Sleep and Dietary Patterns in Pregnancy: Findings from the GUSTO Cohort
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Dietary Intake, Diet Quality and Dietary Pattern Analysis
2.3. Eating Behaviors
2.4. Sleep Quality and Sleep Duration Assessment
2.5. Covariates
2.6. Statistical Analyses
3. Results
3.1. Participants’ Characteristics
3.2. Sleep Quality
3.3. Sleep Duration
3.4. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Capers, P.L.; Fobian, A.D.; Kaiser, K.A.; Borah, R.; Allison, D.B. A systematic review and meta-analysis of randomized controlled trials of the impact of sleep duration on adiposity and components of energy balance. Obes. Rev. 2015, 16, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R. Reduced sleep as an obesity risk factor. Obes. Rev. 2009, 10, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J.P. Sleep patterns, diet quality and energy balance. Physiol. Behav. 2014, 134, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Dashti, H.S.; Scheer, F.A.; Jacques, P.F.; Lamon-Fava, S.; Ordovas, J.M. Short sleep duration and dietary intake: Epidemiologic evidence, mechanisms, and health implications. Adv. Nutr. 2015, 6, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Ocké, M.C. Evaluation of methodologies for assessing the overall diet: Dietary quality scores and dietary pattern analysis. Proc. Nutr. Soc. 2013, 72, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.K.; Chu, Y.L.; Kirk, S.F.; Veugelers, P.J. Are sleep duration and sleep quality associated with diet quality, physical activity, and body weight status? A population-based study of canadian children. Can. J. Public Health 2015, 106, E277–E282. [Google Scholar] [CrossRef] [PubMed]
- Bel, S.; Michels, N.; De Vriendt, T.; Patterson, E.; Cuenca-García, M.; Diethelm, K.; Gutin, B.; Grammatikaki, E.; Manios, Y.; Leclercq, C.; et al. Association between self-reported sleep duration and dietary quality in european adolescents. Br. J. Nutr. 2013, 110, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Haghighatdoost, F.; Karimi, G.; Esmaillzadeh, A.; Azadbakht, L. Sleep deprivation is associated with lower diet quality indices and higher rate of general and central obesity among young female students in Iran. Nutrition 2012, 28, 1146–1150. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Mikic, A.; Pietrolungo, C.E. Effects of diet on sleep quality. Adv. Nutr. 2016, 7, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Hogenkamp, P.S.; Nilsson, E.; Nilsson, V.C.; Chapman, C.D.; Vogel, H.; Lundberg, L.S.; Zarei, S.; Cedernaes, J.; Rangtell, F.H.; Broman, J.E.; et al. Acute sleep deprivation increases portion size and affects food choice in young men. Psychoneuroendocrinology 2013, 38, 1668–1674. [Google Scholar] [CrossRef] [PubMed]
- Kant, A.K.; Graubard, B.I. Association of self-reported sleep duration with eating behaviors of American adults: NHANES 2005–2010. Am. J. Clin. Nutr. 2014, 100, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Mossavar-Rahmani, Y.; Jung, M.; Patel, S.R.; Sotres-Alvarez, D.; Arens, R.; Ramos, A.; Redline, S.; Rock, C.L.; Van Horn, L. Eating behavior by sleep duration in the Hispanic community health study/study of Latinos. Appetite 2015, 95, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; DeRoo, L.A.; Sandler, D.P. Eating patterns and nutritional characteristics associated with sleep duration. Public Health Nutr. 2010, 14, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.; Shin, J.; Kim, M.Y.; Kim, Y.H.; Lee, J.; Kil, K.C.; Moon, H.B.; Lee, G.; Sa-Jin, K.; Kim, B.I. Sleep disturbances in korean pregnant and postpartum women. J. Psychosom. Obstet. Gynaecol. 2012, 33, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Sivertsen, B.; Hysing, M.; Dorheim, S.K.; Eberhard-Gran, M. Trajectories of maternal sleep problems before and after childbirth: A longitudinal population based study. BMC Pregnancy Childbirth 2015, 15, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchison, B.L.; Stone, P.R.; McCowan, L.M.; Stewart, A.W.; Thompson, J.M.; Mitchell, E.A. A postal survey of maternal sleep in late pregnancy. BMC Pregnancy Childbirth 2012, 12, 144. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.W.; Brown, R.; Nitzke, S.; Smith, B.; Eghtedary, K. Stress, sleep, depression and dietary intakes among low-income overweight and obese pregnant women. Matern. Child Health J. 2015, 19, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Soh, S.E.; Tint, M.T.; Gluckman, P.D.; Godfrey, K.M.; Rifkin-Graboi, A.; Chan, Y.H.; Stunkel, W.; Holbrook, J.D.; Kwek, K.; Chong, Y.S.; et al. Cohort profile: Growing up in Singapore towards healthy outcomes (GUSTO) birth cohort study. Int. J. Epidemiol. 2014, 43, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.M.; Ingwersen, L.A.; Vinyard, B.T.; Moshfegh, A.J. Effectiveness of the US department of agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am. J. Clin. Nutr. 2003, 77, 1171–1178. [Google Scholar] [PubMed]
- Health Promotion Board. Energy and Nutrient Composition of Food; Health Promotion Board: Singapore, 2011. [Google Scholar]
- United States Department of Agriculture. USDA Food Composition Database; USDA: Baltimore, MD, USA, 2011.
- Han, C.Y.; Colega, M.; Quah, E.P.L.; Chan, Y.H.; Godfrey, K.M.; Kwek, K.; Saw, S.M.; Gluckman, P.D.; Chong, Y.S.; Chong, M.F.F. A healthy eating index to measure diet quality in pregnant women in Singapore: A cross-sectional study. BMC Nutr. 2015, 1, 39. [Google Scholar] [CrossRef]
- Chia, A.R.; de Seymour, J.V.; Colega, M.; Chen, L.W.; Chan, Y.H.; Aris, I.M.; Tint, M.T.; Quah, P.L.; Godfrey, K.M.; Yap, F.; et al. A vegetable, fruit, and white rice dietary pattern during pregnancy is associated with a lower risk of preterm birth and larger birth size in a multiethnic Asian cohort: The growing up in Singapore towards healthy outcomes (GUSTO) cohort study. Am. J. Clin. Nutr. 2016, 104, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Loy, S.L.; Chan, J.K.; Wee, P.H.; Colega, M.T.; Cheung, Y.B.; Godfrey, K.M.; Kwek, K.; Saw, S.M.; Chong, Y.S.; Natarajan, P.; et al. Maternal circadian eating time and frequency are associated with blood glucose concentrations during pregnancy. J. Nutr. 2017, 147, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Loy, S.L.; Cheng, T.S.; Colega, M.T.; Cheung, Y.B.; Godfrey, K.M.; Gluckman, P.D.; Kwek, K.; Saw, S.M.; Chong, Y.S.; Padmapriya, N.; et al. Predominantly night-time feeding and maternal glycaemic levels during pregnancy. Br. J. Nutr. 2016, 115, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Backhaus, J.; Junghanns, K.; Broocks, A.; Riemann, D.; Hohagen, F. Retest reliability and validity of the Pittsburgh sleep quality index in primary insomnia. J. Psychosom. Res. 2002, 53, 737–740. [Google Scholar] [CrossRef]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Katz, E.S.; LKheirandish-Gozal, L.; et al. National sleep foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health 2015, 1, 40–43. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013; p. 302. [Google Scholar]
- Willett, W.C. Nutritional Epidemiology; NYO Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Jaussent, I.; Dauvilliers, Y.; Ancelin, M.L.; Dartigues, J.F.; Tavernier, B.; Touchon, J.; Ritchie, K.; Besset, A. Insomnia symptoms in older adults: Associated factors and gender differences. Am. J. Geriatr. Psychiatry 2011, 19, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health. BioFactors 2013, 39, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, R.; Asakura, K.; Kobayashi, S.; Suga, H.; Sasaki, S. Three-generation Study of Women on Diets and Health Study Group. Low intake of vegetables, high intake of confectionary, and unhealthy eating habits are associated with poor sleep quality among middle-aged female japanese workers. J. Occup. Health 2014, 56, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, S.; Sakurai, M.; Nakamura, K.; Morikawa, Y.; Miura, K.; Nakashima, M.; Yoshita, K.; Ishizaki, M.; Kido, T.; Naruse, Y.; et al. Associations between rice, noodle, and bread intake and sleep quality in japanese men and women. PLoS ONE 2014, 9, e105198. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.W.; Li, Y.; Winkelman, J.W.; Hu, F.B.; Rimm, E.B.; Gao, X. Probable insomnia is associated with future total energy intake and diet quality in men. Am. J. Clin. Nutr. 2016, 104, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.H.; Grant, A.S.; Thomson, C.A.; Tinker, L.; Hale, L.; Brennan, K.M.; Woods, N.F.; Chen, Z. Short sleep duration is associated with decreased serum leptin, increased energy intake, and decreased diet quality in postmenopausal women. Obesity 2014, 22, E55–E61. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Diet quality as assessed by the healthy eating index, the alternate healthy eating index, the dietary approaches to stop hypertension score, and health outcomes: A systematic review and meta-analysis of cohort studies. J. Acad. Nutr. Diet. 2015, 115, 780–800. [Google Scholar] [CrossRef] [PubMed]
- Sotos-Prieto, M.; Bhupathiraju, S.N.; Mattei, J.; Fung, T.T.; Li, Y.; Pan, A.; Willett, W.C.; Rimm, E.B.; Hu, F.B. Association of changes in diet quality with total and cause-specific mortality. N. Engl. J. Med. 2017, 377, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Kurotani, K.; Kochi, T.; Nanri, A.; Eguchi, M.; Kuwahara, K.; Tsuruoka, H.; Akter, S.; Ito, R.; Pham, N.M.; Kabe, I.; et al. Dietary patterns and sleep symptoms in Japanese workers: The Furukawa nutrition and health study. Sleep Med. 2015, 16, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.S.; Moore Simas, T.A.; Pagoto, S.L.; Person, S.D.; Rosal, M.C.; Waring, M.E. Sleep duration and diet quality among women within 5 years of childbirth in the united states: A cross-sectional study. Matern. Child Health J. 2016, 20, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.; Dregan, A. A population-based investigation into the self-reported reasons for sleep problems. PLoS ONE 2014, 9, e101368. [Google Scholar] [CrossRef] [PubMed]
- Baskin, R.; Hill, B.; Jacka, F.N.; O’Neil, A.; Skouteris, H. The association between diet quality and mental health during the perinatal period. A systematic review. Appetite 2015, 91, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Cudd, T.A.; Meininger, C.J.; Spencer, T.E. Maternal nutrition and fetal development. J. Nutr. 2004, 134, 2169–2172. [Google Scholar] [PubMed]
- Cai, S.; Tan, S.; Gluckman, P.D.; Godfrey, K.M.; Saw, S.M.; Teoh, O.H.; Chong, Y.S.; Meaney, M.J.; Kramer, M.S.; Gooley, J.J.; et al. Sleep quality and nocturnal sleep duration in pregnancy and risk of gestational diabetes mellitus. Sleep 2017, 40, zsw058. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.Y.; Lee, P.L.; Lin, J.W.; Lee, C.N. Cross-sectional and longitudinal associations between sleep and health-related quality of life in pregnant women: A prospective observational study. Int. J. Nurs. Stud. 2016, 56, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.M.; Bhatia, S.K.; Bhatia, S.K.; Khawaja, I.S. Insomnia during pregnancy: Diagnosis and Rational Interventions. Pak. J. Med. Sci. 2016, 32, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Block, G. A review of validations of dietary assessment methods. Am. J. Epidemiol. 1982, 115, 492–505. [Google Scholar] [CrossRef] [PubMed]
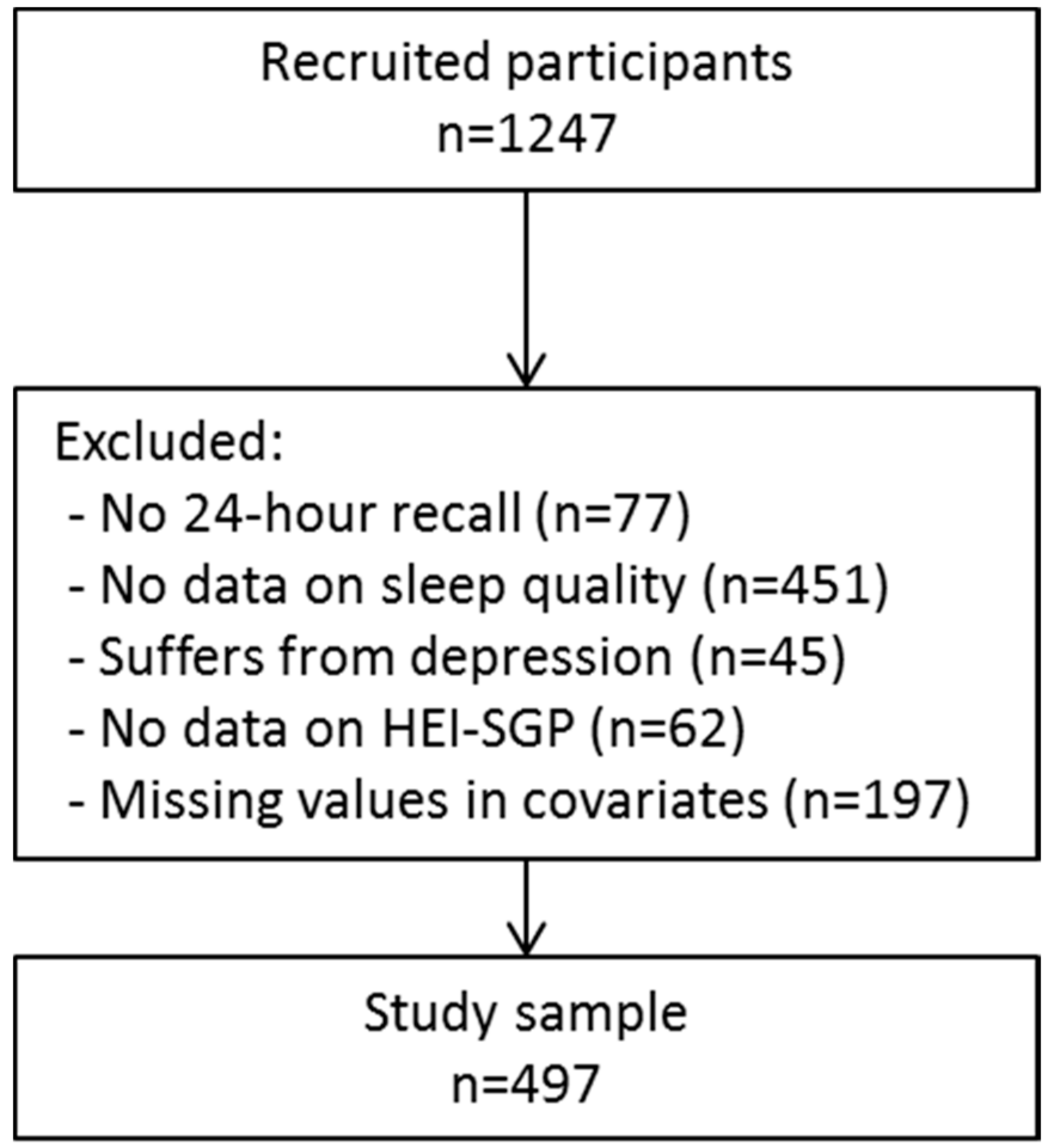
| Poor Sleep Quality (PSQI > 5) | Good Sleep Quality (PSQI ≤ 5) | p Value | |
|---|---|---|---|
| N | 271 | 226 | |
| Age (y) | 30.8 (4.5) | 30.7 (5.0) | 0.942 |
| Pre-pregnancy BMI (kg/m2) | 22.8 (4.2) | 22.5 (4.5) | 0.463 |
| Sleep duration (hours) | 6.6 (1.4) | 8.0 (1.2) | <0.001 |
| Energy intake (kcal) | 1902 (551) | 1875 (573) | 0.596 |
| Education | 0.130 | ||
| Primary/secondary | 70, 25.8% | 48, 21.2% | |
| Postsecondary | 37, 13.7% | 22, 9.7% | |
| University | 164, 60.5% | 156, 69.0% | |
| Household income | 0.171 | ||
| S$0–1999 | 37, 13.7% | 21, 9.3% | |
| S$2000–5999 | 79, 29.2% | 59, 26.1% | |
| ≥S$6000 | 155, 57.2% | 146, 64.6% | |
| Ethnicity | 0.018 | ||
| Chinese | 127, 46.9% | 137, 60.6% | |
| Malay | 87, 32.1% | 52, 23.0% | |
| Indian | 56, 20.7% | 37, 16.4% | |
| Pre-pregnancy physical active | 171, 63.6% | 146, 64.6% | 0.811 |
| Physical active during pregnancy | 61, 22.5% | 74, 32.7% | 0.011 |
| pre-pregnancy alcohol use | 168, 62.0% | 140, 62.0% | 0.992 |
| Alcohol use during pregnancy | 3, 1.1% | 5, 2.2% | 0.332 |
| First child | 117, 43.2% | 106, 46.9% | 0.405 |
| Gestational Diabetes | 55, 21.8% | 36, 16.9% | 0.182 |
| Probable anxiety | 76, 28.0% | 27, 12.0% | <0.001 |
| Model 1 | Model 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Poor Sleep Quality (n = 271) | Good Sleep Quality (n = 226) | p for Difference | Poor Sleep Quality (n = 271) | Good Sleep Quality (n = 226) | p for Difference | |||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |||
| Diet quality (HEI-SGP scores) | 52.0 | 50.4, 53.6 | 54.6 | 52.9, 56.3 | 0.032 | 52.4 | 50.8, 54.0 | 54.5 | 52.7, 56.2 | 0.096 |
| VFR | −0.15 | −0.26, −0.04 | 0.03 | −0.09, 0.15 | 0.039 | −0.13 | −0.24, −0.02 | 0.02 | −0.10, 0.14 | 0.087 |
| SFN | 0.03 | −0.07, 0.13 | −0.14 | −0.25, −0.03 | 0.024 | 0.03 | −0.07, 0.13 | −0.15 | −0.26, −0.04 | 0.018 |
| PCP | 0.03 | −0.09, 0.14 | 0.00 | −0.13, 0.12 | 0.749 | 0.04 | −0.08, 0.16 | −0.01 | −0.14, 0.12 | 0.621 |
| Eating behaviors | ||||||||||
| Night-time fasting interval (h) | 9.83 | 9.64, 10.00 | 9.86 | 9.66, 10.06 | 0.819 | 8.80 | 9.62, 9.99 | 9.84 | 9.63, 10.05 | 0.800 |
| Frequency of consumption occasions | 4.12 | 3.99, 4.25 | 4.26 | 4.11, 4.40 | 0.169 | 4.14 | 4.01, 428 | 4.27 | 4.12, 4.42 | 0.228 |
| Discretionary calories (kcal) † | 382.6 | 346.0, 419.2 | 330.5 | 290.3, 370.6 | 0.063 | 375.9 | 3387, 413.0 | 338.6 | 297.7, 379.4 | 0.198 |
| Night-time eating (TE%) | 33.9 | 31.8, 36.1 | 32.7 | 30.3, 35.0 | 0.476 | 33.7 | 31.5, 35.8 | 33.1 | 30.7, 35.5 | 0.732 |
| Model 1 | Model 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sleep Duration ≤ 6 h (n = 134) | Sleep Duration > 6 h (n = 363) | p | Sleep Duration ≤ 6 h (n = 134) | Sleep Duration > 6 h (n = 363) | p | |||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |||
| Diet quality (HEI-SGP scores) | 52.3 | 50.1, 54.6 | 53.5 | 52.1, 54.8 | 0.405 | 53.0 | 50.8, 55.3 | 53.5 | 52.1, 54.9 | 0.723 |
| VFR | −0.15 | −0.30, 0.01 | −0.04 | −0.13, 0.06 | 0.245 | −0.13 | −0.28, 0.03 | −0.04 | −0.13, 0.06 | 0.353 |
| SFN | 0.01 | −0.13, 0.15 | −0.07 | −0.15,0.02 | 0.378 | 0.00 | −0.15, 0.14 | −0.07 | −0.15, 0.02 | 0.449 |
| PCP | −0.02 | −0.18, 0.14 | 0.03 | −0.07, 0.13 | 0.627 | −0.01 | −0.18, 0.15 | 0.03 | −0.07, 0.13 | 0.659 |
| Eating behaviors | ||||||||||
| Night-time fasting interval (h) | 9.75 | 9.49, 10.01 | 9.87 | 9.72, 10.03 | 0.418 | 9.7 | 9.5, 10.0 | 9.9 | 9.7, 10.0 | 0.365 |
| Frequency of consumption occasions | 4.11 | 3.92, 4.30 | 4.21 | 4.10, 4.32 | 0.368 | 4.15 | 3.96, 4.33 | 4.22 | 4.11, 4.33 | 0.514 |
| discretionary calories (kcal) † | 361.6 | 309.1, 414.1 | 357.9 | 326.3, 389.6 | 0.908 | 358.4 | 306.1, 410.7 | 359.1 | 327.6, 390.6 | 0.982 |
| Night-time eating (TE%) | 33.2 | 30.2, 36.3 | 33.4 | 31.6, 35.3 | 0.920 | 33.1 | 30.1, 36.2 | 33.5 | 31.6, 35.3 | 0.854 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Lee, L.; Chia, A.-R.; Loy, S.L.; Colega, M.; Tham, E.K.H.; Cai, S.; Yap, F.; Godfrey, K.M.; Teoh, O.H.; Goh, D.; et al. Sleep and Dietary Patterns in Pregnancy: Findings from the GUSTO Cohort. Int. J. Environ. Res. Public Health 2017, 14, 1409. https://doi.org/10.3390/ijerph14111409
Van Lee L, Chia A-R, Loy SL, Colega M, Tham EKH, Cai S, Yap F, Godfrey KM, Teoh OH, Goh D, et al. Sleep and Dietary Patterns in Pregnancy: Findings from the GUSTO Cohort. International Journal of Environmental Research and Public Health. 2017; 14(11):1409. https://doi.org/10.3390/ijerph14111409
Chicago/Turabian StyleVan Lee, Linde, Ai-Ru Chia, See Ling Loy, Marjorelee Colega, Elaine K. H. Tham, Shirong Cai, Fabian Yap, Keith M. Godfrey, Oon Hoe Teoh, Daniel Goh, and et al. 2017. "Sleep and Dietary Patterns in Pregnancy: Findings from the GUSTO Cohort" International Journal of Environmental Research and Public Health 14, no. 11: 1409. https://doi.org/10.3390/ijerph14111409





