The Content of Structural and Trace Elements in the Knee Joint Tissues
Abstract
:1. Introduction
2. Material and Methods
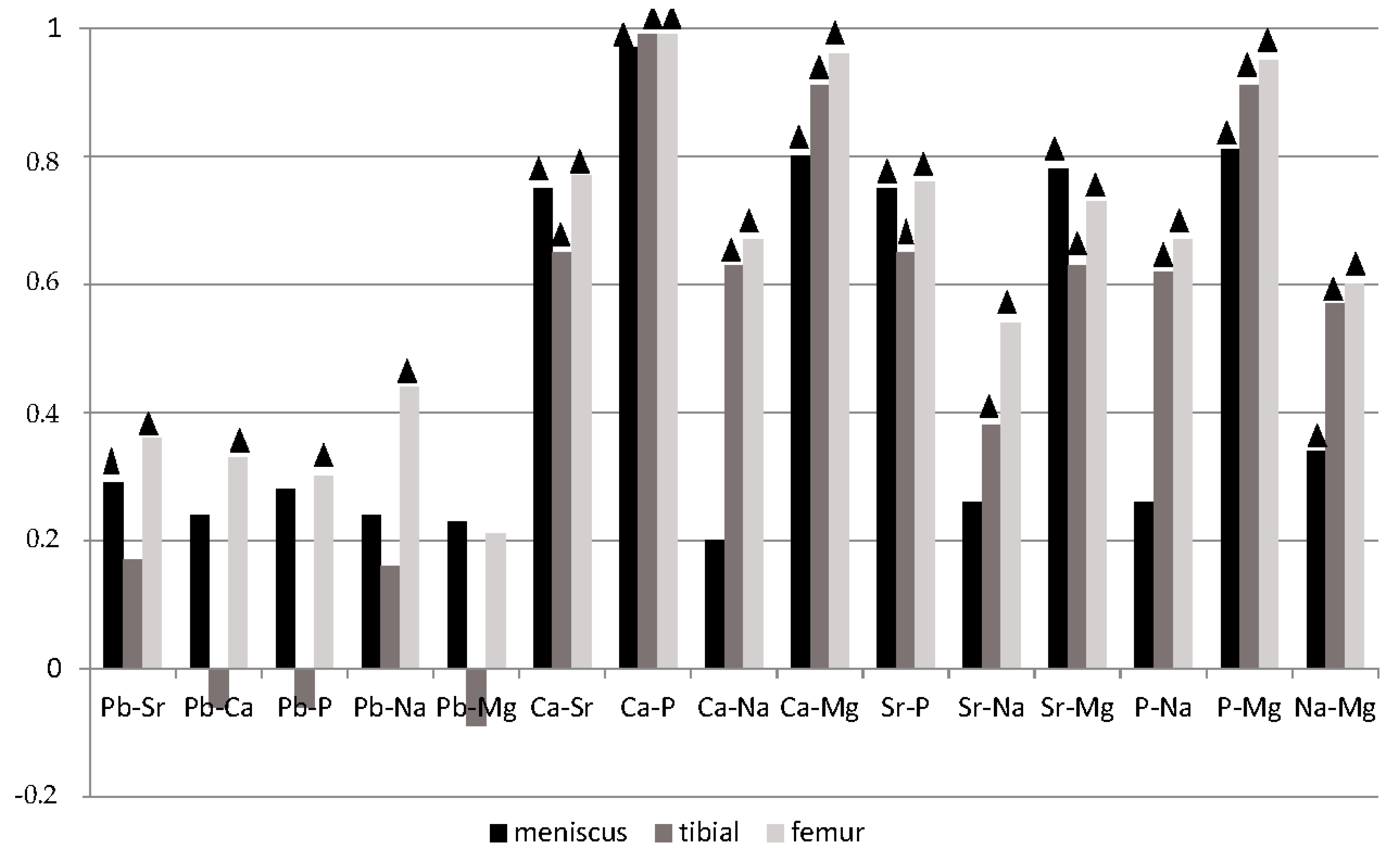
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Berglund, M.; Akesson, A.; Bjellerup, P.; Vahter, M. Metal—Bone interactions. Toxicol. Lett. 2000, 112, 219–222. [Google Scholar] [CrossRef]
- Brodziak-Dopierała, B.; Kwapuliński, J.; Kusz, D.; Gajda, Z.; Sobczyk, K. Interactions between concentrations of chemical elements in human femoral heads. Arch. Environ. Contam. Toxicol. 2009, 57, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.W.; Kuo, S.M.; Chou, C.H.; Lee, T.C. Determination of 14 elements in Taiwanese bones. Sci. Total Environ. 2000, 255, 45–54. [Google Scholar] [CrossRef]
- Branca, F.; Vatuena, S. Calcium, physical activity and bone health—Building bones for a stronger future. Public Health Nutr. 2001, 4, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, G.F.; Fowler, B.A.; Nordberg, M.; Friberg, L.T. Handbook on the Toxicology of Metals; Elsevier: London, UK, 2008. [Google Scholar]
- Theobald, H.E. Dietary calcium and health. Nutr. Bull. 2005, 30, 237–277. [Google Scholar] [CrossRef]
- Blair, H.C.; Schlesinger, P.H.; Huang, C.L.H.; Zaidi, M. Calcium signaling and calcium transport in bone disease. Subcell. Biochem. 2007, 45, 539–562. [Google Scholar] [PubMed]
- Brodziak-Dopierała, B.; Kwapuliński, J.; Sobczyk, K.; Wiechuła, D. Distribution of magnesium, calcium, sodium and potassium in tissues of the hip joint. Magnes. Res. 2013, 26, 125–131. [Google Scholar] [PubMed]
- Barbagallo, M.; Belvedere, M.; Dominguez, L.J. Magnesium homeostasis and aging. Magnes. Res. 2009, 22, 235–246. [Google Scholar] [PubMed]
- Uenishi, K. Nutrition and bone health. Conclus. Clin. Calcium 2010, 20, 940–943. [Google Scholar] [PubMed]
- Yamamoto, S.; Uenishi, K. Nutrition and bone health. Magnesium-rich foods and bone health. Clin. Calcium 2010, 20, 768–774. [Google Scholar] [PubMed]
- Rude, R.K.; Gruber, H.E.; Wei, L.Y.; Frausto, A.; Mills, B.G. Magnesium deficiency: Effect on bone and mineral metabolism in the mouse. Calcif. Tissue Int. 2003, 72, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Odabasi, E.; Turan, M.; Aydin, A.; Akay, C.; Kutlu, M. Magnesium, zinc, copper, manganese, and selenium levels in postmenopausal women with osteoporosis. Can magnesium play a key role in osteoporosis? Ann. Acad. Med. Singap. 2008, 37, 564–567. [Google Scholar] [PubMed]
- New, S.A.; Robins, S.P.; Cambpell, M.K. Dietary influences on bone mass and bone metabolism: Further evidence of a positive link between fruit and vegetable consumption and bone health? Am. J. Clin. Nutr. 2000, 71, 142–151. [Google Scholar] [PubMed]
- Tucker, K.L.; Hannan, M.T.; Chen, H.; Cupples, L.A.; Wilson, D.P.; Kiel, D.P. Potassium, magnesium and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am. J. Clin. Nutr. 1999, 69, 727–736. [Google Scholar] [PubMed]
- Janning, C.; Willbold, E.; Vogt, C.; Nellesen, J.; Meyer-Lindenberg, A.; Windhagen, H.; Thorey, F.; Witte, F. Magnesium hydroxide temporarily enhancing osteoblast activity and decreasing the osteoclast number in peri-implant bone remodeling. Acta Biomater. 2010, 6, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Breslau, N.A.; McGuire, J.L.; Zerwekh, J.E.; Pak, C.Y.C. The role of dietary sodium on renal excretion and intestinal absorption of calcium and on vitamin D metabolism. J. Clin. Endocrinol. Metab. 1982, 55, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Heaney, P.R. Bone health. Am. J. Clin. Nutr. 2007, 85, 300–303. [Google Scholar]
- Kaye, K.E. Bone health and oral health. J. Am. Dent. Assoc. 2007, 138, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.R.; Beaupré, G.S. Skeletal Function and Form: Mechanobiology of Skeletal Development, Aging and Regeneration; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Nielsen, P.S. The biological role of strontium. Bone 2004, 35, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Mays, S. Bone strontium: Calcium ratios and duration of breastfeeding in a Mediaeval skeletal population. J. Archaeol. Sci. 2003, 30, 731–741. [Google Scholar] [CrossRef]
- Marie, P.J.; Ammann, P.; Boivin, G.; Rey, C. Mechanisms of action and therapeutic potential of strontium in bone. Calcif. Tissue Int. 2001, 69, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shen, Y.; Pan, H.; Lin, K.; Liu, X.W.; Darvell, B.; Lu, W.W.; Chang, J.; Deng, L.; Wang, D.; et al. Effects of strontium in modified biomaterials. Acta Biomater. 2011, 7, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Reginster, J.Y. Strontium ranelate in osteoporosis. Curr. Pharm. Design. 2002, 8, 1907–1916. [Google Scholar] [CrossRef]
- Buehler, J.; Chappuis, P.; Saffar, J.L.; Tsouderos, Y.; Vignery, A. Strontium ranelate inhibits bone resorption while maintaining bone formation in alveolar bone in monkeys (Macaca fascicularis). Bone 2001, 29, 76–79. [Google Scholar] [CrossRef]
- Peng, S.; Zhou, G.; Luk, K.D.K.; Cheung, K.M.C.; Li, Z.Y.; Lam, W.M.; Zhou, Z.; Lu, W.W. Strontium promotes osteogenic differentiation of mesenchymal stem cells through the Ras/MAPK signaling pathway. Cell. Phys. Biochem. 2009, 23, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Dahl, S.G.; Allain, P.; Marie, P.J.; Mauras, Y.; Boivin, G.; Ammann, P.; Tsouderos, Y.; Delmas, P.D.; Christiansen, C. Incorporation and distribution of strontium in bone. Bone. 2001, 28, 446–453. [Google Scholar] [CrossRef]
- Cabrera, W.E.; Schrooten, I.; De Broe, M.E.; D’Haese, P.C. Strontium and bone. J. Bone Min. Res. 1999, 14, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Fabig, A.; Herrmann, B. Trace elements in buried human bone: Intra-population variability of Sr/Ca and Ba/Ca. Naturwissenschaften 2002, 89, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Zaichick, V.; Tzaphlidou, M. Determination of calcium, phosphorus, and the calcium/phosphorus ratio in cortical bone from the human femoral neck by neutron activation analysis. Appl. Radiat. Isot. 2002, 56, 781–786. [Google Scholar] [CrossRef]
- Tzaphlidou, M.; Zaichick, V. Calcium, phosphorus, calcium-phosphorus ratio in rib bone of healthy humans. Biol. Trace Elem. Res. 2003, 93, 63–74. [Google Scholar] [CrossRef]
- Vuorinen, H.S.; Pihlman, S.; Rauhamaa-Mussalo, H.; Tapper, U.; Varrela, T. Trace and heavy metal analyses of a skeletal population representing the town people in Turku (Abo), Finland in the 16th–17th centuries: With special reference to gender age and social background. Sci. Total Environ. 1996, 177, 145–160. [Google Scholar] [CrossRef]
- Tzaphlidou, M. Bone architecture: Collagen structure and calcium/phosphorus maps. J. Biol. Phys. 2008, 34, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Tzaphlidou, M.; Zaichick, V. Sex and age related Ca/P ratio in cortical bone of iliac crest of healthy humans. J. Radioanal. Nucl. Chem. 2004, 259, 347–349. [Google Scholar] [CrossRef]
- Kosugi, H.; Hanihara, K.; Suzuki, T.; Himeno, S.I.; Kawabe, T.; Hongo, T.; Morita, M. Elemental composition of ancient Japanese bones. Sci. Total Environ. 1986, 52, 93–107. [Google Scholar] [CrossRef]
- Kwapuliński, J.; Brodziak, B.; Bogunia, M. Relative changes of elements in human osseous tissue. Bull. Environ. Contam. Toxicol. 2003, 70, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Iversen, B.F.; Therkildsen, M.H. Contribution of the trabecular component to mechanical strength and bone mineral content of the femoral neck. An experimental study on cadaver bones. Scand. J. Clin. Lab. Investig. 1988, 48, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Boskey, L.A. Bone composition: Relationship to bone fragility and antiosteoporotic drug effects. BoneKEy Rep. 2013, 2, 447. [Google Scholar] [CrossRef] [PubMed]
- Krachler, M.; Domej, W. Clinical laboratory parameters in osteoarthritic knee-joint effusions correlated to trace element concentrations. Biol. Trace Elem. Res. 2001, 79, 139–148. [Google Scholar] [CrossRef]
- Naza, M.M.A. Relationship between serum levels of some trace elements, disease duration and severity in patients with knee osteoarthritis. Pharmacol. Pharm. 2015, 6, 489–495. [Google Scholar]
- Krachler, M.; Domej, W.; Irgolic, K.J. Concentrations of trace elements in osteoarthritic knee-joint effusions. Biol. Trace Elem. Res. 2000, 75, 253–263. [Google Scholar] [CrossRef]
- Lanocha-Arendarczyk, N.; Kosik-Bogacka, D.I.; Prokopowicz, A.; Kalisinska, E.; Sokolowski, S.; Karaczun, M.; Zietek, P.; Podlasińska, J.; Pilarczyk, B.; Tomza-Marciniak, A.; et al. The effect of risk factors on the levels of chemical elements in the tibial plateau of patients with osteoarthritis following knee surgery. Biomed. Res. Int. 2015, 2015, 650282. [Google Scholar] [CrossRef] [PubMed]
- Zioła-Frankowska, A.; Kubaszewski, Ł.; Dąbrowski, M.; Kowalski, A.; Rogala, P.; Strzyżewski, W.; Łabędź, W.; Uklejewski, R.; Novotny, K.; Kanicky, V.; et al. The content of the 14 metals in cancellous and cortical bone of the hip joint affected by osteoarth. Biomed. Res. Int. 2015, 815648, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Helliwell, T.R.; Kelll, S.A.; Walsh, H.P.J.; Klenerman, L.; Haines, J.; Clark, R.; Roberts, N.B. Elemental analysis of femoral bone from patients with fractured neck of femur or osteoarthrosis. Bone 1996, 18, 151–157. [Google Scholar] [CrossRef]
- Milachowski, A.K. Investigation of ischaemic necrosis of the femoral head with trace elements. Int. Orthop. 1988, 12, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, A.; Kubaszewski, Ł.; Frankowski, M.; Wilk-Frańczuk, M.; Zioła-Frankowska, A.; Czabak-Garbacz, R.; Kaczmarczyk, J.; Gasik, R. Analysis of trace element in intervertebral disc by Atomic Absorption Spectrometry techniques in degenerative disc disease in the Polish population. Ann. Agric. Environ. Med. 2015, 22, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Garcia, F.; Ortega, A.; Domingo, J.L.; Corbella, J. Accumulation of metals in autopsy tissues of subjects living in Tarragona county, Spain. J. Environ. Sci. Health. 2001, 36, 1767–1786. [Google Scholar] [CrossRef]
- Yoshinaga, J.; Suzuki, T.; Morita, M.; Hayakawa, M. Trace elements in ribs of elderly people and elemental variation in the presence of chronic diseases. Sci. Total Environ. 1995, 162, 239–252. [Google Scholar] [CrossRef]
- Budis, H.; Kalisinska, E.; Lanocha, N.; Kosik-Bogacka, D.; Sokolowski, S.; Dobiecki, K.; Kolodziej, L.; Bohatyrewicz, A. The concentration of manganese, iron, and strontium in hip joint bone obtained from patient undergoing hip replacement surgery. J. Trace Elem. Med. Biol. 2014, 28, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Zaichick, V.; Zaichick, S.; Karandashev, V.; Nosenko, S. The effect of age and gender on Al, B, Ba, Ca, Cu, Fe, K, Li, Mg, Mn, Na, P, S, Sr, V, and Zn contents in rib bone of healthy humans. Biol. Trace Elem. Res. 2009, 129, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Zaichick, S.; Zaichick, V. The effect of age and gender on 38 chemical element contents in human femoral neck investigated by instrumental neutron activation analysis. Biol. Trace Elem. Res. 2010, 137, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Grynpas, M.D.; Pritzker, K.P.; Hancock, R.G.V. Neutron activation analysis of bulk and selected trace elements in bone using low flux SLOWPOKE reactor. Biol. Trace Elem. Res. 1987, 13, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Takata, M.K.; Saiki, M.; Sumita, N.M.; Saldiva, P.H.N.; Pasqualucci, C.A. Trace element determinations in human cortical and trabecular bones. J. Radioanal. Nucl. Chem. 2005, 264, 5–8. [Google Scholar] [CrossRef]
- Scancar, J.; Milacic, R.; Bendikt, M.; Bukovec, P. Determination of trace elements and calcium in bone of the human iliac crest by atomic absorption spectrometry. Clin. Chim. Acta. 2000, 293, 187–197. [Google Scholar] [CrossRef]
- Wiechuła, D.; Jurkiewicz, A.; Loska, K. An assessment of natural concentrations of selected metals in the bone tissues of the femur head. Sci. Total Environ. 2008, 15, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Zaichick, V.; Tzaphlidou, M. Calcium and phosphorus concentrations and the calcium/phosphorus ratio in trabecular bone from the femoral neck of healthy humans as determined by neutron activation analysis. Appl. Radiat. Isot. 2003, 58, 623–627. [Google Scholar] [CrossRef]
- Stenstrom, M.; Olander, B.; Lehto-Axtelius, D.; Madsen, J.E.; Nordsletten, L.; Carlsson, G.A. Bone mineral density and bone structure parameters as predictors of bone strength: An analysis using computerized microtomography and gastrectomy-induced osteopenia in the rat. J. Biomech. 2000, 33, 289–297. [Google Scholar] [CrossRef]
| Parameters | Whole Population n = 50 | Females n = 36 | Males n = 14 |
|---|---|---|---|
| Age (years) | |||
| AM ± SD | 67.46 ± 7.11 | 67.22 ± 7,09 | 68.07 ± 7.20 |
| range | 54–78 | 54–78 | 56–78 |
| Body weight (kg) | |||
| AM ± SD | 83.54 ± 14.56 | 81.45 ± 14.19 | 88.58 ± 14.56 |
| range | 54–115 | 54–115 | 66–108 |
| Height (cm) | |||
| AM ± SD | 164.37 ± 9.32 | 160.24 ± 6.14 | 174.33 ± 8.11 |
| range | 149–189 | 149–173 | 165–189 |
| Smokers (n, %) | |||
| - nonsmokers | 20 (40%) | 19 (38%) | 1 (2%) |
| - smokers | 21 (42%) | 10 (20%) | 11 (22%) |
| - smokers in the past | 9 (18%) | 5 (10%) | 4 (8%) |
| Place of residence (%) | |||
| Village | 11 (22%) | 7 (14%) | 4 (8%) |
| Town | 39 (78%) | 29 (58%) | 10 (20%) |
| Knee (%) | |||
| Left | 24 (48%) | 18 (36%) | 6 (12%) |
| Right | 26 (52%) | 18 (36%) | 8 (16%) |
| Beginning pain (years, %) | |||
| <5 | 16 (32%) | 11 (22%) | 5 (10%) |
| <10 | 21 (42%) | 15 (30%) | 6 (12%) |
| >10 | 13 (26%) | 10 (20%) | 3 (9%) |
| Earlier knee endoprosthesis (%) | |||
| Yes | 13 (26%) | 10 (20%) | 3 (6%) |
| No | 37 (74%) | 26 (52%) | 11 (22%) |
| Degenerative changes in the other knee (%) | |||
| Yes | 33 (66%) | 23 (46%) | 10 (20%) |
| No | 17 (34%) | 13 (26%) | 4 (8%) |
| Contact with chemicals in the workplace | |||
| (factory PVC-polyvinylchloride, zinc smelter) (%) | 3 (6%) | 1 (2%) | 2 (4%) |
| Meniscus | Tibia | Femur | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AM ± SD | Med. | Range | CV | AM ± SD | Med. | Range | CV | AM ± SD | Med. | Range | CV | |
| Female | ||||||||||||
| Sr (mg/kg) | 1.26 ± 0.77 | 1.06 | 0.27–3.46 | 61 | 26.69 ± 10.44 | 25.52 | 11.47–56.73 | 39 | 24.57 ± 10.13 | 23.26 | 8.82–55.98 | 41 |
| Pb (mg/kg) | 0.24 ± 0.18 | 0.17 | 0.11–1.05 | 77 | 2.16 ± 1.66 | 1.73 | 0.42–7.02 | 77 | 2.41 ± 1.97 | 1.74 | 0.12–9.40 | 82 |
| Mg (mg/kg) | 78.74 ± 36.88 | 68.27 | 37.78–209.94 | 47 | 1549.45 ± 366.84 | 1562.44 | 889.72–2192.12 | 24 | 1453.84 ± 414.94 | 1437.11 | 756.10–2194.35 | 29 |
| Na (%) | 0.18 ± 0.07 | 0.18 | 0.10–0.46 | 37 | 0.54 ± 0.09 | 0.54 | 0.34–0.72 | 17 | 0.53 ± 0.08 | 0.52 | 0.32–0.68 | 16 |
| P (%) | 0.19 ± 0.20 | 0.14 | 0.03–0.96 | 103 | 5.51 ± 1.50 | 5.53 | 2.88–8.62 | 27 | 5.00 ± 1.60 | 4.7 | 2.53–7.65 | 32 |
| Ca (%) | 0.34 ± 0.26 | 0.27 | 0.04–1.06 | 76 | 12.21 ± 3.29 | 12.19 | 6.33–19.23 | 27 | 11.12 ± 3.41 | 10.42 | 5.89–17.15 | 31 |
| Male | ||||||||||||
| Sr (mg/kg) | 1.93 ± 1.80 | 1.25 | 0.56-5.69 | 94 | 26.52 ± 9.76 | 27.33 | 12.57–47.09 | 37 | 24.66 ± 11.08 | 27 | 8.55–40.28 | 45 |
| Pb (mg/kg) | 0.53 ± 0.63 | 0.19 | 0.13–1.73 | 120 | 3.99 ± 2.61 | 3.29 | 0.50–9.75 | 65 | 3.22 ± 2.59 | 2.9 | 0.13–10.03 | 80 |
| Mg (mg/kg) | 152.74 ± 193.64 | 65.52 | 41.02–612.32 | 127 | 1568.89 ± 397.14 | 1591.00 | 955.84–2195.89 | 25 | 1330.60 ± 547.40 | 1471.24 | 370.02–2138.12 | 41 |
| Na (%) | 0.28 ± 0.21 | 0.17 | 0.12–0.70 | 74 | 0.58 ± 0.14 | 0.57 | 0.41–0.81 | 23 | 0.52 ± 0.16 | 0.54 | 0.19–0.85 | 32 |
| P (%) | 0.30 ± 0.42 | 0.12 | 0.03–1.18 | 139 | 5.59 ± 1.87 | 5.96 | 3.00–8.80 | 33 | 5.21 ± 1.92 | 5.51 | 2.10–7.83 | 37 |
| Ca (%) | 0.25 ± 0.23 | 0.2 | 0.01–0.92 | 93 | 12.37 ± 3.91 | 13.17 | 6.92–18.93 | 32 | 11.56 ± 4.19 | 12.25 | 5.17–17.40 | 36 |
| Total | ||||||||||||
| Sr (mg/kg) | 1.44 ± 1.17 | 1.07 | 0.27–5.69 | 81 | 26.64 ± 10.15 | 25.9 | 11.47–56.73 | 38 | 24.60 ± 10.29 | 23.95 | 8.55–55.98 | 42 |
| Pb (mg/kg) | 0.32 ± 0.38 | 0.18 | 0.11–1.73 | 121 | 2.67 ± 2.11 | 2.1 | 0.42–9.75 | 79 | 2.64 ± 2.16 | 2.05 | 0.12–10.03 | 82 |
| Mg (mg/kg) | 99.46 ± 109.75 | 67.95 | 37.78–612.32 | 110 | 1554.89 ± 371.55 | 1572.40 | 889.72–2195.89 | 24 | 1419.34 ± 453.43 | 1443.62 | 370.02–2194.35 | 32 |
| Na (%) | 0.21 ± 0.13 | 0.18 | 0.10–0.70 | 61 | 0.55 ± 0.11 | 0.54 | 0.34–0.81 | 19 | 0.52 ± 0.11 | 0.54 | 0.19–0.85 | 21 |
| P (%) | 0.22 ± 0.28 | 0.13 | 0.03–1.18 | 125 | 5.53 ± 1.60 | 5.58 | 2.88–8.80 | 29 | 5.06 ± 1.68 | 4.84 | 2.10–7.83 | 33 |
| Ca (%) | 0.31 ± 0.25 | 0.26 | 0.01–1.06 | 80 | 12.26 ± 3.43 | 12.45 | 6.33–19.23 | 28 | 11.25 ± 3.61 | 10.7 | 5.17–17.40 | 32 |
| AM ± SD | Med. | Range | CV | |
|---|---|---|---|---|
| non-smoker | ||||
| Sr (mg/kg) | 17.20 ± 14.25 | 17.77 | 0.27–56.73 | 83 |
| Pb (mg/kg) | 1.99 ± 2.34 | 1.35 | 0.11–10.03 | 118 |
| Mg (mg/kg) | 1019.25 ± 732.19 | 1158.10 | 37.78–2192.12 | 72 |
| Na (%) | 0.42 ± 0.19 | 0.49 | 0.10–0.73 | 45 |
| P (%) | 3.58 ± 2.74 | 3.70 | 0.03–8.80 | 77 |
| Ca (%) | 7.90 ± 6.14 | 8.55 | 0.04–19.23 | 78 |
| smoker | ||||
| Sr (mg/kg) | 18.88 ± 14.14 | 20.90 | 0.61–47.09 | 75 |
| Pb (mg/kg) | 1.72 ± 1.61 | 1.13 | 0.12–5.46 | 94 |
| Mg (mg/kg) | 976.41 ± 697.07 | 1165.12 | 47.72–2195.89 | 71 |
| Na (%) | 0.40 ± 0.20 | 0.44 | 0.12–0.81 | 51 |
| P (%) | 3.33 ± 2.49 | 3.98 | 0.07–7.67 | 75 |
| Ca (%) | 7.35 ± 5.46 | 8.77 | 0.17–16.42 | 74 |
| Sr/Ca (×10−3) | Ca/P | Pb/Ca | ||
|---|---|---|---|---|
| Men | Femur | 0.22 | 2.22 | 0.03 |
| Men | Meniscus | 0.28 | 2.14 | 0.10 |
| Men | Tibial | 0.21 | 2.21 | 0.03 |
| Women | Femur | 0.24 | 2.23 | 0.02 |
| Women | Meniscus | 0.34 | 1.99 | 0.07 |
| Women | Tibial | 0.23 | 2.22 | 0.02 |
| Women | 0.24 | 2.21 | 0.02 | |
| Men | 0.22 | 2.21 | 0.03 | |
| Total | 0.23 | 2.21 | 0.02 | |
| Femur | 0.23 | 2.23 | 0.02 | |
| Meniscus | 0.31 | 2.08 | 0.10 | |
| Tibial | 0.23 | 2.22 | 0.02 | |
| Smoker | 0.22 | 2.21 | 0.03 | |
| Non-smoker | 0.26 | 2.20 | 0.02 | |
| People >60 years of age | 0.23 | 2.22 | 0.01 | |
| People 61–70 years of age | 0.20 | 2.29 | 0.02 | |
| People <71 years of age | 0.22 | 2.25 | 0.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roczniak, W.; Brodziak-Dopierała, B.; Cipora, E.; Mitko, K.; Jakóbik-Kolon, A.; Konieczny, M.; Babuśka-Roczniak, M. The Content of Structural and Trace Elements in the Knee Joint Tissues. Int. J. Environ. Res. Public Health 2017, 14, 1441. https://doi.org/10.3390/ijerph14121441
Roczniak W, Brodziak-Dopierała B, Cipora E, Mitko K, Jakóbik-Kolon A, Konieczny M, Babuśka-Roczniak M. The Content of Structural and Trace Elements in the Knee Joint Tissues. International Journal of Environmental Research and Public Health. 2017; 14(12):1441. https://doi.org/10.3390/ijerph14121441
Chicago/Turabian StyleRoczniak, Wojciech, Barbara Brodziak-Dopierała, Elżbieta Cipora, Krzysztof Mitko, Agata Jakóbik-Kolon, Magdalena Konieczny, and Magdalena Babuśka-Roczniak. 2017. "The Content of Structural and Trace Elements in the Knee Joint Tissues" International Journal of Environmental Research and Public Health 14, no. 12: 1441. https://doi.org/10.3390/ijerph14121441






