Distributions and Sources of Polycyclic Aromatic Hydrocarbons (PAHs) in Soils around a Chemical Plant in Shanxi, China
Abstract
:1. Introduction
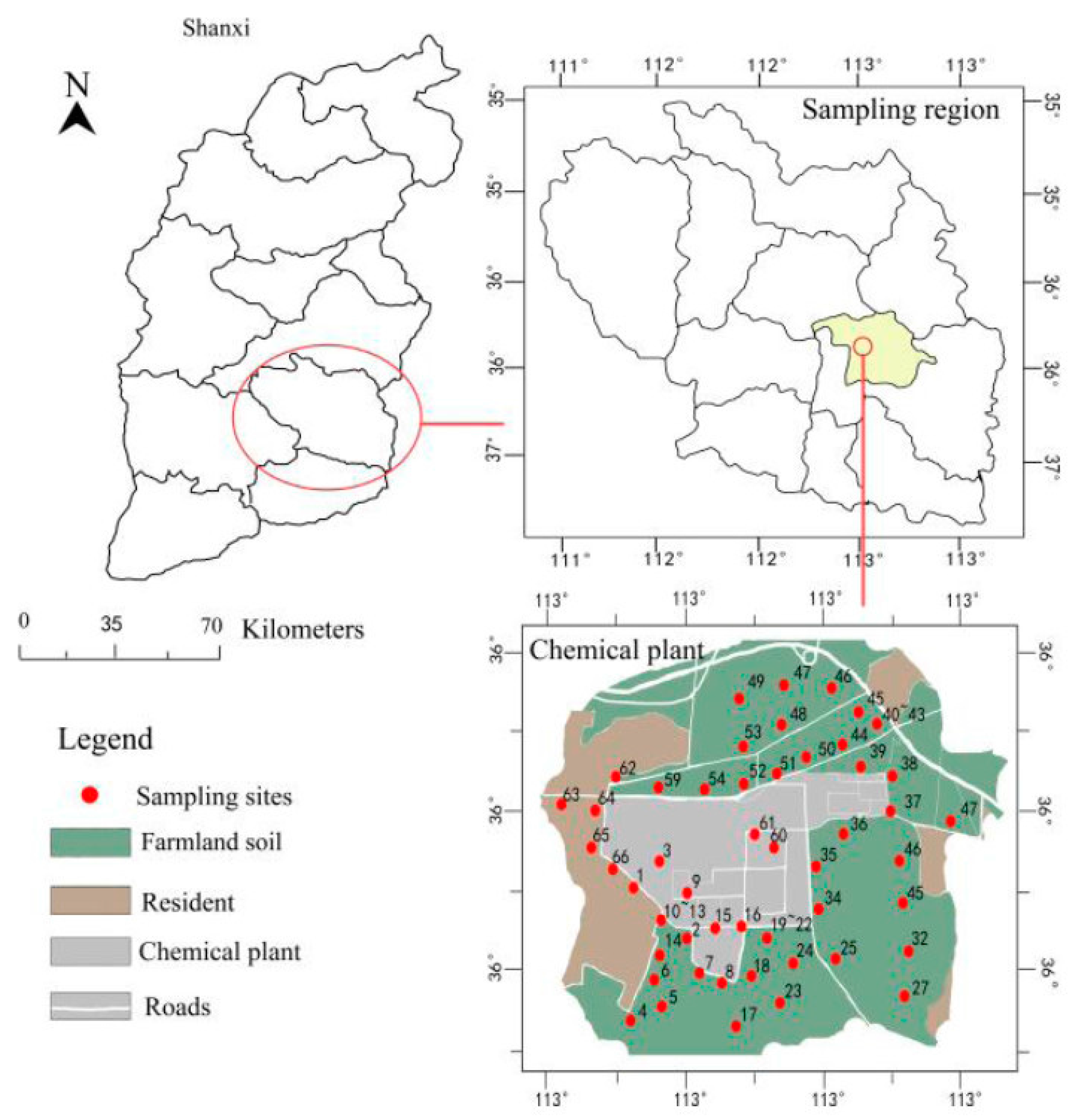
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Extraction
2.3. PAHs Analysis
2.4. Quality Control
2.5. Data Analysis
3. Results
3.1. Characteristics of PAHs Concentrations in Surface Soils
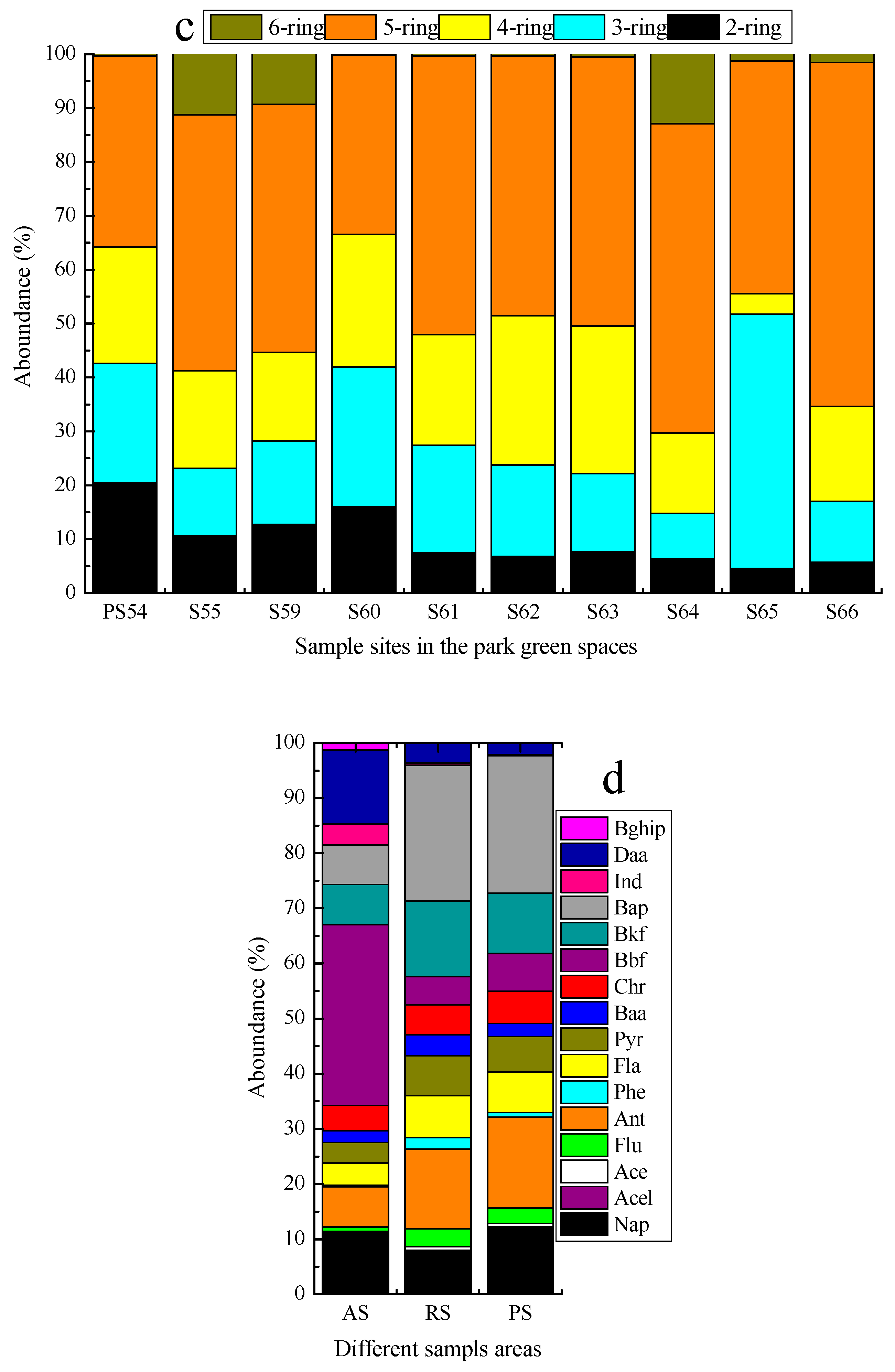
3.2. Characteristics of PAHs Component in Surface Soils
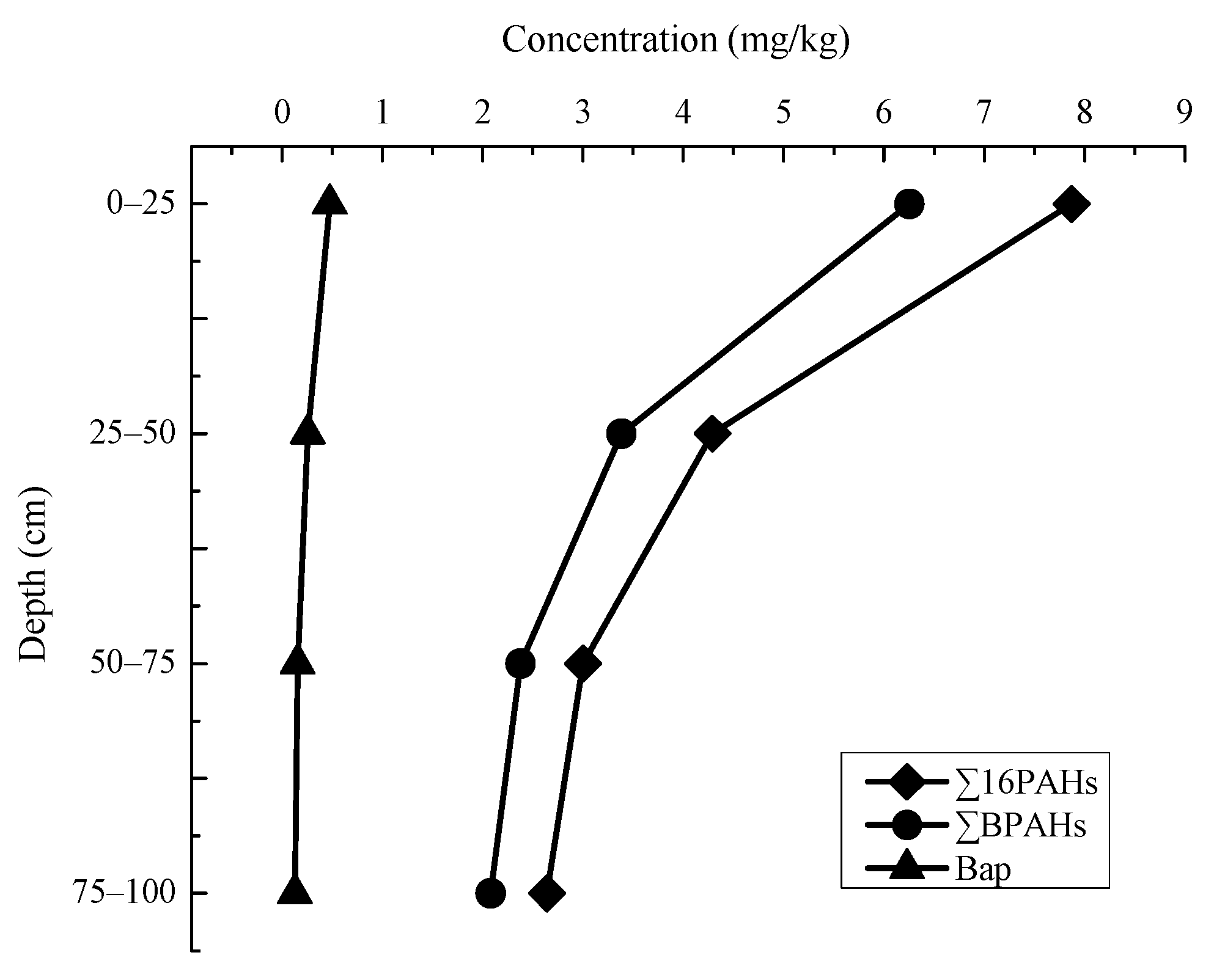
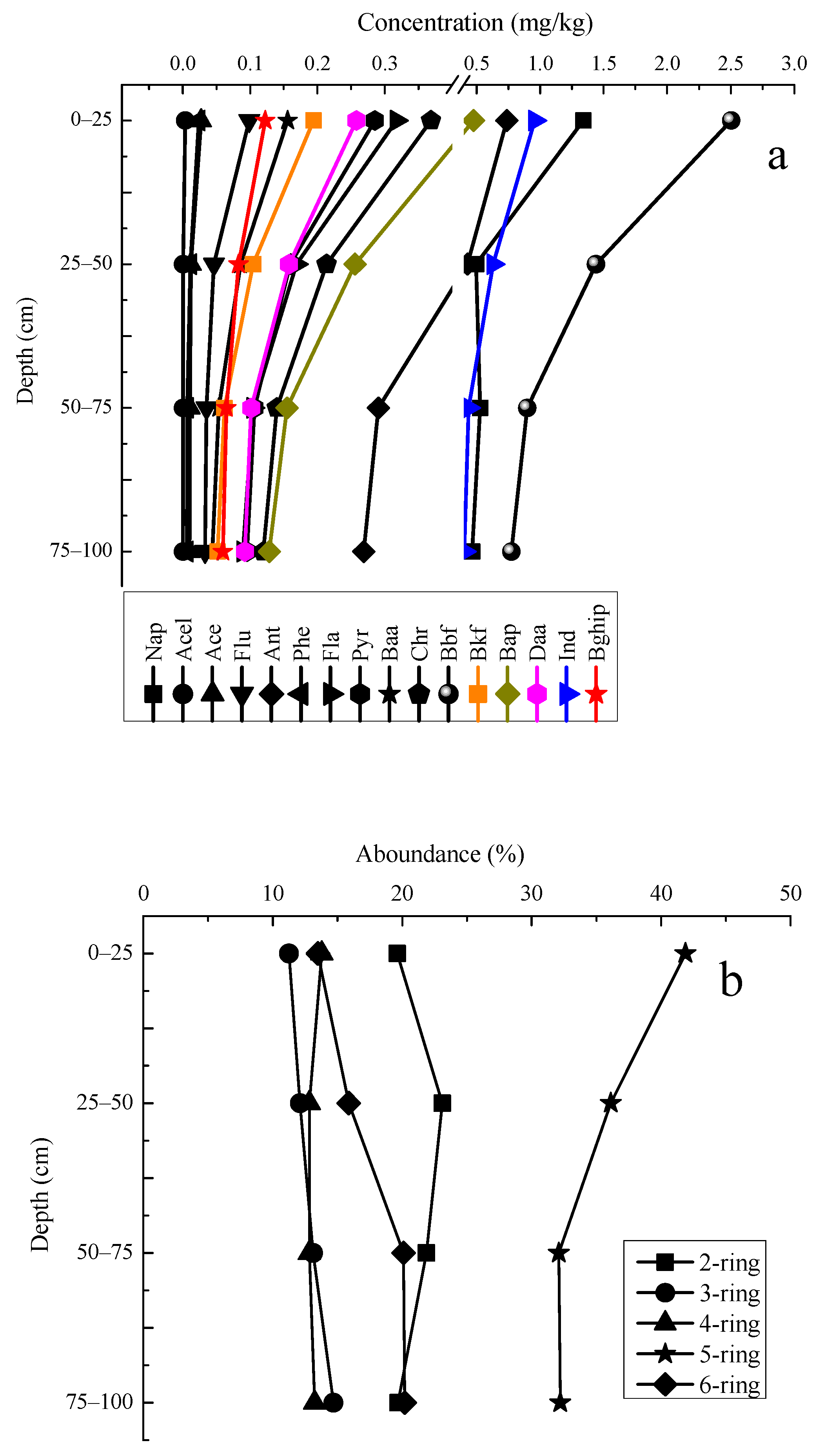
3.3. Characteristics of PAHs Concentration in Vertical Distribution
3.4. Characteristics of PAHs Component in Vertical Distribution
3.5. Identification of PAH Sources
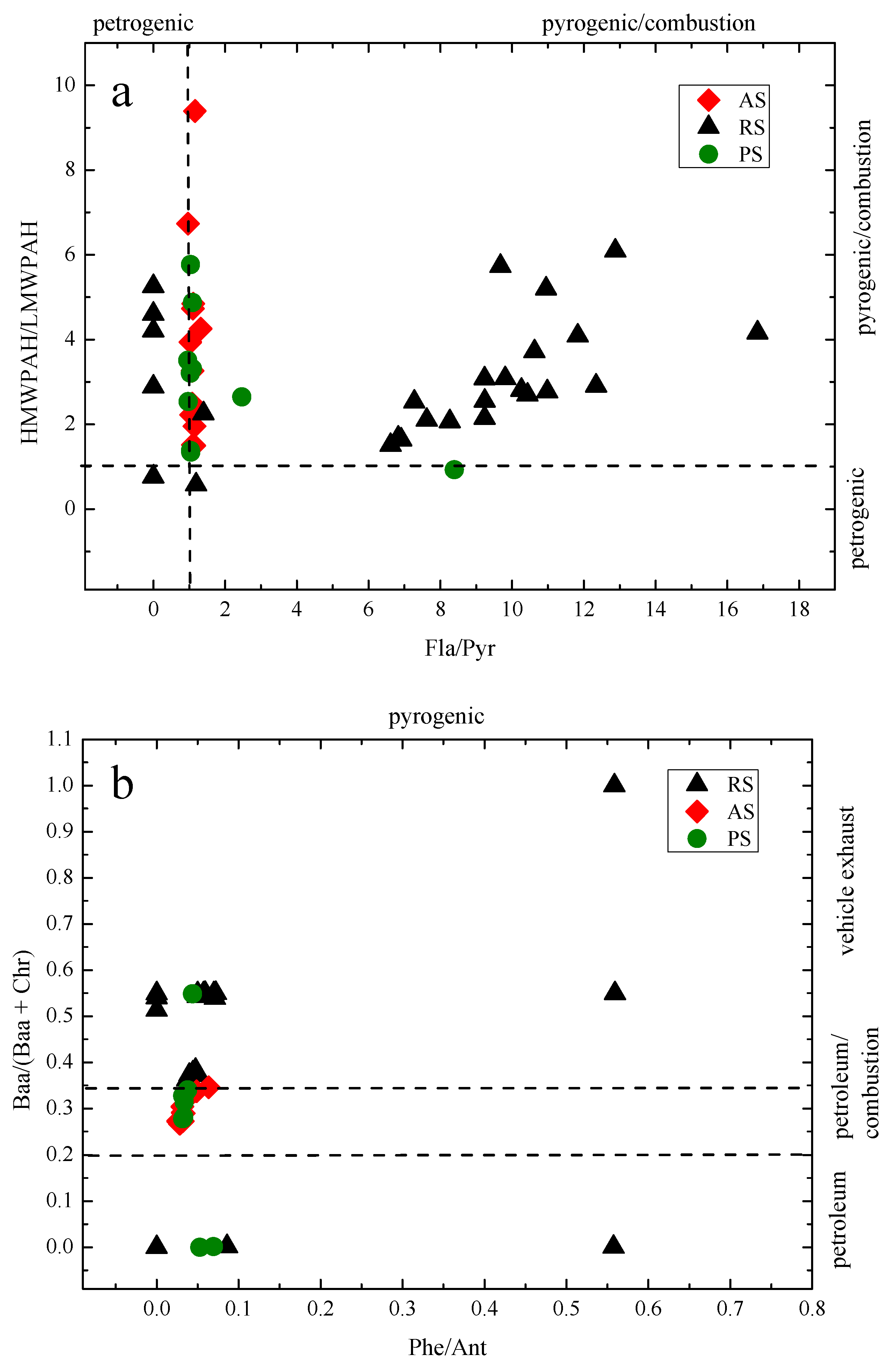
3.5.1. Diagnostic Ratio
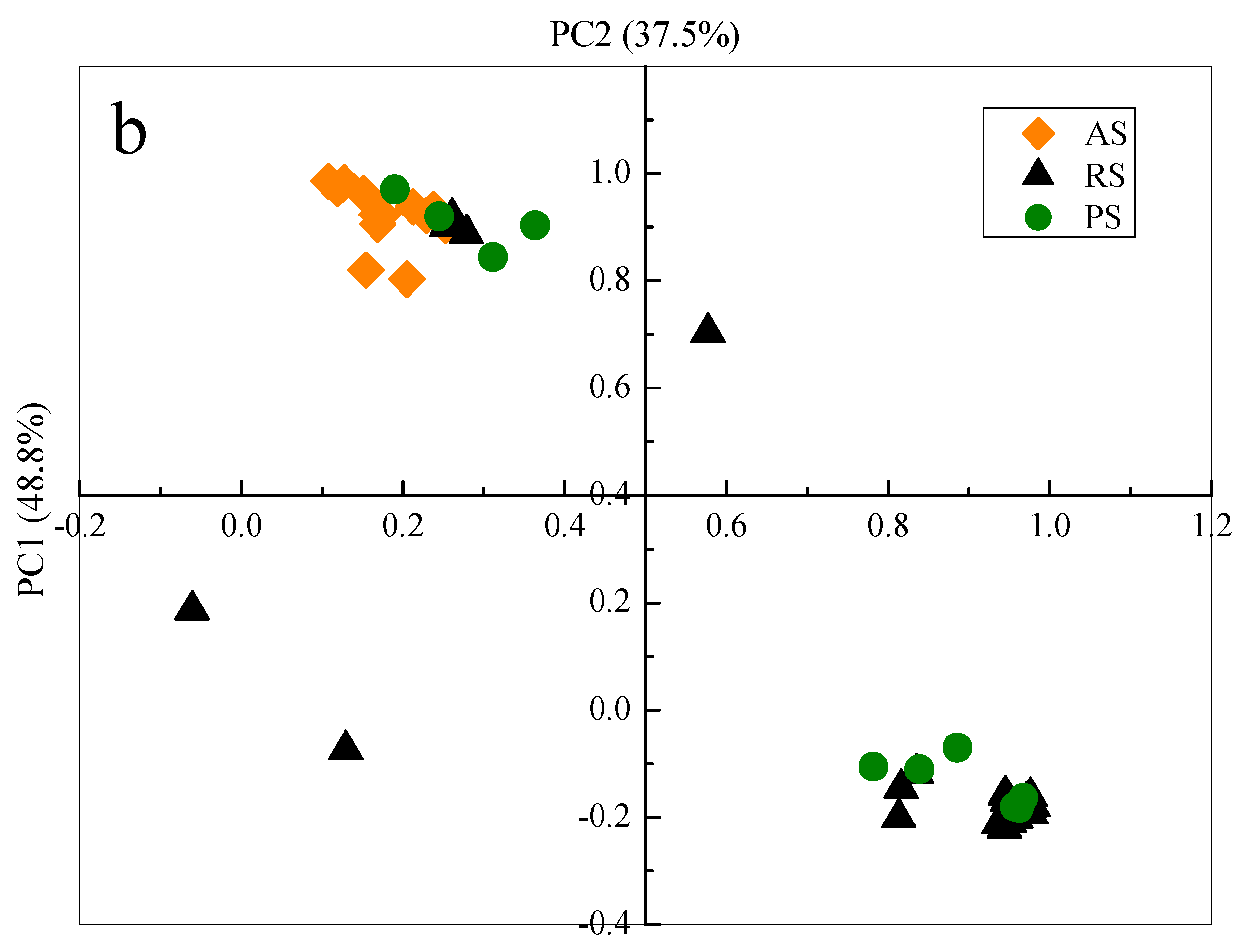
3.5.2. Principal Component Analysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- National Research Council (US). Committee on Research Advisory to the US Department of Agriculture. Report of the Committee on Research Advisory to the US Department of Agriculture; National Academies: Washington, DC, USA, 1972. [Google Scholar]
- International Agency for Research on Cancer. IARC Handbooks of Cancer Prevention; International Agency for Research on Cancer: Beverly Hills, CA, USA, 2002. [Google Scholar]
- Loprieno, N. International Agency for Research on Cancer (IARC) monographs on the evaluation of carcinogenic risk of chemicals to man: “Relevance of data on mutagenicity”. Mutat. Res. 1975, 31, 201. [Google Scholar] [CrossRef]
- Srogi, K. Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: A review. Environ. Chem. Lett. 2007, 5, 169–195. [Google Scholar] [CrossRef]
- Derbyshire, F.J.; Davis, A.; Lin, R. Two-Component Concept of Coal Structure. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1991. [Google Scholar]
- Swaine, D.J. Why trace elements are important. Fuel Process. Technol. 2000, 65, 21–33. [Google Scholar] [CrossRef]
- Stout, S.A.; Emsbo-Mattingly, S.D. Concentration and character of PAHs and other hydrocarbons in coals of varying rank–implications for environmental studies of soils and sediments containing particulate coal. Organ. Geochem. 2008, 39, 801–819. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Liu, G.J.; Xue, J. Study of polycyclic aromatic hydrocarbons (PAHs) and its environmental impact in coal and coal combustion products. J. China Coal Soc. 2005, 30, 97–101. [Google Scholar]
- Hiller, E.; Lachká, L.; Jurkovič, L.; Vozár, J. Polycyclic aromatic hydrocarbons in urban soils from kindergartens and playgrounds in Bratislava, the capital city of Slovakia. Environ. Earth Sci. 2015, 73, 7147–7156. [Google Scholar] [CrossRef]
- Jiao, W.; Lu, Y.; Li, J.; Han, J.; Wang, T.; Luo, W.; Shi, Y.; Wang, G. Identification of sources of elevated concentrations of polycyclic aromatic hydrocarbons in an industrial area in Tianjin, China. Environ. Monit. Assess. 2009, 158, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.; Reimann, C.; Finne, T.E.; Ottesen, R.T.; Arnoldussen, A. PAH-concentrations and compositions in the top 2 cm of forest soils along a 120 km long transect through agricultural areas, forests and the city of Oslo, Norway. Environ. Pollut. 2007, 145, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Ran, Y.; Chen, L. Polycyclic aromatic hydrocarbons (PAHs) in rural soils of Dongjiang River Basin: Occurrence, source apportionment, and potential human health risk. J. Soils Sediments 2014, 14, 110–120. [Google Scholar] [CrossRef]
- Aichner, B.; Glaser, B.; Zech, W. Polycyclic aromatic hydrocarbons and polychlorinated biphenyls in urban soils from Kathmandu, Nepal. Organ. Geochem. 2007, 38, 700–715. [Google Scholar] [CrossRef] [Green Version]
- Bucheli, T.D.; Blum, F.; Desaules, A.; Gustafsson, Ö. Polycyclic aromatic hydrocarbons, black carbon, and molecular markers in soils of Switzerland. Chemosphere 2004, 56, 1061–1076. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.H.; Rui, X.P.; Wu, S.H.; Bai, Z.H.; Zhuang, X.L.; Huang, Z.B. Polycyclic aromatic hydrocarbons in the Dagang Oilfield (China): Distribution, sources, and risk assessment. Int. J. Environ. Res. Public Health 2015, 12, 5775–5791. [Google Scholar] [CrossRef] [PubMed]
- Ugwu, K.E.; Ukoha, P.O. Analysis and sources of polycyclic aromatic hydrocarbons in soil and plant samples of a coal mining area in Nigeria. Bull. Environ. Contam. Toxicol. 2016, 96, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.T.; Miao, Y.; Zhang, Y.; Li, Y.C.; Wu, M.H.; Yu, G. Polycyclic aromatic hydrocarbons (PAHs) in urban soils of the megacity Shanghai: Occurrence, source apportionment and potential human health risk. Sci. Total Environ. 2013, 447, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.A.; Li, Z. Scope of the worldwide effort to regulate pesticide contamination in surface soils. J. Environ. Manag. 2014, 146, 420–443. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.A.; Li, Z. Residential surface soil guidance applied worldwide to the pesticides added to the Stockholm Convention in 2009 and 2011. J. Environ. Manag. 2015, 160, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Morillo, E.; Romero, A.S.; Maqueda, C.; Madrid, L.; Ajmone-Marsan, F.; Grcman, H.; Davidson, C.M.; Hursthouse, A.S.; Villaverde, J. Soil pollution by PAHs in urban soils: A comparison of three European cities. J. Environ. Monit. 2007, 9, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Haugland, T.; Ottesen, R.T.; Volden, T. Lead and polycyclic aromatic hydrocarbons (PAHs) in surface soil from day care centres in the city of Bergen, Norway. Environ. Pollut. 2008, 153, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Nadal, M.; Schuhmacher, M.; Domingo, J.L. Levels of metals, PCBs, PCNs and PAHs in soils of a highly industrialized chemical/petrochemical area: Temporal trend. Chemosphere 2007, 66, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.B.; Luo, Y.M.; Wong, M.H.; Zhao, Q.G.; Zhang, G.L. Distributions and concentrations of PAHs in Hong Kong soils. Environ. Pollut. 2006, 141, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Tang, X.Y.; Zhu, Y.G.; Zheng, M.H.; Miao, Q.L. Contamination of polycyclic aromatic hydrocarbons (PAHs) in urban soils in Beijing, China. Environ. Int. 2005, 31, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Bi, X.; Tan, J.; Sheng, G.; Fu, J. The differences of the size distribution of polycyclic aromatic hydrocarbons (PAHs) between urban and rural sites of Guangzhou, China. Atmos. Res. 2005, 78, 190–203. [Google Scholar] [CrossRef]
- Dai, J.; Li, S.; Zhang, Y.; Wang, R.; Yu, Y. Distributions, sources and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in topsoil at Jinan city, China. Environ. Monit. Assess. 2008, 147, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tian, F.; Yang, M.; Liu, C.; Li, Y.F. Application of positive matrix factorization to identify potential sources of PAHs in soil of Dalian, China. Environ. Pollut. 2009, 157, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Wen, D.; Kuang, Y.; Li, J.; Li, J.; Zuo, W. Concentrations of heavy metals and polycyclic aromatic hydrocarbons in needles of Masson pine (Pinus massoniana L.) growing nearby different industrial sources. J. Environ. Sci. 2010, 22, 1006–1013. [Google Scholar] [CrossRef]
- Maliszewska-Kordybach, B.; Klimkowicz-Pawlas, A.; Smreczak, B.; Stuczyński, T. Relationship between soil concentrations of PAHs and their regional emission indices. Water Air Soil Pollut. 2010, 213, 319–330. [Google Scholar] [CrossRef]
- Nielsen, T.; Jørgensen, H.E.; Jensen, F.P.; Larsen, J.C.; Poulsen, M.; Jensen, A.B.; Schramm, J.; Tønnesen, J. Traffic PAH and Other Mutagens in Air in DENMARK; Ministry of Environment and Energy, Danish Environmental Protection: Copenhagen, Demark, 1995.
- Canadian Council of Ministers of the Environment (CCME). Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health. CCME Soil Quality Index 1.0. Technical Report, 2007, 1–10. Available online: http://www.ccme.ca/en/resources/canadian_environmental_quality_guidelines/calculators.html (accessed on 8 October 2017).
- Ministry of Environmental Protection of the People’s Republic of China. Environmental Quality Standard for Soils (GB15618–2008); Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2008.
- Jennings, A.A.; Li, Z. Residential surface soil guidance values applied worldwide to the original 2001 Stockholm Convention POP pesticides. J. Environ. Manag. 2015, 160, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Yunker, M.B.; Macdonald, R.W.; Vingarzan, R.; Mitchell, R.H.; Goyette, D.; Sylvestre, S. PAHs in the Fraser River basin: A critical appraisal of PAH ratios as indicators of PAH source and composition. Organ. Geochem. 2002, 33, 489–515. [Google Scholar] [CrossRef]
- Galarneau, E. Source specificity and atmospheric processing of airborne PAHs: Implications for source apportionment. Atmos. Environ. 2008, 42, 8139–8149. [Google Scholar] [CrossRef]
- Katsoyiannis, A.; Sweetman, A.J.; Jones, K.C. PAH molecular diagnostic ratios applied to atmospheric sources: A critical evaluation using two decades of source inventory and air concentration data from the UK. Environ. Sci. Technol. 2011, 45, 8897–8906. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.R.; Bzdusek, P.A. PAHs in sediments of the Black River and the Ashtabula River, Ohio: Source apportionment by factor analysis. Water Res. 2005, 39, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Mannino, M.R.; Orecchio, S. Polycyclic aromatic hydrocarbons (PAHs) in indoor dust matter of Palermo (Italy) area: Extraction, GC–MS analysis, distribution and sources. Atmos. Environ. 2008, 42, 1801–1817. [Google Scholar] [CrossRef]
- Essumang, D.K.; Kowalski, K.; Sogaard, E.G. Levels, distribution and source characterization of polycyclic aromatic hydrocarbons (PAHs) in top soils and roadside soils in Esbjerg, Denmark. Bull. Environ. Contam. Toxicol. 2011, 86, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska-Kordybach, B.; Smreczak, B.; Klimkowicz-Pawlas, A.; Terelak, H. Monitoring of the total content of polycyclic aromatic hydrocarbons (PAHs) in arable soils in Poland. Chemosphere 2008, 73, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Soclo, H.H.; Garrigues, P.H.; Ewald, M. Origin of polycyclic aromatic hydrocarbons (PAHs) in coastal marine sediments: Case studies in Cotonou (Benin) and Aquitaine (France) areas. Mar. Pollut. Bull. 2000, 40, 387–396. [Google Scholar] [CrossRef]
- Zakaria, M.P.; Takada, H.; Tsutsumi, S.; Ohno, K.; Yamada, J.; Kouno, E.; Kumata, H. Distribution of polycyclic aromatic hydrocarbons (PAHs) in rivers and estuaries in Malaysia: A widespread input of petrogenic PAHs. Environ. Sci. Technol. 2002, 36, 1907–1918. [Google Scholar] [CrossRef] [PubMed]
- Baumard, P.; Budzinski, H.; Michon, Q.; Garrigues, P.; Burgeot, T.; Bellocq, J. Origin and bioavailability of PAHs in the Mediterranean Sea from mussel and sediment records. Estuar. Coast. Shelf Sci. 1998, 47, 77–90. [Google Scholar] [CrossRef]
- Harrison, R.M.; Smith, D.J.T.; Luhana, L. Source apportionment of atmospheric polycyclic aromatic hydrocarbons collected from an urban location in Birmingham, UK. Environ. Sci. Technol. 1996, 30, 825–832. [Google Scholar] [CrossRef]
- Larsen, R.K.; Baker, J.E. Source apportionment of polycyclic aromatic hydrocarbons in the urban atmosphere: A comparison of three methods. Environ. Sci. Technol. 2003, 37, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Li, C.K.; Kamens, R.M. The use of polycyclic aromatic hydrocarbons as source signatures in receptor modeling. Atmos. Environ. Part A Gen. Top. 1993, 27, 523–532. [Google Scholar] [CrossRef]
- Miguel, A.H.; Pereira, P.A.P. Benzo (k) fluoranthene, benzo (ghi) perylene, and indeno (1, 2, 3-cd) pyrene: New tracers of automotive emissions in receptor modeling. Aerosol Sci. Technol. 1989, 10, 292–295. [Google Scholar] [CrossRef]
- Duval, M.M.; Friedlander, S.K. Source Resolution of Polycyclic Aromatic Hydrocarbons in the Los Angeles Atmosphere: Application of a Chemical Species Balance Method with First Order Chemical Decay. Final Report January–December 80; Deptartment of Chemical, Nuclear, and Thermal Engineering, California University: Los Angeles, CA, USA, 1981. [Google Scholar]
- Zhang, K.; Liang, B.; Wang, J.Z.; Guan, Y.F.; Zeng, E.Y. Polycyclic aromatic hydrocarbons in upstream riverine runoff of the Pearl River Delta, China: An assessment of regional input sources. Environ. Pollut. 2012, 167, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Khalili, N.R.; Scheff, P.A.; Holsen, T.M. PAH source fingerprints for coke ovens, diesel and, gasoline engines, highway tunnels, and wood combustion emissions. Atmos. Environ. 1995, 29, 533–542. [Google Scholar] [CrossRef]
- Fraser, M.P.; Grosjean, D.; Grosjean, E.; Rasmussen, R.A.; Cass, G.R. Air quality model evaluation data for organics. 1. Bulk chemical composition and gas/particle distribution factors. Environ. Sci. Technol. 1996, 30, 1731–1743. [Google Scholar] [CrossRef]
- Venkataraman, C.; Negi, G.; Sardar, S.B.; Rastogi, R. Size distributions of polycyclic aromatic hydrocarbons in aerosol emissions from biofuel combustion. J. Aerosol Sci. 2002, 33, 503–518. [Google Scholar] [CrossRef]
- Khairy, M.A.; Lohmann, R. Source apportionment and risk assessment of polycyclic aromatic hydrocarbons in the atmospheric environment of Alexandria, Egypt. Chemosphere 2013, 91, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.T.; Bartle, K.D.; Andrews, G.E. The relation between polycyclic aromatic compounds in diesel fuels and exhaust particulates. Fuel 1986, 65, 1150–1158. [Google Scholar] [CrossRef]
- Tsai, J.W.; Bremner, K.H.; Vallee, R.B. Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nat. Neurosci. 2007, 10, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Teng, Y.; Wang, J. Source apportionment of polycyclic aromatic hydrocarbons (PAHs) in surface sediments of the Rizhao coastal area (China) using diagnostic ratios and factor analysis with nonnegative constraints. Sci. Total Environ. 2012, 414, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Knafla, A.; Phillipps, K.A.; Brecher, R.W.; Petrovic, S.; Richardson, M. Development of a dermal cancer slope factor for benzo [a] pyrene. Regul. Toxicol. Pharmacol. 2006, 45, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska-Kordybach, B. Polycyclic aromatic hydrocarbons in agricultural soils in Poland: Preliminary proposals for criteria to evaluate the level of soil contamination. Appl. Geochem. 1996, 11, 121–127. [Google Scholar] [CrossRef]
- Ravindra, K.; Sokhi, R.; Van Grieken, R. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmos. Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef] [Green Version]
- Jin, A.; He, J.; Chen, S.; Huang, G. Distribution and transport of PAHs in soil profiles of different water irrigation areas in Beijing, China. Environ. Sci. Process. Impacts 2014, 16, 1526–1534. [Google Scholar] [CrossRef] [PubMed]
- Barbella, R.; Bertoli, C.; Ciajolo, A.; D’anna, A. Behavior of a fuel oil during the combustion cycle of a direct injection diesel engine. Combust. Flame 1990, 82, 191–198. [Google Scholar] [CrossRef]
- Chen, Y.; Sheng, G.; Bi, X.; Feng, Y.; Mai, B.; Fu, J. Emission factors for carbonaceous particles and polycyclic aromatic hydrocarbons from residential coal combustion in China. Environ. Sci. Technol. 2005, 39, 1861–1867. [Google Scholar] [CrossRef] [PubMed]
- Purushothama, S.; Pan, W.P.; Riley, J.T.; Lloyd, W.G. Analysis of polynuclear aromatic hydrocarbons from coal fly ash. Fuel Process. Technol. 1998, 53, 235–242. [Google Scholar] [CrossRef]
- Mastral, A.M.; Callen, M.S. A review on polycyclic aromatic hydrocarbon (PAH) emissions from energy generation. Environ. Sci. Technol. 2000, 34, 3051–3057. [Google Scholar] [CrossRef]



| PAH Compound | Abbr. | Carcinogenicity Classification | No. of Ring | Agriculture Soils (AS, mg/kg) | Roadside Soils (RS, mg/kg) | Park Green Space Soils (PS, mg/kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | Mean | Median | Range | Mean | Median | Range | Mean | Median | ||||
| Naphthalene | Nap | 2B | 2 | 0.007–4.87 | 1.51 | 1.5 | 1.15–8.93 | 5.17 | 4.7 | 0.337–10.6 | 2.72 | 1.61 |
| Acenaphthylene | Acel | 3 | ND-0.022 | 0.006 | 0.003 | 0.11–0.240 | 0.064 | 0.044 | ND-0.109 | 0.027 | 0.016 | |
| Acenaphthene | Ace | 3 | ND-0.103 | 0.032 | 0.025 | 0.056–1.79 | 0.412 | 0.263 | 0.015–0.517 | 0.126 | 0.08 | |
| Fluorene | Flu | 3 | 0.015–0.399 | 0.104 | 0.077 | 0.405–7.86 | 2.14 | 1.64 | 0.053–2.62 | 0.615 | 0.41 | |
| Anthracene | Ant | 3 | 0.332–4.26 | 0.992 | 0.701 | 3.62–23.0 | 9.45 | 8.36 | 0.485–13.1 | 3.69 | 2.84 | |
| Phenanthrene | Phe | 3 | 0.009–0.168 | 0.038 | 0.024 | ND-12.9 | 1.37 | 0.435 | 0.016–0.907 | 0.181 | 0.12 | |
| Fluoranthene | Fla | 4 | 0.140–2.41 | 0.546 | 0.397 | ND-10.3 | 4.97 | 4.59 | 0.058–6.53 | 1.63 | 1.11 | |
| Pyrene | Pyr | 4 | 0.135–2.49 | 0.502 | 0.341 | 0.288–10.2 | 4.72 | 4.58 | 0.007–6.29 | 1.44 | 1.00 | |
| Benz [a] anthracene | Baa | B | 4 | 0.071–1.71 | 0.288 | 0.149 | ND-6.02 | 2.51 | 2.43 | ND-1.32 | 0.533 | 0.403 |
| Chrysene | Chr | 2B | 4 | 0.190–3.49 | 0.62 | 0.348 | ND-7.96 | 3.54 | 3.74 | 0.216–3.52 | 1.31 | 1.11 |
| Benzo [b] fluoranthene | Bbf | 2B | 5 | 1.36–25.5 | 4.44 | 2.52 | ND-39.0 | 3.35 | 0.327 | ND-6.36 | 1.53 | 0.153 |
| Benzo [k] fluoranthene | Bkf | 2B | 5 | 0.100–9.90 | 0.985 | 0.229 | ND-19.5 | 8.97 | 10.4 | ND-6.01 | 2.45 | 1.73 |
| Benzo [a] pyrene | Bap | B | 5 | 0.232–5.95 | 0.973 | 0.49 | ND-35.3 | 16.1 | 15.8 | 0.435–22.1 | 5.55 | 4.01 |
| Dibenz [a,h] anthracene | Daa | B | 5 | 0.150–3.10 | 0.514 | 0.265 | 0.010–2.61 | 0.276 | 0.01 | 0.010–0.299 | 0.045 | 0.01 |
| Indeno[1,2,3-cd]pyrene | Ind | 2B | 6 | 0.566–11.6 | 1.83 | 0.927 | 0.087–39.5 | 2.34 | 0.087 | 0.087–2.07 | 0.469 | 0.087 |
| Benzo [g,h,i] perylene | Bghip | 6 | 0.007–0.343 | 0.163 | 0.142 | 0.007–0.642 | 0.043 | 0.007 | 0.007 | 0.007 | 0.007 | |
| ∑16PAHs | 3.87–76.0 | 12.6 | 7.55 | 35.4–116. | 65.5 | 60.7 | 5.93–66.5 | 22.3 | 19.9 | |||
| ∑BPAHs | 3.22–66.1 | 11.2 | 5.99 | 20.2–94.2 | 42.3 | 39.4 | 3.82–36.4 | 14.6 | 16.8 | |||
| PAHs | Diagnostic Ratio | Sources | Reference |
|---|---|---|---|
| Ant/(Ant + Phe) | <0.1 | Petroleum | [14] |
| >0.1 | Combustion | ||
| Fla/(Fla + Pyr) | <0.4 | Petroleum | [34] |
| 0.4–0.5 | Liquid fossil fuelcombustion | ||
| >0.5 | Coal, wood or grass combustion | ||
| Baa/(Baa + Chr) | <0.2 | Petroleum | [37] |
| 0.2–0.35 | Petroleum or combustion | ||
| >0.35 | Combustion | ||
| Phe/Ant | <10 | Pyrogenic | [40] |
| <15 | Petrogenic | ||
| Ind/(Ind + Bghip) | <0.2 | Petrogenic | [38] |
| 0.2–0.5 | Fuel combustion(vehicle and crude oil) | ||
| >0.5 | Grass/coal/wood combustion | ||
| Bap/(Bap + Chr) | <0.2 | Petroleum | [39,40] |
| 0.2–0.35 | Coal, wood or grass combustion | ||
| >0.35 | Vehicular combustion | ||
| HMW/LMW PAHs | >1 | Pyrogenic | [41] |
| <1 | Petrogenic | [42] | |
| Fla/Pyr | <1 | Petrogenic | [43] |
| >1 | Pyrogenic | [39] | |
| Bap/Bghip | >0.6 | Traffic | [40] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, H.; Wang, Q.; Zhao, N.; Jin, B.; Zhuang, X.; Bai, Z. Distributions and Sources of Polycyclic Aromatic Hydrocarbons (PAHs) in Soils around a Chemical Plant in Shanxi, China. Int. J. Environ. Res. Public Health 2017, 14, 1198. https://doi.org/10.3390/ijerph14101198
Jiao H, Wang Q, Zhao N, Jin B, Zhuang X, Bai Z. Distributions and Sources of Polycyclic Aromatic Hydrocarbons (PAHs) in Soils around a Chemical Plant in Shanxi, China. International Journal of Environmental Research and Public Health. 2017; 14(10):1198. https://doi.org/10.3390/ijerph14101198
Chicago/Turabian StyleJiao, Haihua, Qi Wang, Nana Zhao, Bo Jin, Xuliang Zhuang, and Zhihui Bai. 2017. "Distributions and Sources of Polycyclic Aromatic Hydrocarbons (PAHs) in Soils around a Chemical Plant in Shanxi, China" International Journal of Environmental Research and Public Health 14, no. 10: 1198. https://doi.org/10.3390/ijerph14101198






