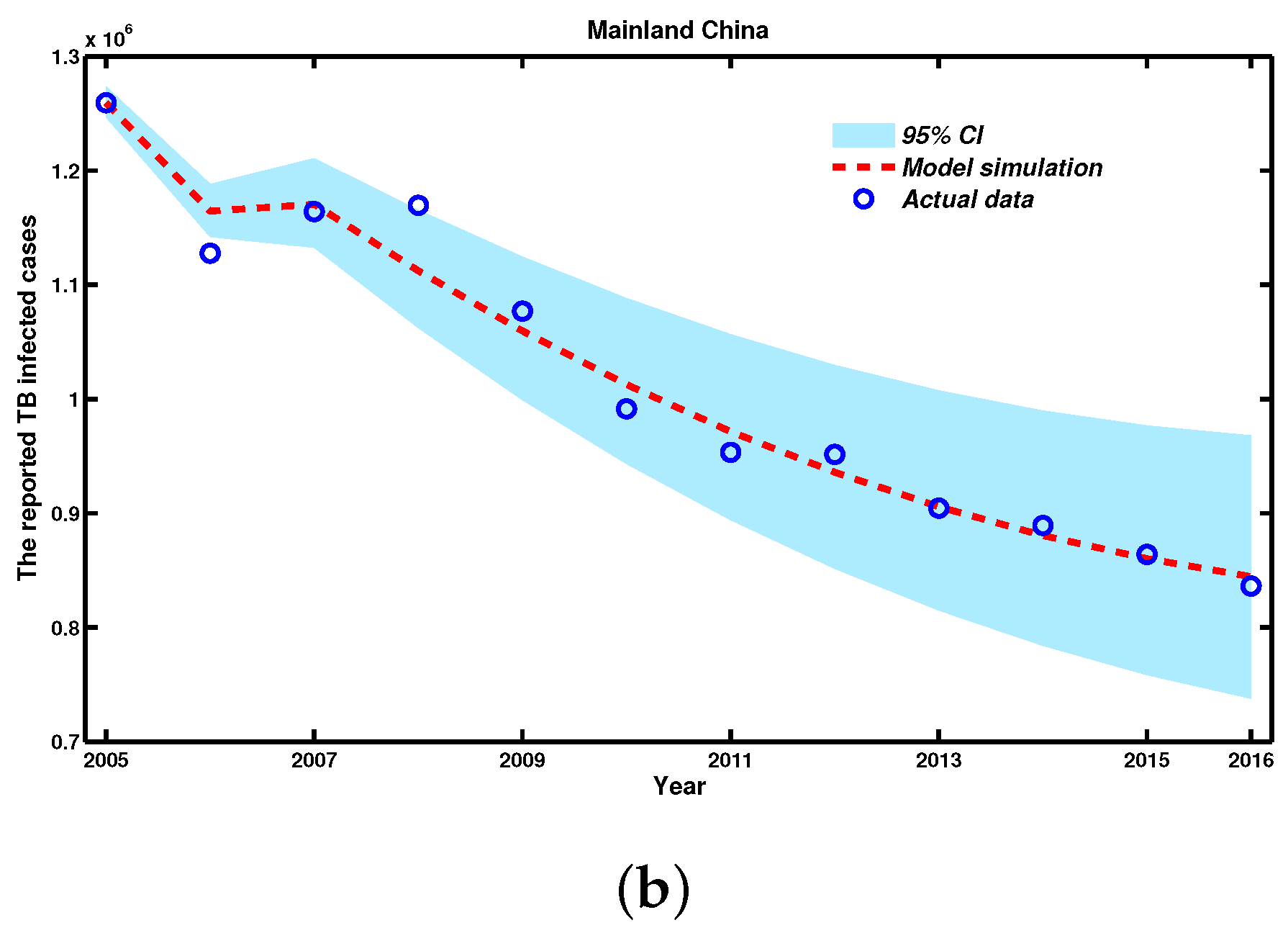
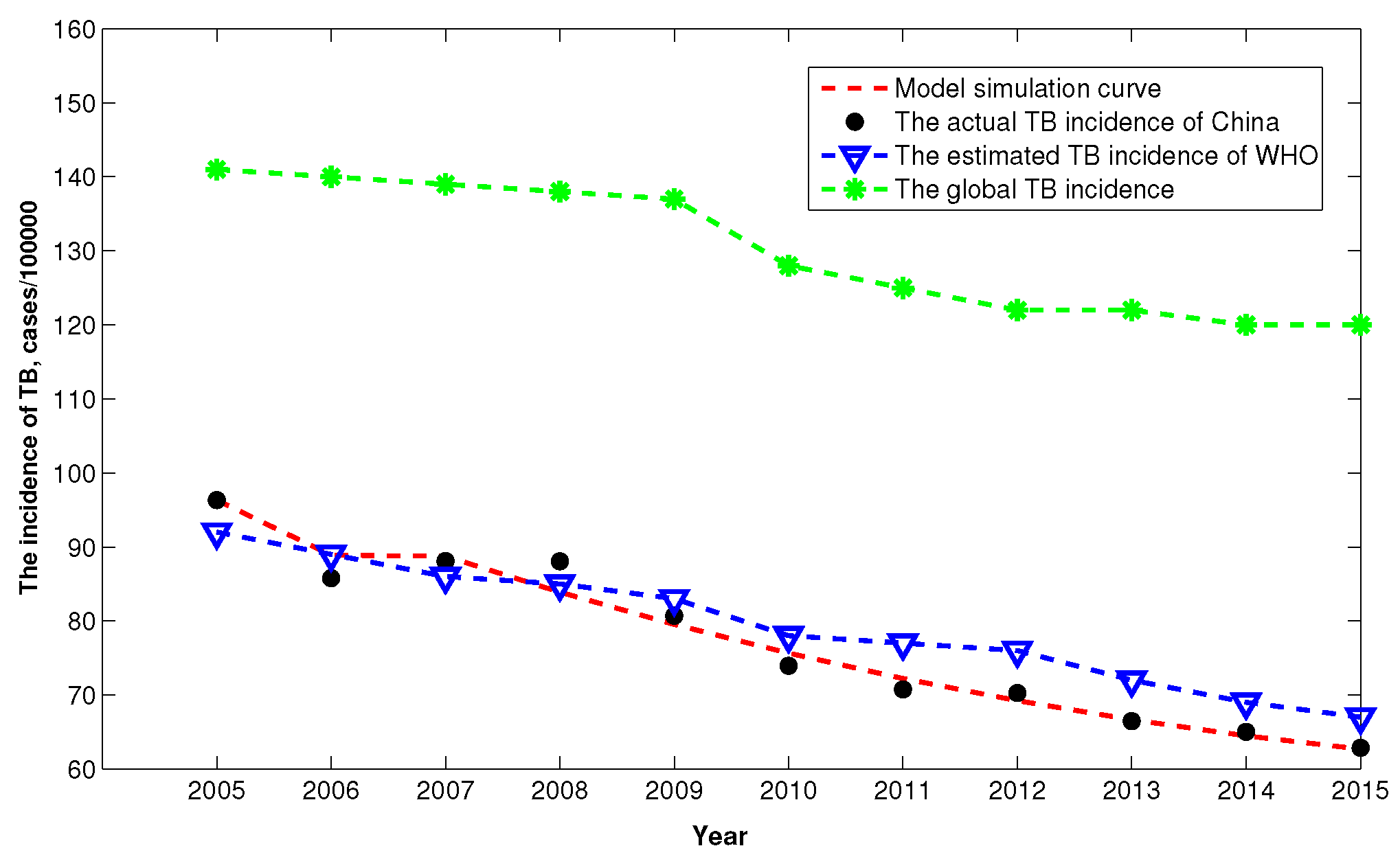
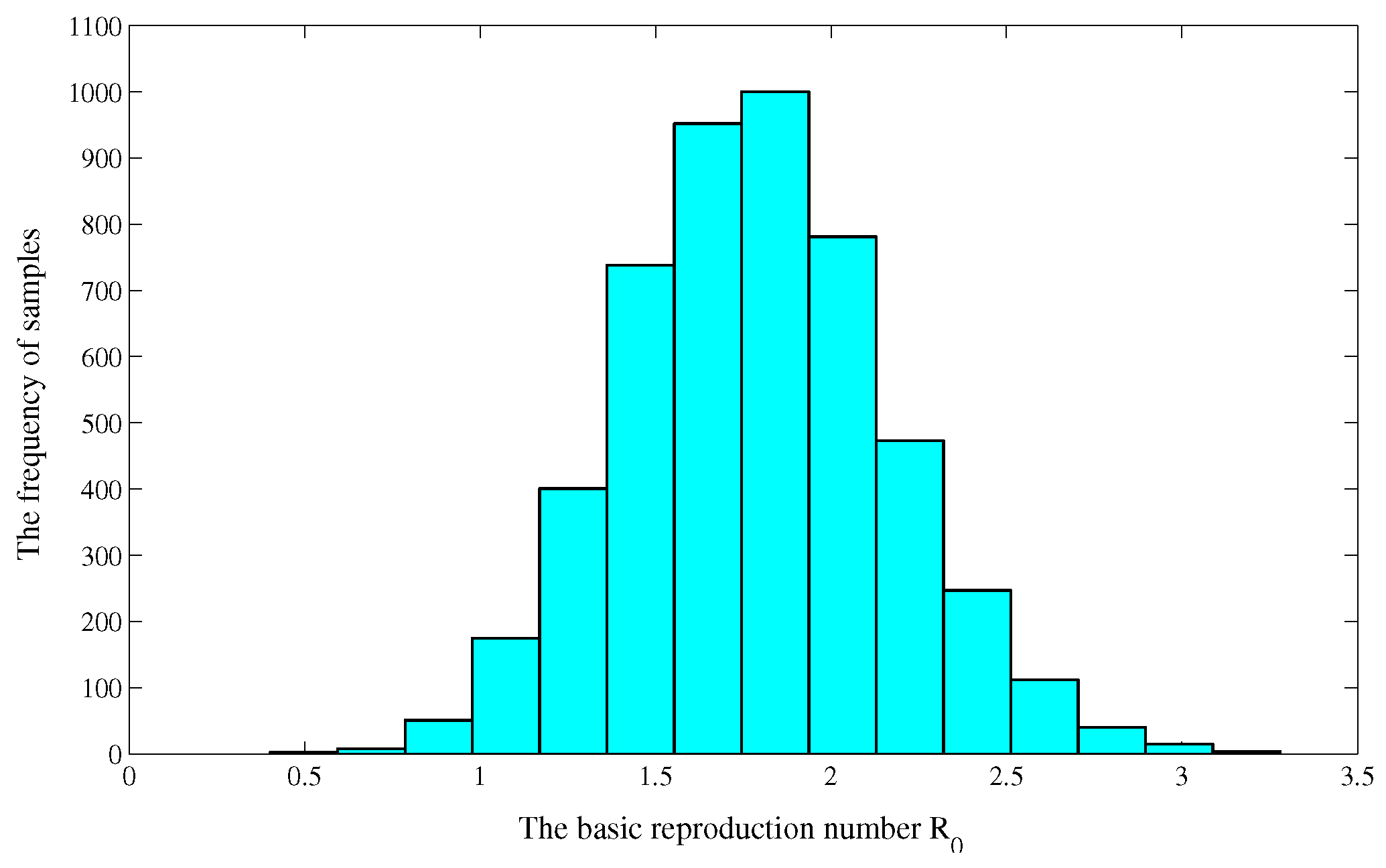
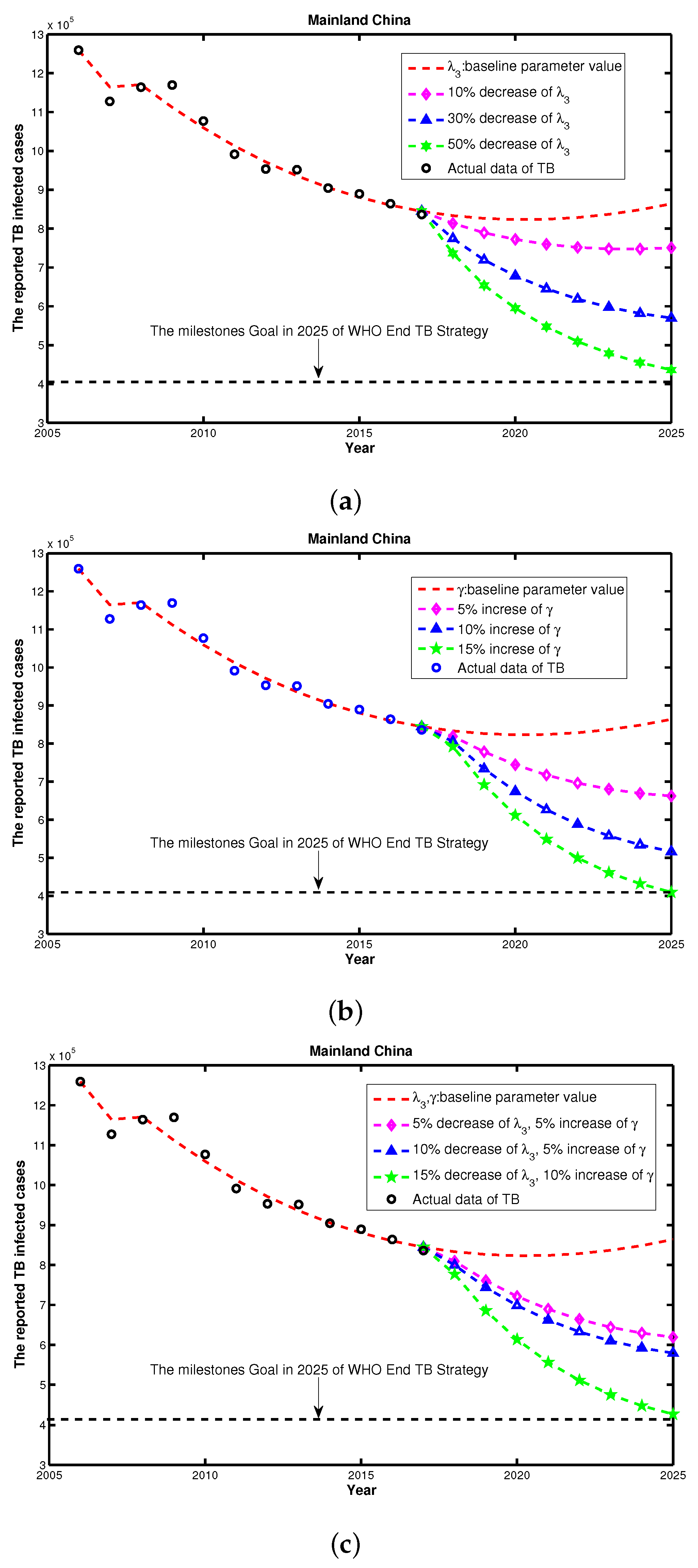
| Year | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 |
| Cases | 1,259,308 | 1,127,571 | 1,163,959 | 1,169,540 | 953,275 | 951,508 |
| Year | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 |
| Cases | 1,076,938 | 991,350 | 889,381 | 864,015 | 864,015 | 836,236 |
| Parameter | Value | Confidence Interval | Source |
|---|---|---|---|
| A | [32] | ||
| d | (0.00688, 0.00702) | [32] | |
| (0.0013, 0.0021) | [32] | ||
| (0.0023, 0.0024) | [32] | ||
| [32] | |||
| − | Fitting | ||
| − | Fitting | ||
| − | [2] | ||
| p | [10] | ||
| − | [10] | ||
| − | [3] | ||
| v | 6 | − | [33] |
| − | [30] | ||
| − | [3] | ||
| − | Fitting | ||
| − | Fitting | ||
| − | Fitting |
| Parameters | PRCC | p-Value |
|---|---|---|
| A | 0.9961 | 0.0005 |
| −0.7482 | 0.0009 | |
| 0.6141 | ||
| −0.5265 | 0.0001 | |
| 0.5195 | ||
| 0.4820 | ||
| 0.1808 | 0.0036 | |
| 0.0175 | 0.0012 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Li, M.; Yuan, S. Analysis of Transmission and Control of Tuberculosis in Mainland China, 2005–2016, Based on the Age-Structure Mathematical Model. Int. J. Environ. Res. Public Health 2017, 14, 1192. https://doi.org/10.3390/ijerph14101192
Zhao Y, Li M, Yuan S. Analysis of Transmission and Control of Tuberculosis in Mainland China, 2005–2016, Based on the Age-Structure Mathematical Model. International Journal of Environmental Research and Public Health. 2017; 14(10):1192. https://doi.org/10.3390/ijerph14101192
Chicago/Turabian StyleZhao, Yu, Mingtao Li, and Sanling Yuan. 2017. "Analysis of Transmission and Control of Tuberculosis in Mainland China, 2005–2016, Based on the Age-Structure Mathematical Model" International Journal of Environmental Research and Public Health 14, no. 10: 1192. https://doi.org/10.3390/ijerph14101192





