Application of Circular Bubble Plume Diffusers to Restore Water Quality in a Sub-Deep Reservoir
Abstract
:1. Introduction
2. Materials and Methods
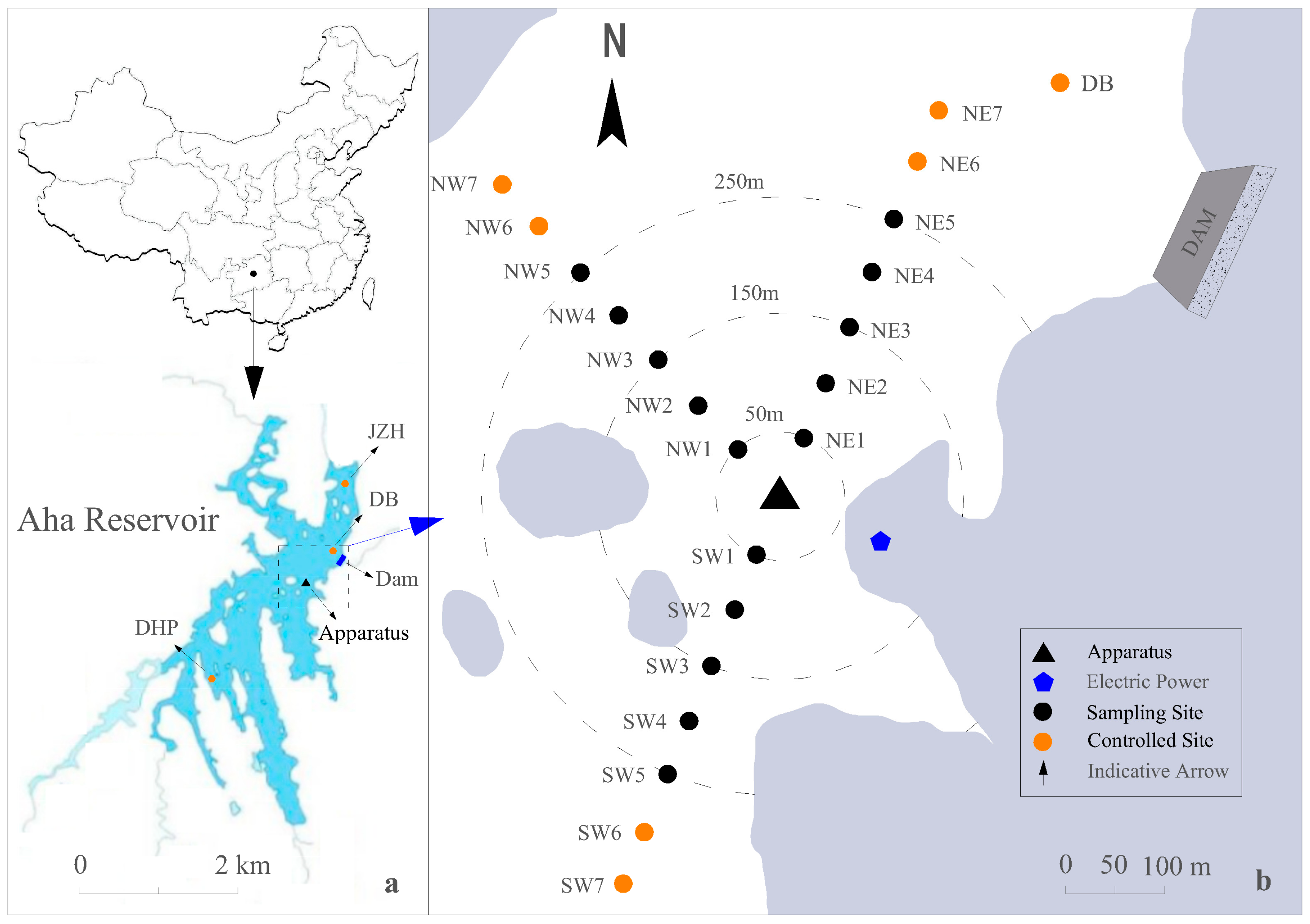
2.1. Study Area
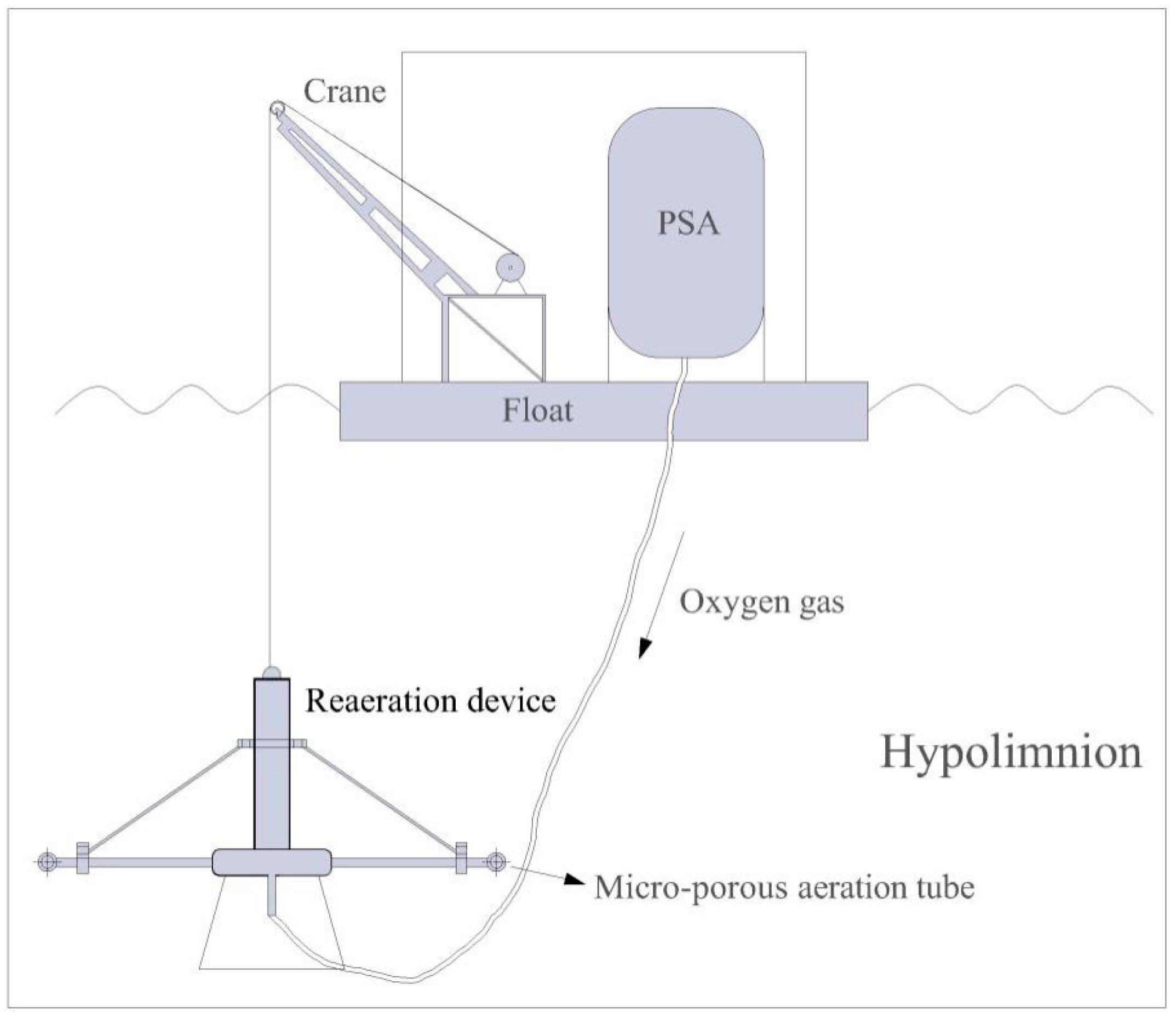
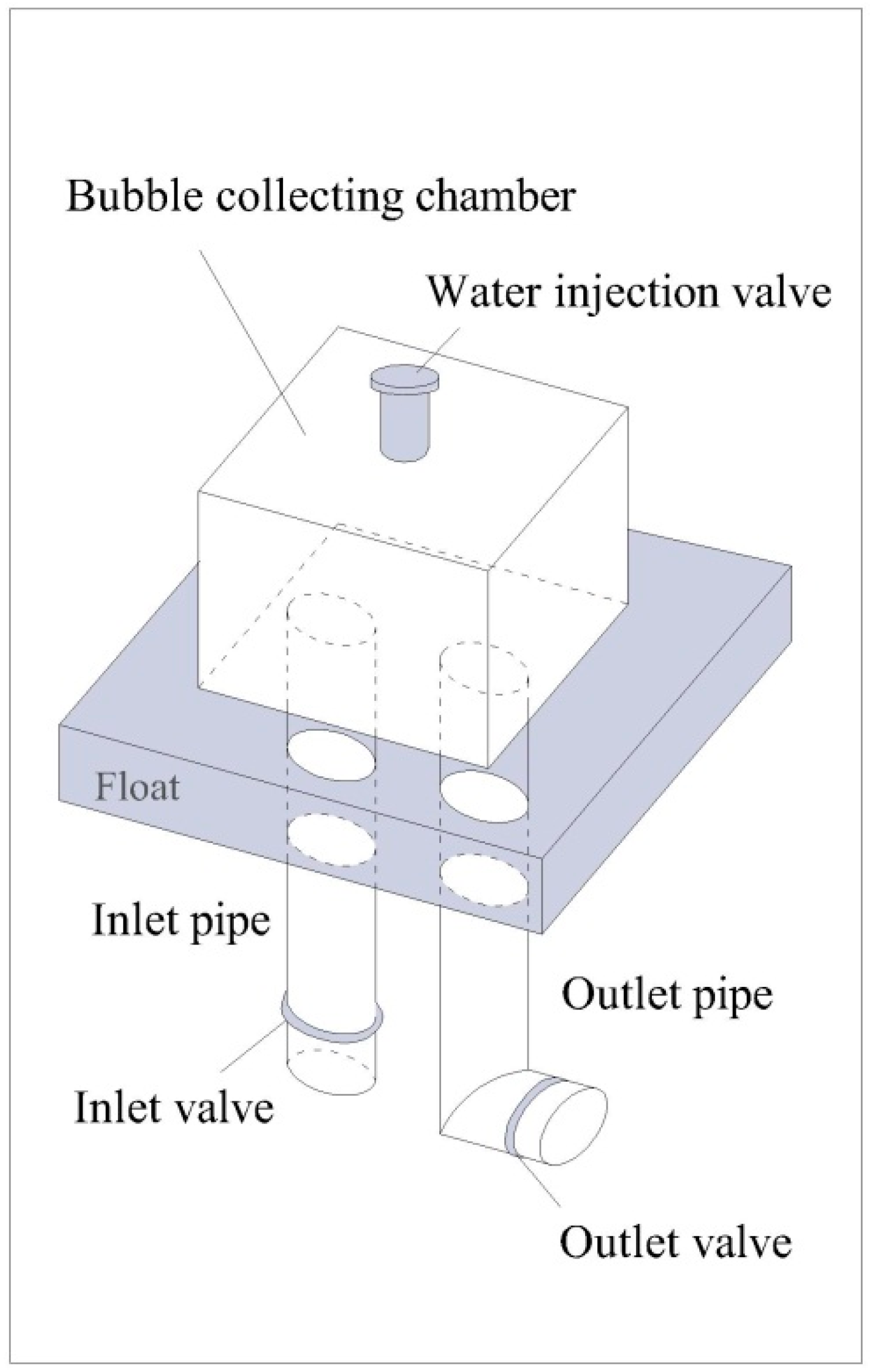
2.2. Reaeration Apparatus
2.3. Experimental Design
2.4. Water Chemistry Analysis
2.5. Oxygen Dissolution Efficiency Calculation
3. Results and Discussion
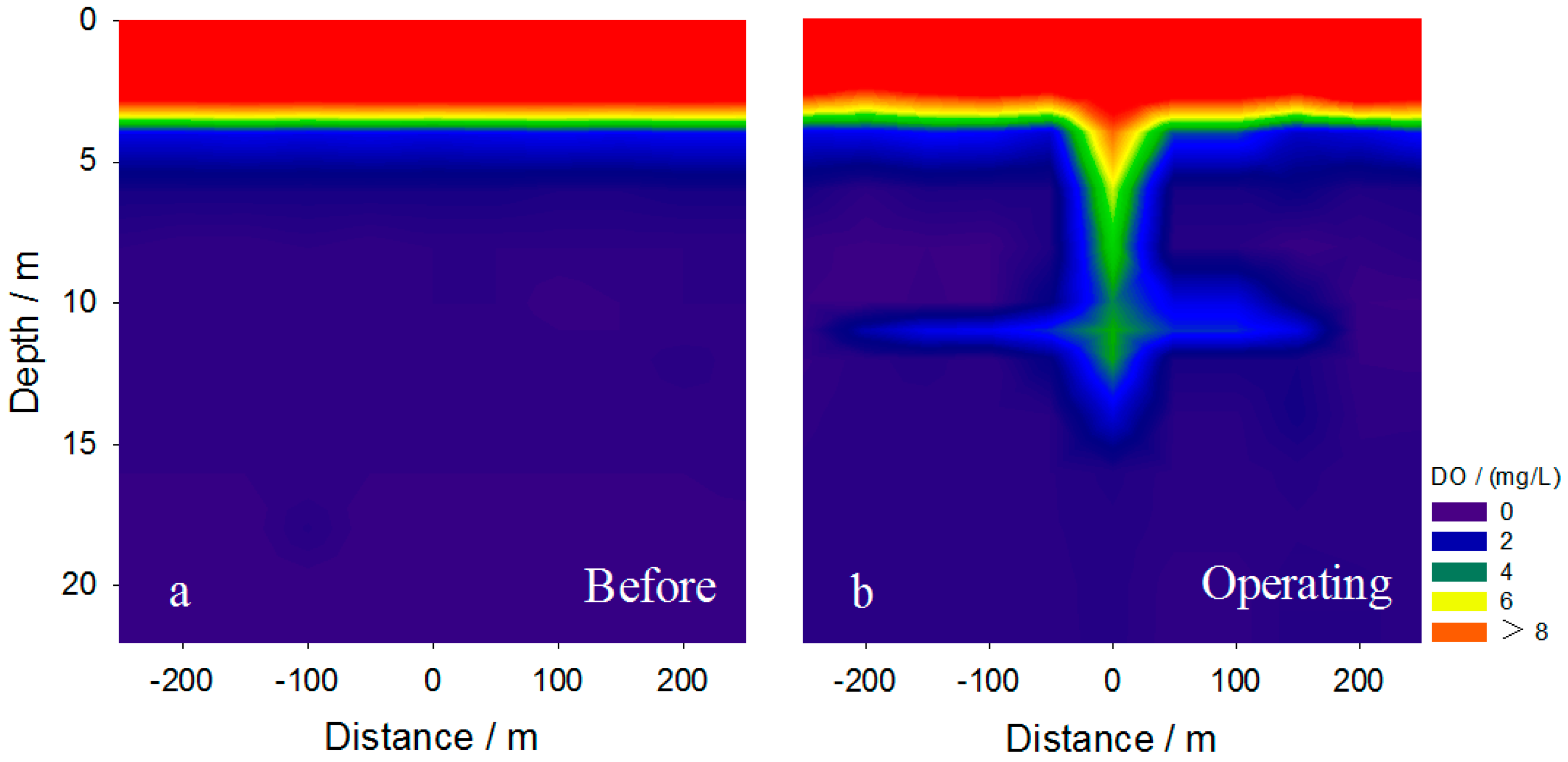
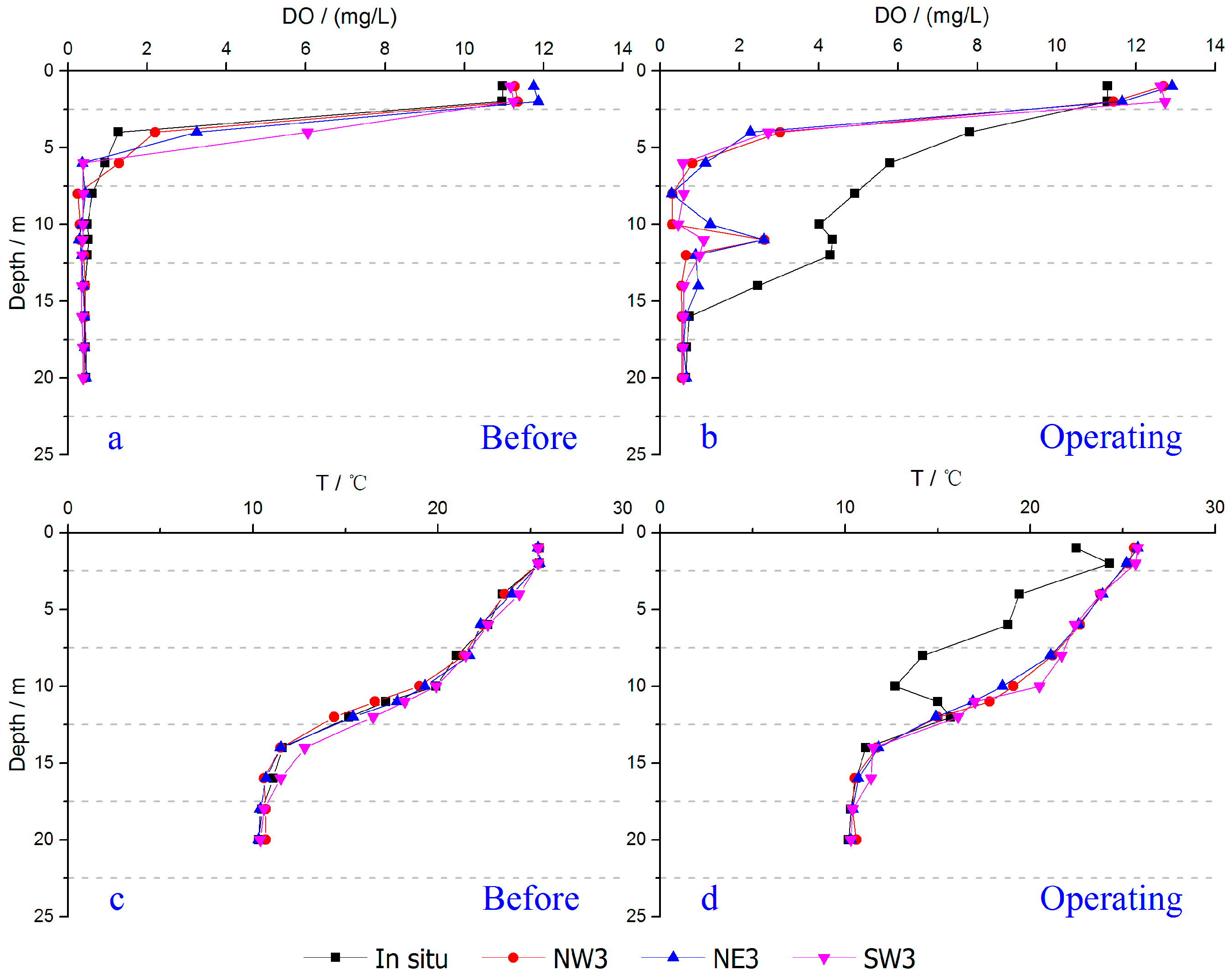
3.1. Variations of DO within the Hypolimnion
3.2. Mass Balance of Oxygen
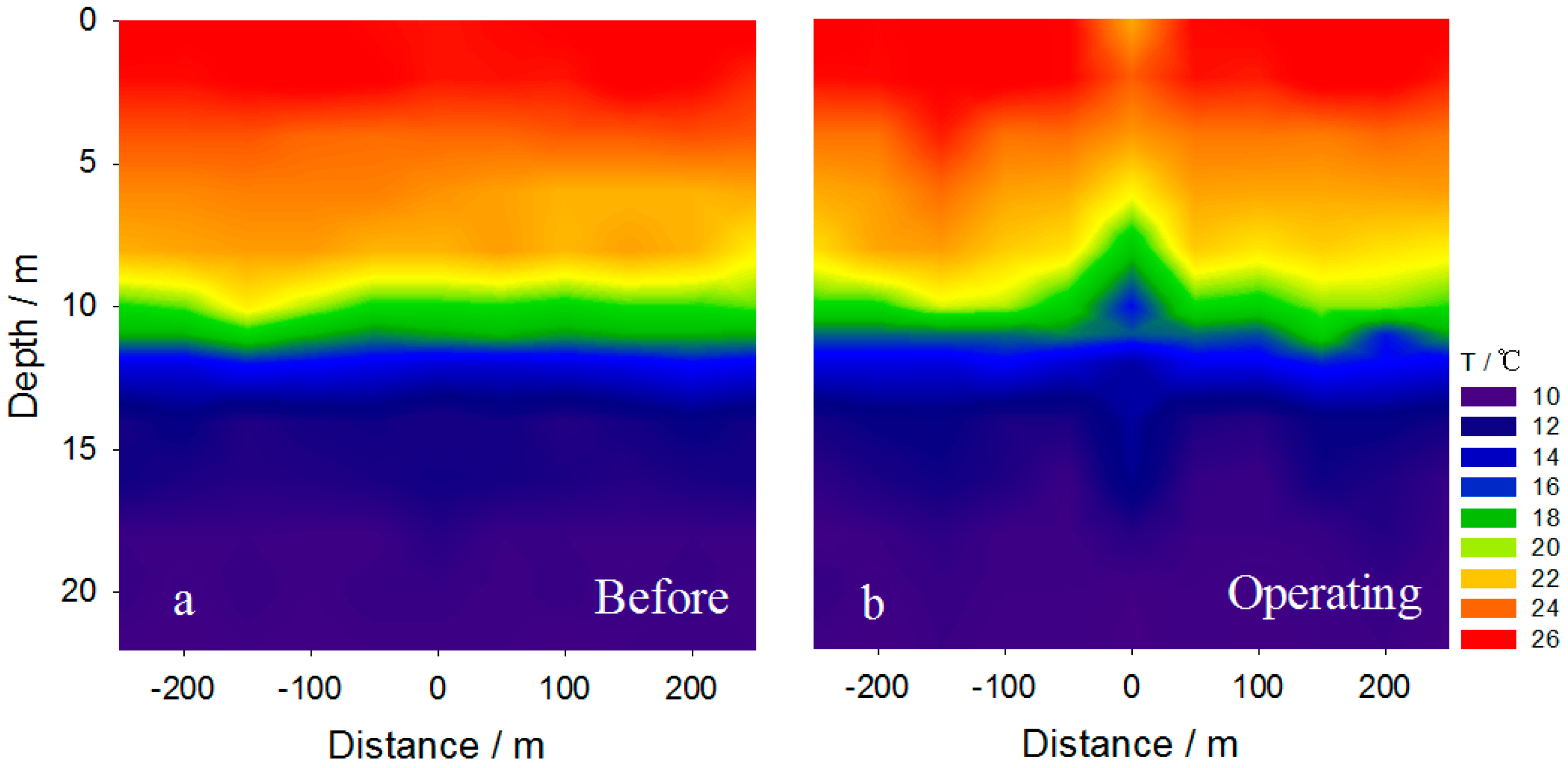
3.3. Effect on the Stability of Thermal Stratification
3.4. Intrusion Characteristics
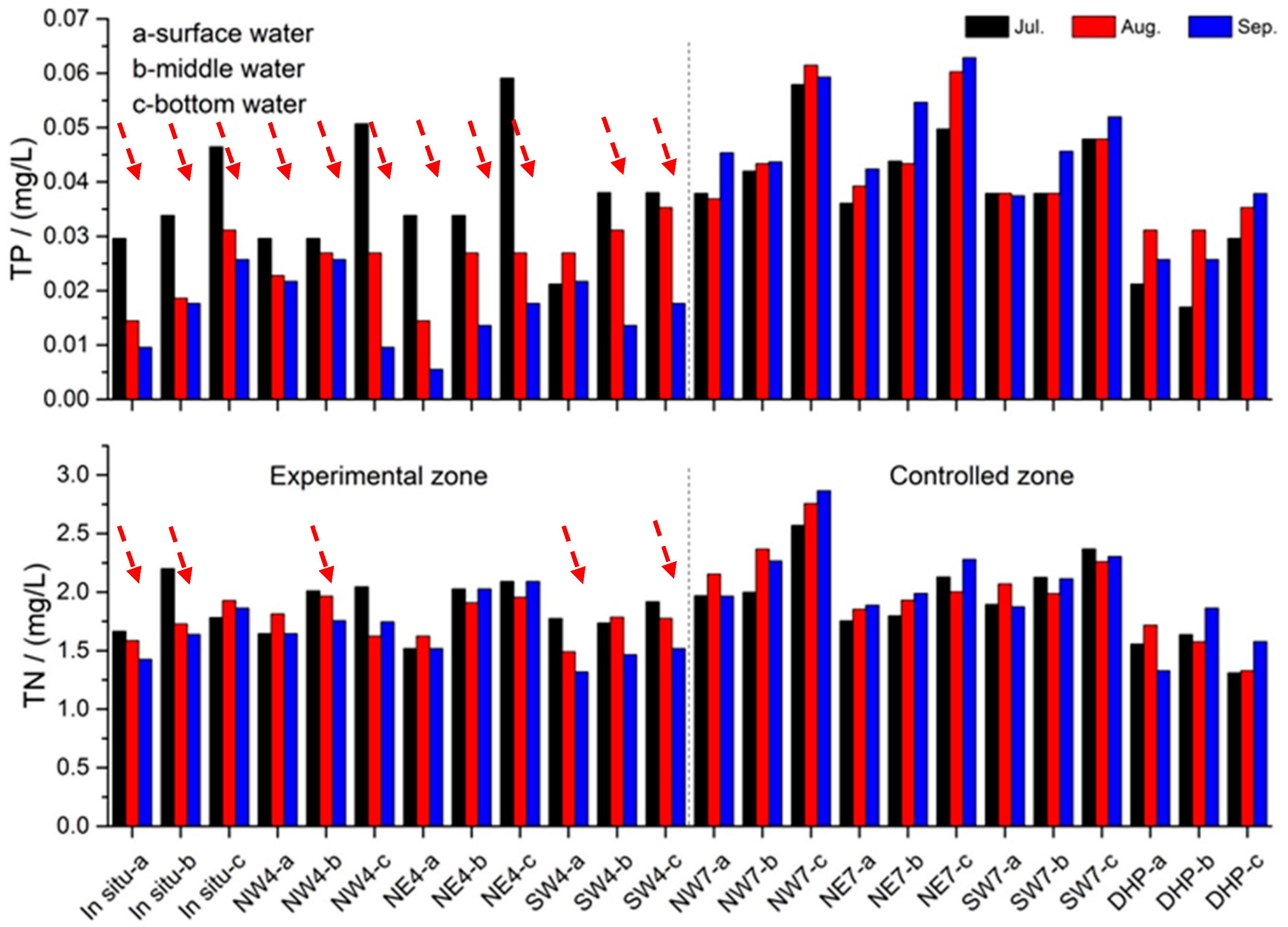
3.5. Effect on the Water Quality
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Higashino, M.; Stefan, H.G. Sedimentary microbial oxygen demand for laminar flow over a sediment bed of finite length. Water Res. 2005, 39, 3153–3166. [Google Scholar] [CrossRef] [PubMed]
- Gantzer, P.A.; Bryant, L.D.; Little, J.C. Effect of hypolimnetic oxygenation on oxygen depletion rates in two water-supply reservoirs. Water Res. 2009, 43, 1700–1710. [Google Scholar] [CrossRef] [PubMed]
- Mackenthun, A.A.; Stefan, H.G. Effect of flow velocity on sediment oxygen demand: Experiments. J. Environ. Eng. 1998, 124, 222–230. [Google Scholar] [CrossRef]
- Rippey, B.; McSorley, C. Oxygen depletion in lake hypolimnia. Limnol. Oceanogr. 2009, 54, 905–916. [Google Scholar] [CrossRef]
- Anderson, D.M. Approaches to monitoring, control and management of harmful algal blooms (habs). Ocean Coast. Manag. 2009, 52, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Hallegraeff, G.M. A review of harmful algal blooms and their apparent global increase. Phycologia 1993, 32, 79–99. [Google Scholar] [CrossRef]
- Walker, W.W. Use of hypolimnetic oxygen depletion rate as a trophic state index for lakes. Water Resour. Res. 1979, 15, 1463–1470. [Google Scholar] [CrossRef]
- Bormans, M.; Maršálek, B.; Jančula, D. Controlling internal phosphorus loading in lakes by physical methods to reduce cyanobacterial blooms: A review. Aquat. Ecol. 2016, 50, 407–422. [Google Scholar] [CrossRef]
- Dent, S.R.; Beutel, M.W.; Gantzer, P.; Moore, B.C. Response of methylmercury, total mercury, iron and manganese to oxygenation of an anoxic hypolimnion in North Twin Lake, Washington. Lake Reserv. Manag. 2014, 30, 119–130. [Google Scholar] [CrossRef]
- Gantzer, P.A.; Bryant, L.D.; Little, J.C. Controlling soluble iron and manganese in a water-supply reservoir using hypolimnetic oxygenation. Water Res. 2009, 43, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Hupfer, M.; Lewandowski, J. Oxygen controls the phosphorus release from lake sediments—A long lasting paradigm in limnology. Int. Rev. Hydrobiol. 2008, 93, 415–432. [Google Scholar] [CrossRef]
- Munger, Z.W.; Carey, C.C.; Gerling, A.B.; Hamre, K.D.; Doubek, J.P.; Klepatzki, S.D.; McClure, R.P.; Schreiber, M.E. Effectiveness of hypolimnetic oxygenation for preventing accumulation of Fe and Mn in a drinking water reservoir. Water Res. 2016, 106, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Naeher, S.; Gilli, A.; North, R.P.; Hamann, Y.; Schubert, C.J. Tracing bottom water oxygenation with sedimentary mn/fe ratios in Lake Zurich, Switzerland. Chem. Geol. 2013, 352, 125–133. [Google Scholar] [CrossRef]
- Beutel, M.W.; Horne, A.J. A review of the effects of hypolimnetic oxygenation on lake and reservoir water quality. Lake Reserv. Manag. 1999, 15, 285–297. [Google Scholar] [CrossRef]
- Mehner, T.; Diekmann, M.; Gonsiorczyk, T.; Kasprzak, P.; Koschel, R.; Krienitz, L.; Rumpf, M.; Schulz, M.; Wauer, G. Rapid recovery from eutrophication of a stratified lake by disruption of internal nutrient load. Ecosystems 2008, 11, 1142–1156. [Google Scholar] [CrossRef]
- Chen, J.A.; Yang, H.Q.; Zhang, D.D.; Xu, D.; Luo, J.; Wang, J.F. A particular river-whiting phenomenon caused by discharge of hypolimnetic water from a stratified reservoir. PLoS ONE 2015, 10, e0137860. [Google Scholar] [CrossRef] [PubMed]
- Petts, G.E. Impounded Rivers: Perspectives for Ecological Management; John Wiley and Sons: New York, NY, USA, 1984. [Google Scholar]
- WCD (World Commission on Dams). Dams and Development: A New Framework for Decision Making; Earthscan: London, UK, 2001. [Google Scholar]
- Rosenberg, D.M.; McCully, P.; Pringle, C.M. Global-scale environmental effects of hydrological alterations: Introduction. BioScience 2000, 50, 746–751. [Google Scholar] [CrossRef]
- Bosshard, P. China dams the world. World Policy J. 2009, 26, 43–51. [Google Scholar] [CrossRef]
- Xiao, X.Z.; Fan, T.S.; Huang, K.X.; Luo, Z.L.; Shi, J.Z. Chronicles of Guizhou Province: Chronicles of Water Conservancy; Fangzhi Press: Beijing, China, 1997. [Google Scholar]
- Chen, J.A.; Wang, J.F.; Yu, J.; Zeng, Y.; Yang, H.Q.; Zhang, R.Y.; Guo, J.Y. Eco-environmental characteristics of reservoirs in southwest China and thier research prospects. Earth Environ. 2017, 45, 115–125. (In Chinese) [Google Scholar]
- Genkai-Kato, M.; Carpenter, S.R. Eutrophication due to phosphorus recycling in relation to lake morphometry, temperature, and macrophytes. Ecology 2005, 86, 210–219. [Google Scholar] [CrossRef]
- Cong, H.B.; Huang, T.L.; Chai, B.B.; Zhao, J.W. A new mixing–oxygenating technology for water quality improvement of urban water source and its implication in a reservoir. Renew. Energy 2009, 34, 2054–2060. [Google Scholar] [CrossRef]
- Fast, A.W. Effects of artifical destratification on primary production and zoobenthos of captian reservoir, California. Water Resour. Res. 1973, 9, 607–623. [Google Scholar] [CrossRef]
- Heinzmann, B. Restoration concept for lake tegel, a major drinking and bathing water resource in a densely populated area. Environ. Sci. Technol. 1994, 28, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.L.; Ma, Y.; Cong, H.B.; Tan, P. Application of the technology of water lifting and aeration on improving water quality in a deep canyon reservoir—A case study from northern China. Densalination Water Treat. 2014, 52, 1636–1646. [Google Scholar] [CrossRef]
- Little, J.C. Hypolimnetic aerators: Predicting oxygen transfer and hydrodynamics. Water Res. 1995, 29, 2475–2482. [Google Scholar] [CrossRef]
- McGinnis, D.F.; Little, J.C. Bubble dynamics and oxygen transfer in a speece cone. Water Sci. Technol. 1998, 37, 285–292. [Google Scholar]
- Wuest, A.; Norman, H.B. Bubble plume modeling for restoration. Water Resour. Res. 1992, 28, 3235–3250. [Google Scholar] [CrossRef]
- Singleton, V.L.; Little, J.C. Designing hypolimnetic aeration and oxygenation systems—A review. Environ. Sci. Technol. 2006, 40, 7512–7520. [Google Scholar]
- McGinnis, D.F.; Lorke, A.; Wuest, A.; Stockli, A.; Little, J.C. Interaction between a bubble plume and the near field in a stratified lake. Water Resour. Res. 2004, 40. [Google Scholar] [CrossRef]
- Prepas, E.; Field, K.; Murphy, T.; Johnson, W.; Burke, J.; Tonn, W. Introduction to the amisk lake project: Oxygenation of a deep, eutrophic lake. Can. J. Fish. Aquat. Sci. 1997, 54, 2105–2110. [Google Scholar] [CrossRef]
- Moore, B.C.; Cross, B.K.; Beutel, M.; Dent, S.; Preece, E.; Swanson, M. Newman lake restoration: A case study part III. Hypolimnetic oxygenation. Lake Reserv. Manag. 2012, 28, 311–327. [Google Scholar] [CrossRef]
- Mostefa, G.; Ahmed, K.; Abdelkader, D. Study of the oxygen transfer efficiencies in the different methods used in the technique of hypolimnetic aeration. Adv. Mater. Res. 2012, 452, 1014–1019. [Google Scholar]
- Feng, X.B.; Bai, W.Y.; Shang, L.H.; He, T.R.; Qiu, G.L.; Yan, H.Y. Mercury speciation and distribution in aha reservoir which was contaminated by coal mining activities in Guiyang, Guizhou, China. Appl. Geochem. 2011, 26, 213–221. [Google Scholar] [CrossRef]
- Song, L.T.; Liu, C.Q.; Wang, Z.L.; Zhu, X.K.; Teng, Y.G.; Liang, L.L.; Tang, S.H.; Li, J. Iron isotope fractionation during biogeochemical cycle: Information from suspended particulate matter (spm) in aha lake and its tributaries, Guizhou, China. Chem. Geol. 2011, 280, 170–179. [Google Scholar] [CrossRef]
- Wang, F.S.; Liu, C.Q.; Liang, X.B.; Wei, Z.Q. Remobilization of trace metals induced by microbiological activities near sediment-water interface, Aha Lake, Guiyang. Chin. Sci. Bull. 2003, 48, 2352–2356. [Google Scholar] [CrossRef]
- Matzinger, A.; Müller, B.; Niederhauser, P.; Schmid, M.; Wüest, A. Hypolimnetic oxygen consumption by sediment-based reduced substances in former eutrophic lakes. Limnol. Oceanogr. 2010, 55, 2073–2084. [Google Scholar] [CrossRef]
- Kristiansen, K.; Kristensen, E.; Jensen, M. The influence of water column hypoxia on the behaviour of manganese and iron in sandy coastal marine sediment. Estuar. Coast. Shelf Sci. 2002, 55, 645–654. [Google Scholar] [CrossRef]
- Roitz, J.; Flegal, A.; Bruland, K. The biogeochemical cycling of manganese in san francisco bay: Temporal and spatial variations in surface water concentrations. Estuar. Coast. Shelf Sci. 2002, 54, 227–239. [Google Scholar] [CrossRef]
- Bryant, L.D.; Hsu-Kim, H.; Gantzer, P.A.; Little, J.C. Solving the problem at the source: Controlling mn release at the sediment-water interface via hypolimnetic oxygenation. Water Res. 2011, 45, 6381–6392. [Google Scholar] [CrossRef] [PubMed]
- Muller, B.; Bryant, L.D.; Matzinger, A.; Wuest, A. Hypolimnetic oxygen depletion in eutrophic lakes. Environ. Sci. Technol. 2012, 46, 9964–9971. [Google Scholar] [CrossRef] [PubMed]
- Prepas, E.; Burke, J. Effects of hypolimnetic oxygenation on water quality in amisk lake, alberta, a deep, eutrophic lake with high internal phosphorus loading rates. Can. J. Fish. Aquat. Sci. 1997, 54, 2111–2120. [Google Scholar] [CrossRef]
- Meng, B.; Feng, X.B.; Chen, C.X.; Qiu, G.L.; Sommar, J.; Guo, Y.N.; Liang, P.; Wan, Q. Influence of eutrophication on the distribution of total mercury and methylmercury in hydroelectric reservoirs. J. Environ. Qual. 2010, 39, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Karl, E.; Karl, E.; Lancelot, C.; Gene, E.; Gene, E. Controlling eutrophication: Nitrogen and phosphorus. Science 2009, 123, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Ryther, J.H.; Dunstan, W.M. Nitrogen, phosphorus, and eutrophication in the coastal marine environment. Science 1971, 171, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Long, S.X.; Yang, Y.; Xia, P.H.; Chen, C.; Liu, Z.W.; Ma, J.R.; Dong, X.; He, T.R.; Yun, G. Accumulation of metals in zooplankton from karst plateau reservoirs with different eutrophic status in guizhou province, PR China. Crustaceana 2016, 89, 537–557. [Google Scholar] [CrossRef]
- Hupfer, M.; Gächter, R.; Giovanoli, R. Transformation of phosphorus species in settling seston and during early sediment diagenesis. Aquat. Sci.-Res. Across Bound. 1995, 57, 305–324. [Google Scholar] [CrossRef]
- Nowlin, W.H.; Evarts, J.L.; Vanni, M.J. Release rates and potential fates of nitrogen and phosphorus from sediments in a eutrophic reservoir. Freshw. Biol. 2005, 50, 301–322. [Google Scholar] [CrossRef]
- Beutel, M.W. Inhibition of ammonia release from anoxic profundal sediments in lakes using hypolimnetic oxygenation. Ecol. Eng. 2006, 28, 271–279. [Google Scholar] [CrossRef]
- Li, Q.H. Biological Prevention Project for Water Intake (Emergency for Cyanobacteria) of the Aha Reservoir—Monitoring of Algae and Algal Toxins; Guizhou Normal University: Guiyang, China, 2017; p. 26. [Google Scholar]
| Sites | July | September | PND | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NH4+ | Total Fe | Total Mn | NH4+ | Total Fe | Total Mn | NH4+ | Total Fe | Total Mn | |
| In situ | 0.48–0.71 | 37.6–67.5 | 2.4–12.0 | 0.16–0.34 | 4.40–91.4 | 0.4–1.4 | 30–71 | 74–94 | 74–88 |
| NW4 | 0.47–0.54 | 8.10–45.4 | 0.8–1.5 | 0.08–0.27 | 0.10–13.4 | 0.2–0.4 | 49–84 | 94–99 | 53–86 |
| NE4 | 0.47–0.57 | 5.10–45.7 | 0.6–1.3 | 0.14–0.32 | 0.40–33.5 | 0.1–0.8 | 43–71 | 27–92 | 36–81 |
| SW4 | 0.56–0.59 | 11.3–42.4 | 1.1–4.6 | 0.14–0.32 | 3.30–10.8 | 0.3–0.5 | 46–76 | 79–91 | 74–88 |
| DB | 0.34–0.35 | 15.7–27.4 | 1.4–2.2 | 0.18–0.22 | 13.6–58.3 | 1.1–1.8 | 38–47 | 51–60 | 17–21 |
| JZH/DHP * | 0.42–0.45 | 23.3–29.7 | 1.8–2.1 | 0.14–0.34 | 11.4–33.0 | 1.0–1.2 | 19–69 | 10–51 | 35–49 |
| Jul. 2015 | Aug. 2015 | Sep. 2015 | Jul. 2016 | Aug. 2016 | Sep. 2016 | |
|---|---|---|---|---|---|---|
| TP (mg/L) | ||||||
| Upper | 0.03 | 0.05 | 0.04 | 0.03 | 0.01 | 0.01 |
| Middle | 0.03 | 0.06 | 0.05 | 0.03 | 0.02 | 0.02 |
| Bottom | 0.06 | 0.07 | 0.05 | 0.05 | 0.03 | 0.03 |
| AVG | 0.04 | 0.06 | 0.05 | 0.04 | 0.02 | 0.02 |
| TN (mg/L) | ||||||
| Upper | 1.8 | 2.3 | 2.1 | 1.7 | 1.6 | 1.4 |
| Middle | 2.0 | 2.8 | 2.3 | 2.2 | 1.7 | 1.6 |
| Bottom | 2.1 | 3.0 | 2.1 | 1.8 | 1.9 | 1.9 |
| AVG | 2.0 | 2.7 | 2.2 | 1.9 | 1.7 | 1.6 |
| Jul. | Aug. | Sep. | Aug. & Sep. | Aug. & Sep. | Jul. | Jul. & Aug. | Aug. & Sep. | Jul. & Sep. | Jul. | Aug. & Sep. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E.Z. | E.Z. | E.Z. | E.Z. | C.S. | C.S. | E.Z. | E.Z. | E.Z. | E.Z. & C.S. | E.Z. & C.S. | ||||||
| TP | |t| | p | |t| | p | |t| | p | |t| | p | |t| | p | ||||||
| 0.04 | 0.03 | 0.02 | 0.02 | 0.04 | 0.04 | 3.3 | 0.003 | 3.2 | 0.004 | 5.7 | 9 × 10‒6 | 0.28 | 0.8 | 8.3 | 1 × 10‒10 | |
| TN | |t| | p | |t| | p | |t| | p | |t| | p | |t| | p | ||||||
| 1.9 | 1.8 | 1.7 | 1.7 | 2.0 | 1.9 | 1.3 | 0.2 | 1.2 | 0.3 | 2.2 | 0.04 | 0.49 | 0.6 | 3.4 | 0.001 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, C.; Chen, J.; Wang, J.; Guo, J.; Yu, J.; Yu, P.; Yang, H.; Liu, Y. Application of Circular Bubble Plume Diffusers to Restore Water Quality in a Sub-Deep Reservoir. Int. J. Environ. Res. Public Health 2017, 14, 1298. https://doi.org/10.3390/ijerph14111298
Lan C, Chen J, Wang J, Guo J, Yu J, Yu P, Yang H, Liu Y. Application of Circular Bubble Plume Diffusers to Restore Water Quality in a Sub-Deep Reservoir. International Journal of Environmental Research and Public Health. 2017; 14(11):1298. https://doi.org/10.3390/ijerph14111298
Chicago/Turabian StyleLan, Chen, Jingan Chen, Jingfu Wang, Jianyang Guo, Jia Yu, Pingping Yu, Haiquan Yang, and Yong Liu. 2017. "Application of Circular Bubble Plume Diffusers to Restore Water Quality in a Sub-Deep Reservoir" International Journal of Environmental Research and Public Health 14, no. 11: 1298. https://doi.org/10.3390/ijerph14111298





