1. Background
Osteoporosis is characterized by the deterioration of bone mass and microarchitecture, leading to impaired bone strength and subsequently fragility fracture [
1]. Osteoporotic fractures are defined as low-impact fractures resulting from low-level trauma, such as a fall from a standing height or less that would not ordinarily result in fracture [
2,
3]. However, osteoporotic fracture is not straightforwardly defined, which sometimes causes misunderstanding. For example, although large studies have shown that nearly all types of fractures occur more often in patients with low bone mineral density (BMD) irrespective of the site [
4,
5], a low BMD alone might not fully detect the risk of osteoporotic fractures [
4] and fractures are not always associated with low BMD [
6]. In addition, under such definition, bone fragility does not presumably contribute to fractures associated with a high-level trauma. In a study that compared the BMD of a random sample of women who sustained fractures in either low- or high-level trauma events, the results revealed that, in a high-energy trauma, patients with osteoporosis are more prone to fracture than those without osteoporosis [
7]. The exclusion of high-level trauma fractures may result in the underestimation of the contribution of osteoporosis to fractures [
7].
The BMD measured at the lumbar spine and hip is currently the standard assessment tool in diagnosing osteoporosis. The relationship between low BMD and major osteoporotic fractures, including the spine [
8,
9], hip [
10,
11], humerus [
12,
13], and forearm [
14], has already been established. Considering that advanced age and low body weight are strongly associated with low BMD and increased risk of bony fracture [
6,
15,
16], the World Health Organization (WHO) developed the Osteoporosis Self-Assessment Tool for Asians (OSTA) score calculated using the following formula: (body weight (kg) − age (year)) × 0.2 to identify women at risk for osteoporosis [
17]. A significant positive correlation was found between the OSTA index and T-scores of BMD measured by dual energy X-ray absorptiometry at the femoral neck [
18,
19]. In this developmental study, OSTA performed better than other osteoporotic indices by showing a sensitivity of 91%, specificity of 45%, and receiver operating characteristic curve of 0.79 at the cutoff of −1 [
17]. In addition, at the cutoff of −1, the difference in OSTA performance was minimal regardless of using the femoral neck and lumbar spine BMD as reference [
20]. Based on the OSTA scores, patients could be stratified as with low (OSTA > −1), medium (−1 ≥ OSTA ≥ −4), and high risk (OSTA < −4) for sustaining osteoporosis [
21,
22]. It is estimated that the probability of a patient with an OSTA score >−4 not having osteoporosis is 99.3% [
23]. According to OSTA score, the risk of osteoporosis is 61%, 15% and 3% for those patients with high-, medium- and low-risk osteoporosis, respectively [
17]. With strong correlations for the populations in Taiwan [
21,
24], China [
20], Korea [
25], Singapore [
26], Malaysia [
23], Thailand [
27], and Philippines [
28], OSTA has been validated as an effective and feasible screening tool to identify patients at risk for sustaining osteoporosis [
17,
18,
23,
26,
28,
29,
30,
31].
In the emergency department (ED), the terms of fracture modifiers such as “low trauma” and “fragility” are challenging both for the patients and providers. The forces applied to a bone are hardly defined merely from a description of the event. In addition, quantifying the level of applied skeletal force is almost impossible in clinical practice. Over-consideration of this context may be misleading the physician in managing the patients. Currently, little is known regarding the impact of osteoporosis on the fracture patterns of patients with trauma. The main question is, in the event of trauma, is the location of fractures in patients at high- or medium risk for osteoporosis tend to be different from those at low-risk for osteoporosis? Therefore, this study aimed to investigate the fracture patterns of patients with different risks of osteoporosis based on the OSTA score of female trauma patients in a Level I trauma center.
4. Discussion
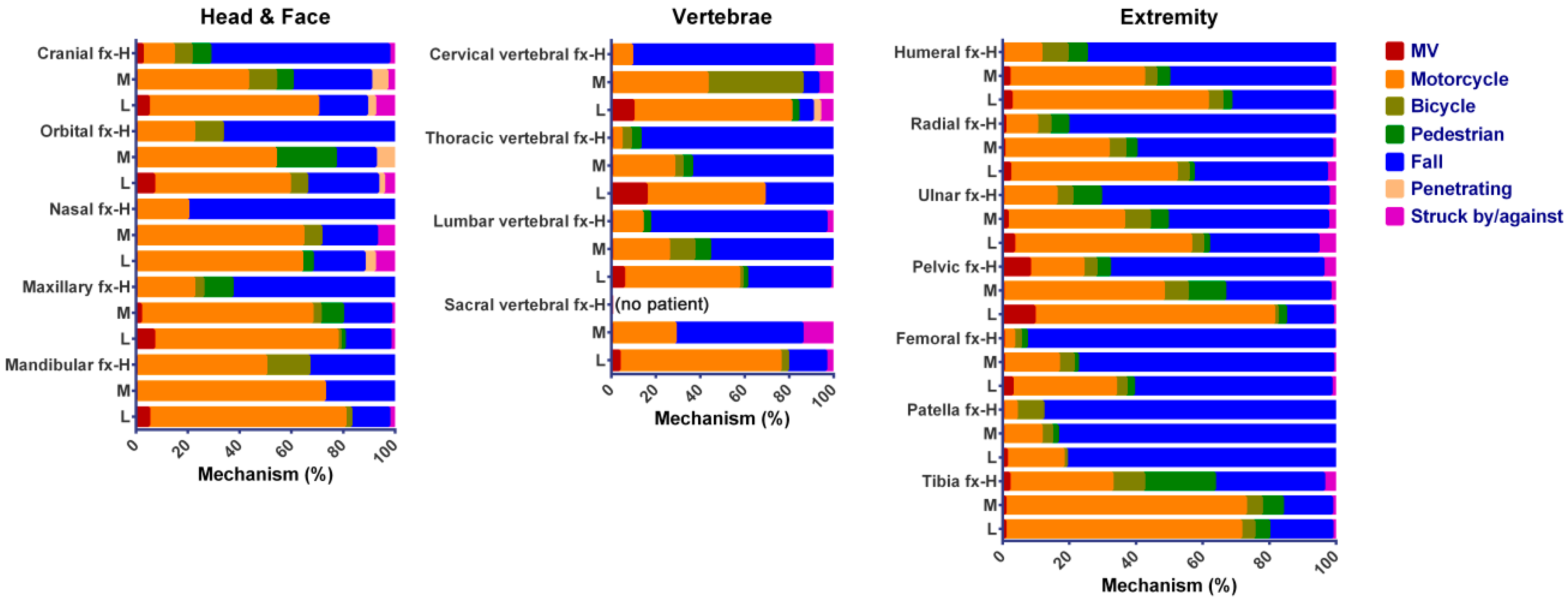
This study compared the clinical fracture patterns of hospitalized female trauma patients, irrespective of injury mechanisms, based on the OSTA classification of associated risk to osteoporosis. In this study, high- and medium-risk patients were significantly older, had higher incidences of comorbidity, and were more frequently injured from a fall and bicycle accident than low-risk patients had. Compared to low-risk patients, high- and medium-risk patients had a higher injury severity and mortality. High-risk, but not medium-risk, patients had a higher proportion of patients admitted to the ICU than low-risk patients had. However, there was no significant difference of hospital LOS between high-risk or medium-risk patients and low-risk patients. After the attenuation of confounding effects to the mechanism of injury in the propensity-score matching, the incidence of fractures was only different in the extremity region between high- or medium-risk patients and low-risk patients. The incidences of femoral fractures are significantly higher in high-risk (OR 3.4; 95% CI 2.73–4.24; p < 0.001) and medium-risk (OR 1.4; 95% CI 1.24–1.54; p < 0.001) patients than low-risk patients had. In addition, high-risk patients had significantly lower odds of humeral, radial, patellar, and tibial fracture than low-risk patients had; however, such lower odds was not found between medium-risk and low-risk patients.
The existing literature suggests that OSTA can suitably be used in screening patients with increased risk of osteoporosis. As OSTA only considers two risk factors, i.e., age and body weight, the fracture pattern is not surprisingly deeply influenced by these two risk factors. With significantly older age in high- and medium-risk patients, higher incidences of comorbidity and injuries from a fall and bicycle accident were encountered more frequently than low-risk patients had. In this study, the percentage of high-risk patients sustaining a fall accident was much higher (80.6%) than that of medium-risk (51.0%) and low-risk (30.6%) patients. In a fall accident, the force impacts directly onto the posterolateral aspect of the greater trochanter, making the femoral neck particularly vulnerable to fractures [
42]. In older age, the proximal femoral fractures occur not necessarily with high energy [
43]. Obviously, the injury mechanisms may have a great impact on the fracture patterns found in patients with different OSTA scores. Therefore, comparison of propensity-score matched populations was performed in this study to reduce the confounding effect of injury mechanisms and the results indicated the incidences of fractures was different mainly in the extremity region between the patients with different risk of osteoporosis.
Regarding the body weight, obesity was once thought to protect people from having a fracture because of the observation that the bone in obese people was less osteoporotic. In a fall, the soft tissue padding may protect obese people against pelvic and hip fractures. In addition, the wrist was also protected from an impact because obese people tend to fall backward or sideways rather than forward, and together with impaired protective reactions to falling [
44]. However, several conditions associated with obesity have adverse effects on bone health through various mechanisms, such as reduced physical activity, co-medications, and decreased 25-hydroxyvitamin D levels and consequent increased serum levels of parathyroid hormones [
44]. Obese patients (body mass index of >30) had been reported to have an increased fracture severity and were more likely to suffer a complex injury resulting from a fall from a standing position [
45]. The Global Longitudinal study of Osteoporosis in Women (GLOW) showed that obese women had higher incidences of ankle and upper leg fractures [
46]. Obesity is increasingly associated with increased risk of fracture, albeit the pathogenesis of fracture in obese individuals have not yet been clearly defined [
15]. The effect of obesity on fracture risk is site-dependent, the risk being increased for some fractures (humerus, ankle, and upper arm) and decreased for others (hip, pelvis, and wrist) [
15]. Furthermore, the relationship between obesity and fracture may also vary by age and ethnicity [
15]. Notably, obesity is not equal to the body weight, which used to calculate OSTA. Therefore, results of obesity researches should be cautiously interpreted.
Common osteoporotic fracture sites include bones that bear weight (such as the spine, pelvis, and hip) or bones that take most of the stress in a fall from a standing height or less (such as upper arm, forearm, and wrist) [
6]. Notably, in this study that included patients with all trauma causes, not all fractures are due to osteoporosis. However, the forces applied to a bone during the accident is hard to define in clinical practice. The force impact on the bone may greatly vary even with the same mechanism. In the study conducted by Sanders et al. who revealed that excluding high trauma fractures may underestimate the prevalence of bone fragility fractures, 77.1% (835/1084) and 22.9% (249/1804) of the patients have low and high trauma, respectively. In this study, the composition of low and high trauma was not recorded in the registered data. Although that could be surmised according to the injury mechanisms in most of the registered patients, this would result in a limitation in the interpretation of results.
In this study, there was a 1.4-fold and 3.4-fold odds of femoral fracture between high- and medium-risk patients as well as between high- and low-risk patients. Fractures due to osteoporosis represent a serious and costly public health problem and lead to disability and increased mortality of the patients [
47]. To reduce these osteoporosis-related fractures, a multidisciplinary approach by the ortho-geriatric model [
48], which involving both an orthopedic surgeon and a geriatrician plus a nursing and physiotherapy team, and a fracture liaison service (FLS) [
49] had been proposed to reduce re-peat fracture risk and mortality. In addition, the extended assessment will include measures of delirium, prevention of malnutrition, treatment of co-morbidities, and a review of medications with the aim to reduce such medications that promote fall risk or interfere with each other [
50]. Some strategies had been implemented to manage the patients with high risk of fracture due to osteoporosis [
47], which included 1. At least one session devoted to education to the patients regarding osteoporosis, fracture risk, and medication choices; 2. Adequate calcium, vitamin D, and weight-bearing and resistance exercise; 3. Consider one of some pharmacologic agents to reduce bone resorption and decrease the risk to fracture; 4. Identify and address non-skeletal risk factors for falling and fracture: problems with vision, hearing, balance, home safety adjustments, avoidance of floor rugs, etc. 5. Periodical reassessment in every 1 to 2 years. The patients with a history of prior fracture represent a high risk group and should be targeted for intervention to reduce future fracture rates [
51]. For example, the uptake of bisphosphonates and the rollout of public health strategies addressing osteoporosis were suggested to reduce the age-standardized incidence of osteoporotic hip fracture for both females and males in Australia [
52].
This study has some other limitations that should be acknowledged. First, in most studies, OSTA demonstrated high sensitivity and low specificity values [
17,
27,
28]. The probability of patients with OSTA score of <−4 having osteoporosis and with an OSTA score of >−4 not having osteoporosis are 53.8% and 99.3%, respectively [
23]. This means that a high percentage of subjects categorized as moderate or high risk for osteoporosis by the OSTA actually have normal bone health status based on the bone densitometry (false-positive). Second, some studies showed that OSTA alone did not satisfactorily predict fracture risks in subjects with pre-existing medical conditions [
53]. Considering the incidences of comorbidities are higher in high-risk and medium-risk patients than that in the low-risk patients, bias would possibly result among the patients with different risk to osteoporosis. Third, this study focused on hospitalized female patients only; however, some fractures at sites other than the hip are manageable without hospital admissions, which may lead to underestimation on the incidence of these fractures and result in a selection bias. Furthermore, the actual reason of the lower rates of humeral, radial, patellar and tibial fractures high-risk patients than those low-risk patients was unknown. We speculated that is because the femoral bone would share most of the force in the extremities during a fall, thus making a higher incidence of femoral fracture but a reciprocal lower incidence of other fractures in extremities. However, there is lack of evidences in supporting such opinions so far. Finally, an inherent selection bias already existed because of the retrospective study design, particularly when considering the impact force of each injury as well as the drugs for treating osteoporosis was not recorded.