Immobilization of Lead Migrating from Contaminated Soil in Rhizosphere Soil of Barley (Hordeum vulgare L.) and Hairy Vetch (Vicia villosa) Using Hydroxyapatite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Soil and Apatite
2.2. Cultivation Using a Rhizobox System
2.3. Analytical Methods
2.4. Statistical Analyses
3. Results
3.1. Shoot Biomass and Lead Uptake from Contaminated Soil
3.2. Soil pH and Water-Extractable Lead in Rhizosphere Soil
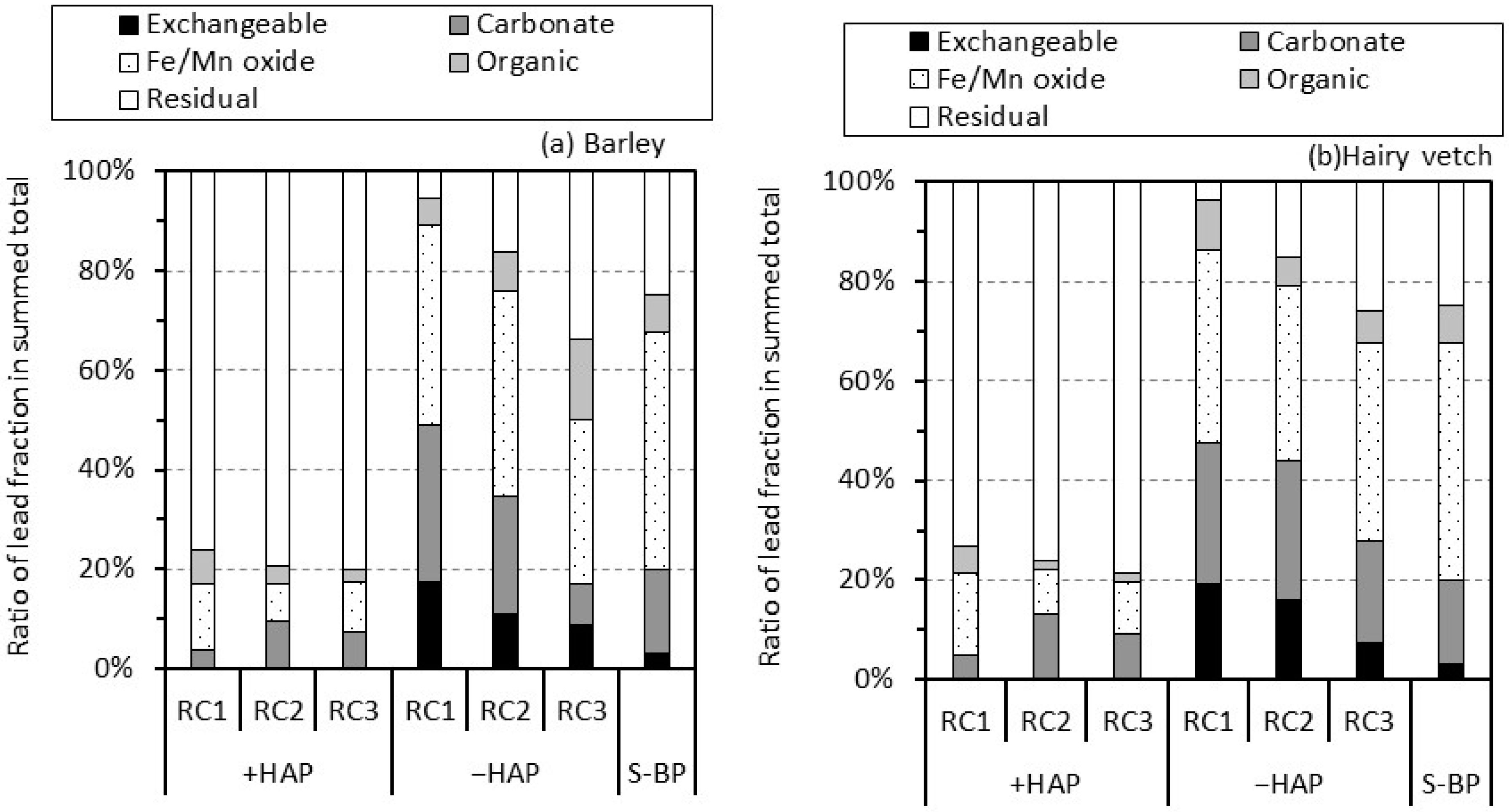
3.3. Lead Phases by Sequential Extraction in Rhizosphere Soil
4. Discussion
4.1. Lead Migration from Contaminated Soil into Uncontaminated Rhizosphere Soil and Its Immobilization by Hydroxyapatite
4.2. Plant Growth in Rhizosphere Soil with and without Hydroxyapatite in Which Lead Migrated from Contaminated Soil
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alvarenga, P.; Goncalves, A.P.; Fernandes, R.M.; de Varennes, A.; Vallini, G.; Duarte, E. Organic residues as immobilizing agents in aided phytostabilization: (I) Effects on soil chemical characteristics. Chemosphere 2009, 74, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Tamura, H.; Kimura, T.; Kinoshita, T.; Matsufuru, H.; Sato, T. Control of lead polluted leachate in a box-scale phytoremediation test using common buckwheat (Fagopyrum esculentum Moench) grownon lead contaminated soil. Environ. Technol. 2007, 28, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Y.; Schulin, R.; Nowack, B. The effects of plants on the mobilization of Cu and Zn in soil columns. Environ. Sci. Technol. 2007, 41, 2770–2775. [Google Scholar] [CrossRef] [PubMed]
- Sayyad, G.; Afyuni, M.; Mousavi, S.F.; Abbaspour, K.C.; Richards, B.K.; Schulin, R. Transport of Cd, Cu, Pb and Zn in a calcareous soil under wheat and safflower cultivation—A column study. Geoderma 2010, 154, 311–320. [Google Scholar] [CrossRef]
- Ensley, B.D. Rationale for use of phytoremediation. In Phytoremediation of Toxic Metals; Raskin, I., Ensley, B.D., Eds.; Wiley: New York, NY, USA, 2000; pp. 3–11. [Google Scholar]
- Kucharski, R.; Sas-Nowosielska, A.; Małkowski, E.; Japenga, J.; Kuperberg, J.M.; Pogrzeba, M.; Krzyżak, J. The use of indigenous plant species and calcium phosphate for the stabilization of highly metalpolluted sites in southern. Pol. Plant Soil 2005, 273, 291–305. [Google Scholar] [CrossRef]
- Ruttens, A.; Colpaert, J.V.; Mench, M.; Boisson, J.; Carleer, R.; Vangronsveld, J. Phytostabilization of a metal contaminated sandy soil. II: Influence of compost and/or inorganic metal immobilizing soil amendments on metal leaching. Environ. Pollut. 2006, 144, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Ruttens, A.; Mench, M.; Colpaert, J.V.; Boisson, J.; Carleer, R.; Vangronsveld, J. Phytostabilization of a metal contaminated sandy soil. I: Influence of compost and/or inorganic metal immobilizing soil amendments on phytotoxicity and plant availability of metals. Environ. Pollut. 2006, 144, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Houben, D.; Pircar, J.; Sonnet, P. Heavy metal immobilization by cost-effective amendments in a contaminated soil: Effects on metal leaching and phytoavailability. J. Geochem. Explor. 2012, 123, 87–94. [Google Scholar] [CrossRef]
- Madej’on, E.; de Mora, A.P.; Felipe, E.; Burgos, P.; Cabrera, F. Soil amendments reduce trace element solubility in a contaminated soil and allow regrowth of natural vegetation. Environ. Pollut. 2006, 139, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M.; Masaki, S.; Sato, T. Single-step extraction to determine soluble lead levels in soil. Int. J. GEOMATE 2012, 3, 375–380. [Google Scholar] [CrossRef]
- Ma, Q.Y.; Logan, T.; Traina, S.J. Lead immobilization from aqueous solutions and contaminated soils using phosphate rocks. Environ. Sci. Technol. 1995, 29, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Scheckel, K.G.; Ryan, J.A. Effects of aging and pH on dissolution kinetics and stability of chloropyromorphite. Environ. Sci. Technol. 2002, 36, 2198–2204. [Google Scholar] [CrossRef] [PubMed]
- Debela, F.; Arocena, J.M.; Thring, R.W.; Whitcombe, T. Organic acid-induced release of lead from pyromorphite and its relevance to reclamation of Pb-contaminated soils. Chemosphere 2010, 80, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils-to mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Katoh, M.; Sato, T. Simultaneous lead and antimony immobilization in shooting range soil by combined application of hydroxyapatite and ferrihydrite. Environ. Technol. 2015, 36, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M.; Makimura, A.; Sato, T. Removal of lead by apatite and its stability in the presence of organic acids. Environ. Technol. 2016, 37, 3036–3045. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Hatsushika, T.; Hayakawa, Y. Synthetic hydroxyapatite employed as inorganic cation exchangers. J. Chem. Soc. Faraday Trans. I 1998, 77, 1059–1062. [Google Scholar] [CrossRef]
- Mavropoulos, E.; Rossi, A.M.; Costa, A.M.; Perez, C.A.C.; Moreira, J.C.; Saldanha, M. Studies of the mechanisms of lead immobilization by hydroxyapatite. Environ. Sci. Technol. 2002, 36, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M.; Matsuoka, H.; Sato, T. Stability of lead immobilized by apatite in lead-contaminated rhizosphere soil of buckwheat (Fagopyrum esculentum) and hairy vetch (Vicia villosa). Int. J. Phytoremediation 2015, 17, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, M.; Ma, Y.; Yang, J. Evaluation of different phosphate amendments on availability of metals in contaminated soil. Ecotoxicol. Environ. Saf. 2007, 67, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Sato, T.; Katoh, M. Formation of a lead insoluble phase using an immobilization material and its maximization in soil under unsaturated moisture conditions. J. Soils Sediments 2017. [Google Scholar] [CrossRef]
- Chen, Z.; Setagawa, M.; Kang, Y.; Sakurai, K.; Aikawa, Y.; Iwasaki, K. Zinc and cadmium uptake from a metalliferous soil by a mixed culture of Athyrium yokoscense and Arabis flagellosa. Soil Sci. Plant Nutr. 2009, 55, 315–324. [Google Scholar] [CrossRef]
- Mart’ınez-Alcal’a, I.; Clemente, R.; Bernal, M.P. Metal availability and chemical properties in the rhizosphere of Lupinus albus L. growing in a high-metal calcareous soil. Water Air Soil Pollut. 2009, 201, 283–293. [Google Scholar] [CrossRef]
- Ryan, J.A.; Zhang, P.; Hesterberg, D.; Chou, J.; Sayers, D.E. Formation of chloropyromorphite in a lead-contaminated soil amended with hydroxyapatite. Environ. Sci. Technol. 2001, 35, 3798–3803. [Google Scholar] [CrossRef] [PubMed]
- Furuta, S.; Katsuki, H.; Komarneni, S. Porous hydroxyapatite monoliths from gypsum waste. J. Mater. Chem. 1998, 8, 2803–2806. [Google Scholar] [CrossRef]
- Ogawa, S.; Katoh, M.; Sato, T. Contribution of hydroxyapatite and ferrihydrite in combined application for the removal of lead and antimony from aqueous solutions. Water Air Soil Pollut. 2014, 225, 2023. [Google Scholar] [CrossRef]
- Toker, M.C. Localization of lead accumulated by barley (Hordeum vulgare L.) root tips and its effects. Commun. Fac. Sci. Univ. Ank. Series C 1998, 6, 53–72. [Google Scholar]
- Matsufuru, H.; Honda, M.; Muto, J.; Tamura, H.; Kojima, J.; Sato, T. Remediation and diffusion prevention of lead (Pb) contaminated soil by plants in a pilot test. J. JSCE Ser. G 2007, 63, 51–57. (In Japanese) [Google Scholar] [CrossRef]
- Gee, G.W.; Bauder, J.M. Partical-size analysis. In Methods of Soil Analysis, Part 1; Klute, A., Ed.; American Society of Agronomy: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Shuman, L.M. Fractionation method for soil microelements. Soil Sci. 1998, 140, 11–22. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1997, 51, 844–851. [Google Scholar] [CrossRef]
- Jones, D.L.; Dennis, P.G.; Owen, A.G.; van Hees, P.A.W. Organic acid behavior in soils—Misconceptions and knowledge gaps. Plant Soil 2003, 248, 31–41. [Google Scholar] [CrossRef]
- Chen, X.; Wright, J.V.; Conca, J.L.; Peurrung, L.M. Effects of pH on heavy metal sorption on mineral apatite. Environ. Sci. Technol. 1997, 31, 624–631. [Google Scholar] [CrossRef]
- Oliva, J.; Cama, J.; Cortina, J.L.; Ayora, C.; De Pablo, J. Biogenic hydroxyapatite (Apatite IITM) dissolution kinetics and metal removal from acid mine drainage. J. Hazard. Mater. 2012, 213, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Katoh, M.; Sato, T. Microscopic range of immobilization between heavy metals and amendment in soil through water migration. Int. J. GEOMATE 2014, 6, 870–877. [Google Scholar] [CrossRef]
- Scheckel, K.G.; Diamond, G.L.; Burgess, M.F.; Klotzbach, J.M.; Maddaloni, M.; Miller, B.W.; Partridge, C.R.; Serda, S.M. Amending soils with phosphate as means to mitigate soil lead hazard: A critical review of the state of the science. J. Toxicol. Environ. Health Part B 2013, 16, 337–380. [Google Scholar] [CrossRef] [PubMed]
- Tlustoš, P.; Száková, J.; Kořínek, K.; Pavlíková, D.; Hanč, A.; Balík, J. The effect of liming on cadmium, lead, and zinc uptake reduction by spring wheat grown in contaminated soil. Plant Soil Environ. 2006, 52, 16. [Google Scholar]
- Davey, B.G.; Mitchell, R.L. The distribution of trace elements in cocksfoot (Dactylis glomerata) at flowering. J. Sci. Food Agric. 1968, 19, 425–431. [Google Scholar] [CrossRef]
- Katoh, M.; Hashimoto, K.; Sato, T. Lead and antimony removal from contaminated soil by phytoremediation combined with an immobilization material. Clean Soil Air Water 2016, 44, 1717–1724. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals―Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]


| Soil | pH | TC a | Water-extractable | Total | Amorphous Fe (g kg−1) | ||
|---|---|---|---|---|---|---|---|
| (g kg−1) | OC b | Pb | Pb | P | |||
| (mg kg−1) | (mg kg−1) | (g kg−1) | (g kg−1) | ||||
| Contaminated soil | 7.2 | 51 | 354 | 11.2 | 25.7 | 0.40 | 2.6 |
| Non-contaminated soil | 5.3 | 16 | 99 | <0.02 | 0.02 | 0.12 | 3.0 |
| Plant | Material | Shoot Weight | Lead Concentration | Lead Uptake |
|---|---|---|---|---|
| (a) | (b) | (c = a × b) | ||
| (mg box−1 DW) | (μg g−1 DW) | (μg box−1 DW) | ||
| Barley | +HAP | 410 ± 90 | 7 ± 2 | 2.7 ± 0.2 |
| −HAP | 240 ± 40 | 90 ± 70 | 18 ± 13 | |
| p value | 0.1513 | 0.2964 | 0.2867 | |
| Hairy vetch | +HAP | 700 ± 100 | 9 ± 1 | 6.1 ± 1.2 |
| −HAP | 244 ± 24 | 24 ± 7 | 5.5 ± 1.3 | |
| p value | 0.0014 ** | 0.1005 | 0.7530 |
| Soil | Barley | Hairy Vetch | ||
|---|---|---|---|---|
| +HAP | −HAP | +HAP | −HAP | |
| RC1 | 6.2 ± 0ab | 5.2 ± 0a | 6.2 ± 0a | 5.2 ± 0a |
| RC2 | 6.3 ± 0a | 5.1 ± 0a | 6.2 ± 0a | 5.0 ± 0b |
| RC3 | 6.1 ± 0b | 5.0 ± 0a | 6.1 ± 0b | 4.9 ± 0c |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katoh, M.; Risky, E.; Sato, T. Immobilization of Lead Migrating from Contaminated Soil in Rhizosphere Soil of Barley (Hordeum vulgare L.) and Hairy Vetch (Vicia villosa) Using Hydroxyapatite. Int. J. Environ. Res. Public Health 2017, 14, 1273. https://doi.org/10.3390/ijerph14101273
Katoh M, Risky E, Sato T. Immobilization of Lead Migrating from Contaminated Soil in Rhizosphere Soil of Barley (Hordeum vulgare L.) and Hairy Vetch (Vicia villosa) Using Hydroxyapatite. International Journal of Environmental Research and Public Health. 2017; 14(10):1273. https://doi.org/10.3390/ijerph14101273
Chicago/Turabian StyleKatoh, Masahiko, Elsya Risky, and Takeshi Sato. 2017. "Immobilization of Lead Migrating from Contaminated Soil in Rhizosphere Soil of Barley (Hordeum vulgare L.) and Hairy Vetch (Vicia villosa) Using Hydroxyapatite" International Journal of Environmental Research and Public Health 14, no. 10: 1273. https://doi.org/10.3390/ijerph14101273





