Cross-Sectional Serological Survey for Leptospira spp. in Beef and Dairy Cattle in Two Districts in Uganda
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Population
2.2. Sample Size
2.3. Serological Analysis
2.4. Statistical Analysis
3. Results
3.1. Data Collection and Description of the Study Population
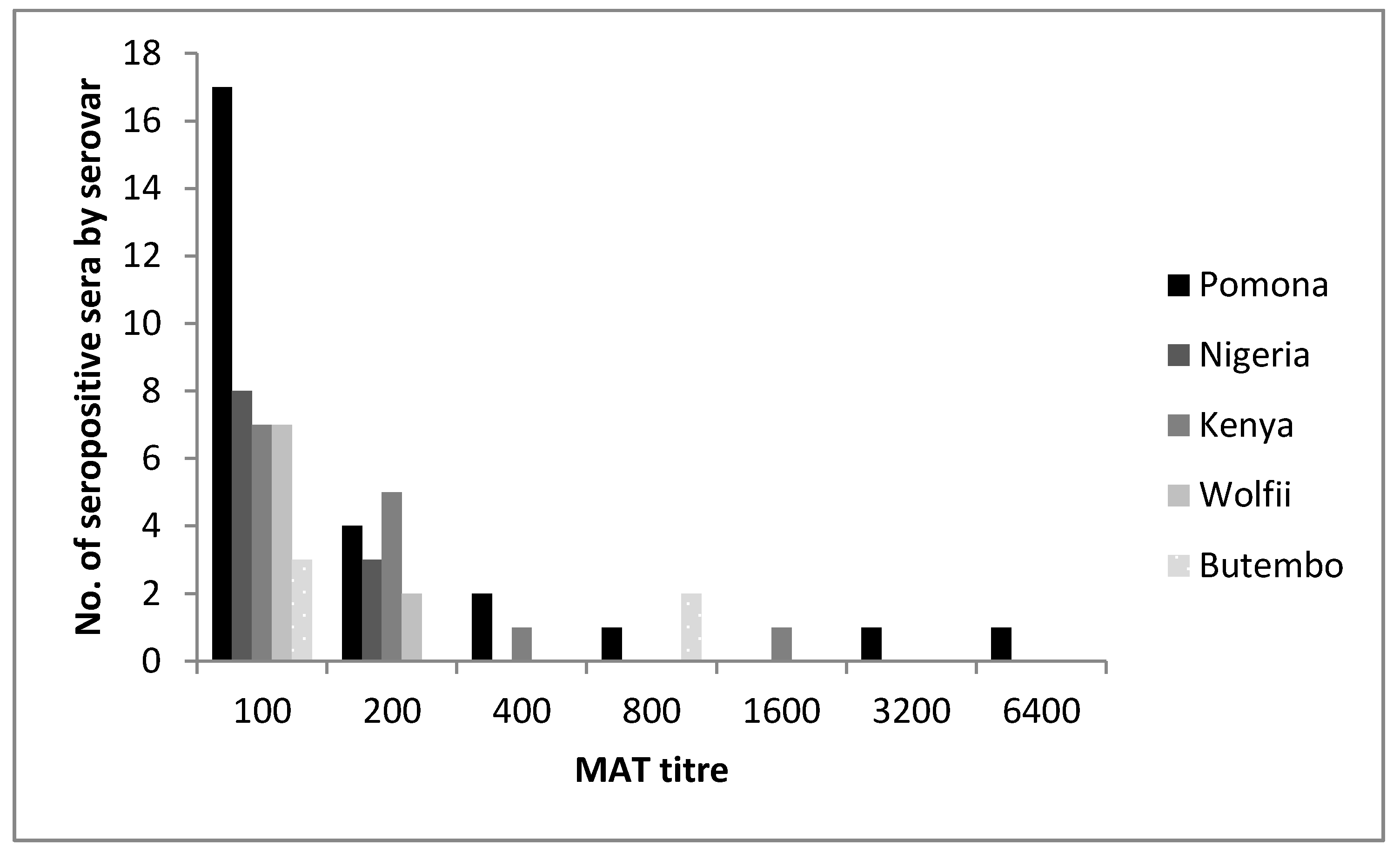
3.2. Serological Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hartskeerl, R.A.; Collares-Pereira, M.; Ellis, W.A. Emergence, control and re-emerging leptospirosis: Dynamics of infection in the changing world. Clin. Microbiol. Infect. 2011, 17, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Adler, B.; de la Pena Moctezuma, A. Leptospira and leptospirosis. Vet. Microbiol. 2010, 140, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Faine, S.; Adler, B.; Bolin, C.; Perolat, P. Leptospira and Leptospirosis; MediSci: Melbourne, Australia, 1999. [Google Scholar]
- Costa, F.; Hagan, J.E.; Calcagno, J.; Kane, M.; Torgerson, P.; Martinez-Silveira, M.S.; Stein, C.; Abela-Ridder, B.; Ko, A.I. Global morbidity and mortality of leptospirosis: A systematic review. PLoS Negl. Trop. Dis. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Ezeh, A.O.; Kmety, E.; Ellis, W.A.; Addo, P.B.; Adesiyun, A.A. A new leptospiral serovar in the pyrogenes serogroup isolated in nigeria. Rev. Sci. Tech. 1990, 9, 1195–1196. [Google Scholar] [CrossRef] [PubMed]
- Dreyfus, A.; Dyal, J.W.; Pearson, R.; Kankya, C.; Kajura, C.; Alinaitwe, L.; Kakooza, S.; Pelican, K.M.; Travis, D.A.; Mahero, M.; et al. Leptospira seroprevalence and risk factors in health centre patients in hoima district, western uganda. PLoS Negl. Trop. Dis. 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Millan, J.; Chirife, A.D.; Kalema-Zikusoka, G.; Cabezon, O.; Muro, J.; Marco, I.; Cliquet, F.; Leon-Vizcaino, L.; Wasniewski, M.; Almeria, S.; et al. Serosurvey of dogs for human, livestock, and wildlife pathogens, uganda. Emerg. Infect. Dis. 2013, 19, 680–682. [Google Scholar] [CrossRef] [PubMed]
- Atherstone, C.; Picozzi, K.; Kalema-Zikusoka, G. Seroprevalence of leptospira hardjo in cattle and african buffalos in southwestern uganda. Am. J. Trop. Med. Hyg. 2014, 90, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Allan, K.J.; Biggs, H.M.; Halliday, J.E.B.; Kazwala, R.R.; Maro, V.P.; Cleaveland, S.; Crump, J.A. Epidemiology of leptospirosis in africa: A systematic review of a neglected zoonosis and a paradigm for ‘one health’ in africa. PLoS Negl. Trop. Dis. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Uganda Bureau of Statistics (UBOS). The National Population and Housing Census 2014—Area Specific Profile Series; UBOS: Kampala, Uganda, 2017. [Google Scholar]
- UBOS; MAAIF Uganda Bureau of Statistics; Ministry of Agriculture Animal Industries and Fisheries. A Summary Report of the National Livestock Census, 2008; UBOS: Kampala, Uganda, 2009.
- Kansiime, M.K.; Wambugu, S.K.; Shisanya, C.A. Perceived and actual rainfall trends and variability in eastern uganda: Implications for community preparedness and response. J. Natl. Sci. Res. 2013, 8, 2224–3186. [Google Scholar]
- Uganda National Meteorological Authority. Kole Rainfall Data 2015; UNMA: Kampala, Uganda, 2017. [Google Scholar]
- Office International des Epizooties (OIE). Leptospirosis. In Oie Terrestrial Manual; OIE: Paris, France, 2014; pp. 1–15. [Google Scholar]
- Goris, M.G.; Hartskeerl, R.A. Leptospirosis serodiagnosis by the microscopic agglutination test. Curr. Protoc. Microbiol. 2014, 32. [Google Scholar] [CrossRef]
- Charleston, B.; Mioulet, V. Personal Communication; Rodriguez, S., Ed.; Pirbright Institute: Woking, UK, 2015. [Google Scholar]
- Levett, P.N. Leptospirosis. Clin. Microbiol. Rev. 2001, 14, 296–326. [Google Scholar] [CrossRef] [PubMed]
- Fleiss, J.L. Statistical Methods for Rates and Proportions; John Wiley & Sons Inc.: New York, NY, USA, 1981. [Google Scholar]
- Dreyfus, A. Leptospirosis in humans and pastoral livestock in new zealand. Ph.D. Thesis, Massey University, Palmerston North, New Zealand, 20 November 2013. [Google Scholar]
- Dreyfus, A.; Heuer, C.; Wilson, P.; Collins-Emerson, J.; Baker, M.G.; Benschop, J. Risk of infection and associated influenza-like disease among abattoir workers due to two leptospira species. Epidemiol. Infect. 2014, 143, 2095–2105. [Google Scholar] [CrossRef] [PubMed]
- Goris, M. Diagnostic tests for human leptospirosis. In Second Meeting of the European Leptospirosis Society on Leptospirosis and Other Rodent Borne Hemorrhagic Fevers; European Leptospirosis Society Royal Tropical Institute: Amsterdam, The Netherlands, 2015. [Google Scholar]
| Genomospecies | Serogroup | Serovar | Strain |
|---|---|---|---|
| L. interrogans | Sejroe | Hardjo | Hardjoprajitno |
| Sejroe | Wolfii | 3705 | |
| Icterohaemorrhagiae | Icterohaemorrhagiae | RGA | |
| Pomona | Pomona | Pomona | |
| L. kirschneri | Autumnalis | Butembo | Butembo |
| Grippotyphosa | Grippotyphosa | Moskva V | |
| L. borgpetersenii | Ballum | Kenya | Njenga |
| Pyrogenes | Nigeria | Vom |
| Serogroup | Serovar | n Positive 1 | Prevalence 2 % | 95% CI |
|---|---|---|---|---|
| Pomona | Pomona | 26 | 9.45 | 6.4–13.7 |
| Ballum | Kenya | 14 | 5.09 | 2.9–8.6 |
| Pyrogenes | Nigeria | 11 | 4.00 | 2.1–7.2 |
| Sejroe | Wolfii | 9 | 3.27 | 1.6–6.3 |
| Autumnalis | Butembo | 5 | 1.82 | 0.7–4.4 |
| Sejroe | Hardjo | 4 | 1.45 | 0.5–3.9 |
| Icterohaemorrhagiae | Icterohaemorrhagiae | 2 | 0.73 | 0.1–2.9 |
| Grippotyphosa | Grippotyphosa | 1 | 0.36 | 0.0–2.3 |
| District | Sub-County | N Sampled (%) | n Lepto 2 Positive (%) | n Pomona Positive (%) |
|---|---|---|---|---|
| Kole | Aboke | 88 (32.0) | 18 (20.4) | 5 (5.7) |
| Ayer | 71 (25.8) | 11 (15.5) | 6 (8.4) | |
| Mbale | Busoba | 27 (9.8) | 9 (33.3) | 7 (25.9) |
| Nakaloki | 29 (10.5) | 5 (17.2) | 1 (3.4) | |
| Namanyoni 1 | 3 (1.1) | 0 (0.0) | 0 (0.0) | |
| Busiu | 57 (20.7) | 10 (17.5) | 7 (12.3) | |
| Total | 275 (100) | 53 (19.3) | 26 (9.4) |
| Serogroup | Serovar | No of Positive Samples | No (%) of Cross-Reacting 4 Samples | No of Cross-Reactions 5 | Main Cross-Reacting Serovar (No of Cross-Reactions) |
|---|---|---|---|---|---|
| Pomona | Pomona | 26 | 7 (27) | 12 | Butembo (5) |
| Ballum | Kenya | 14 | 5 (36) | 10 | Nigeria (4) |
| Pyrogenes | Nigeria | 11 | 4 (36) | 9 | Kenya (4) |
| Sejroe | Wolfii | 9 | 4 (44) | 7 | Hardjo (3) |
| Autumnalis | Butembo | 5 | 5 (100) | 7 | Pomona (5) |
| Sejroe | Hardjo | 4 | 4 (100) | 4 | Wolfii (3) |
| Ictero 1 | Ictero 1 | 2 | 1 (50) | 6 | |
| Grippo 2 | Grippo 2 | 1 | 1 (100) | 6 | |
| Total | 72 3 | 31 (43) | 61 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dreyfus, A.; Odoch, T.; Alinaitwe, L.; Rodriguez-Campos, S.; Tsegay, A.; Jaquier, V.; Kankya, C. Cross-Sectional Serological Survey for Leptospira spp. in Beef and Dairy Cattle in Two Districts in Uganda. Int. J. Environ. Res. Public Health 2017, 14, 1421. https://doi.org/10.3390/ijerph14111421
Dreyfus A, Odoch T, Alinaitwe L, Rodriguez-Campos S, Tsegay A, Jaquier V, Kankya C. Cross-Sectional Serological Survey for Leptospira spp. in Beef and Dairy Cattle in Two Districts in Uganda. International Journal of Environmental Research and Public Health. 2017; 14(11):1421. https://doi.org/10.3390/ijerph14111421
Chicago/Turabian StyleDreyfus, Anou, Terence Odoch, Lordrick Alinaitwe, Sabrina Rodriguez-Campos, Amanuel Tsegay, Valentine Jaquier, and Clovice Kankya. 2017. "Cross-Sectional Serological Survey for Leptospira spp. in Beef and Dairy Cattle in Two Districts in Uganda" International Journal of Environmental Research and Public Health 14, no. 11: 1421. https://doi.org/10.3390/ijerph14111421





