Mixture Toxicity of Bensulfuron-Methyl and Acetochlor to Red Swamp Crayfish (Procambarus clarkii): Behavioral, Morphological and Histological Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Organisms and Chemicals
2.2. Acute Toxicity Tests
2.3. Histopathology
2.4. Statistical Analysis
3. Results
3.1. Descriptive Behavioral and Morphological Effects
3.2. Lethal Concentration 50 (LC50) of MBA
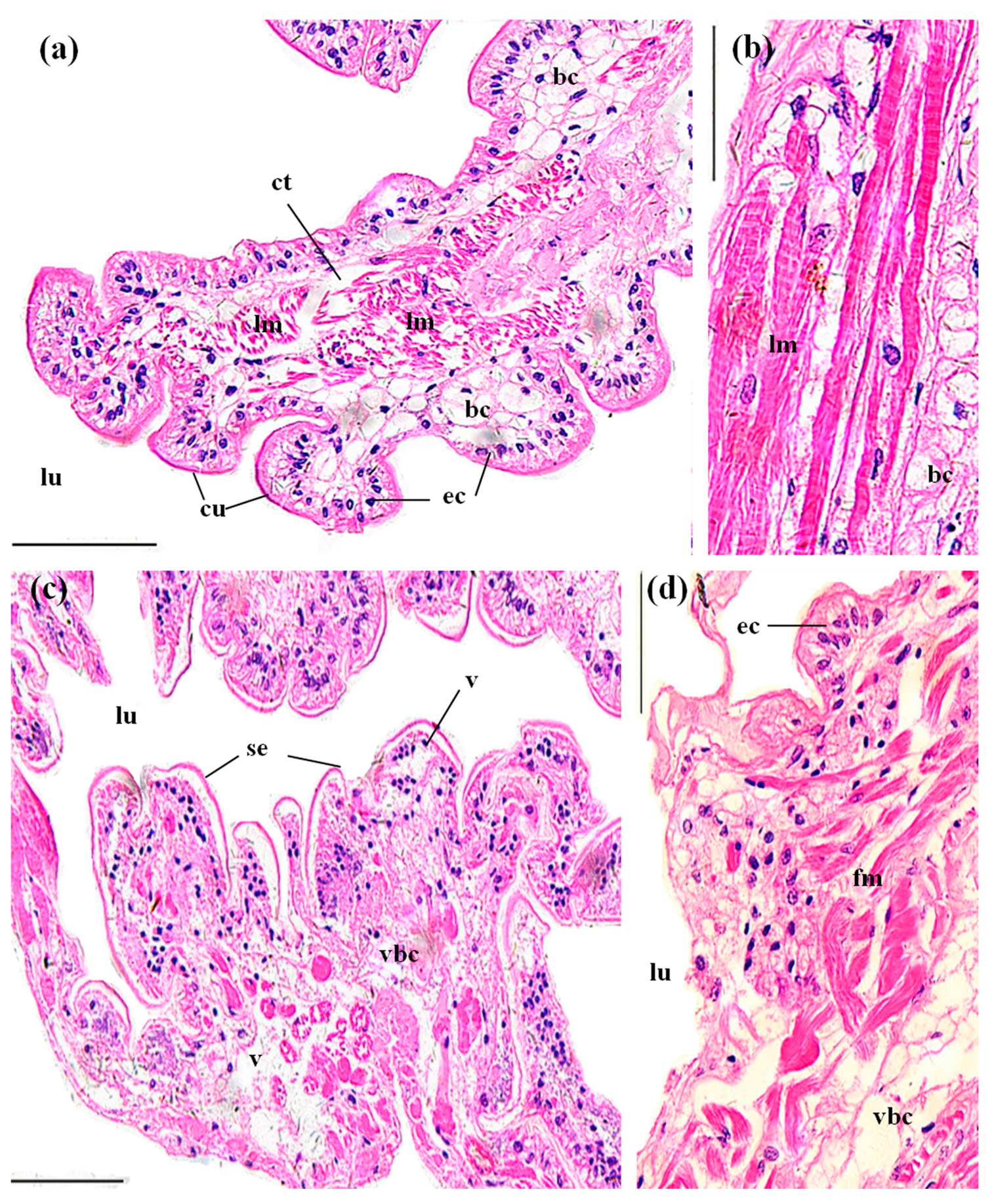
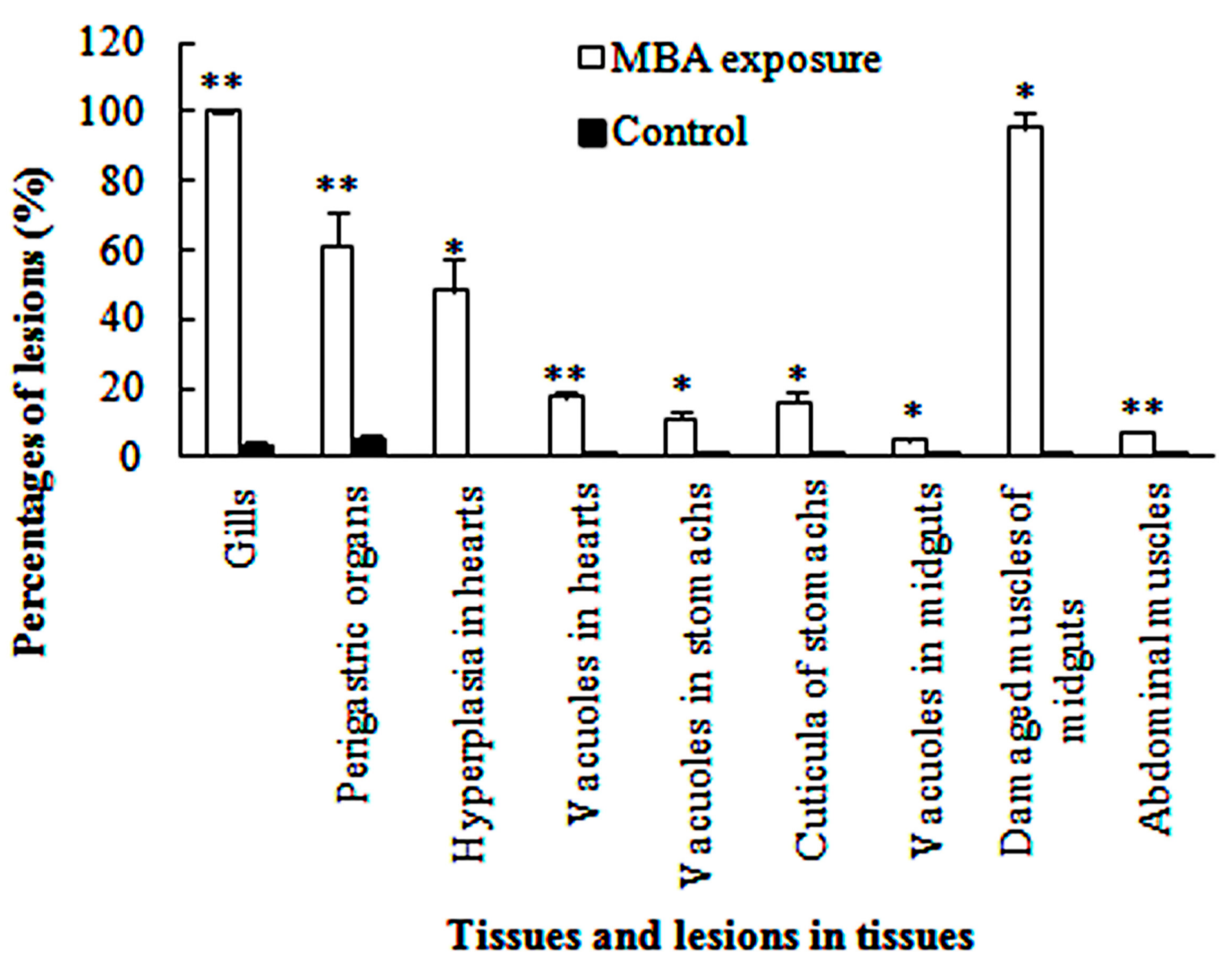
3.3. Histopathological Effects of MBA
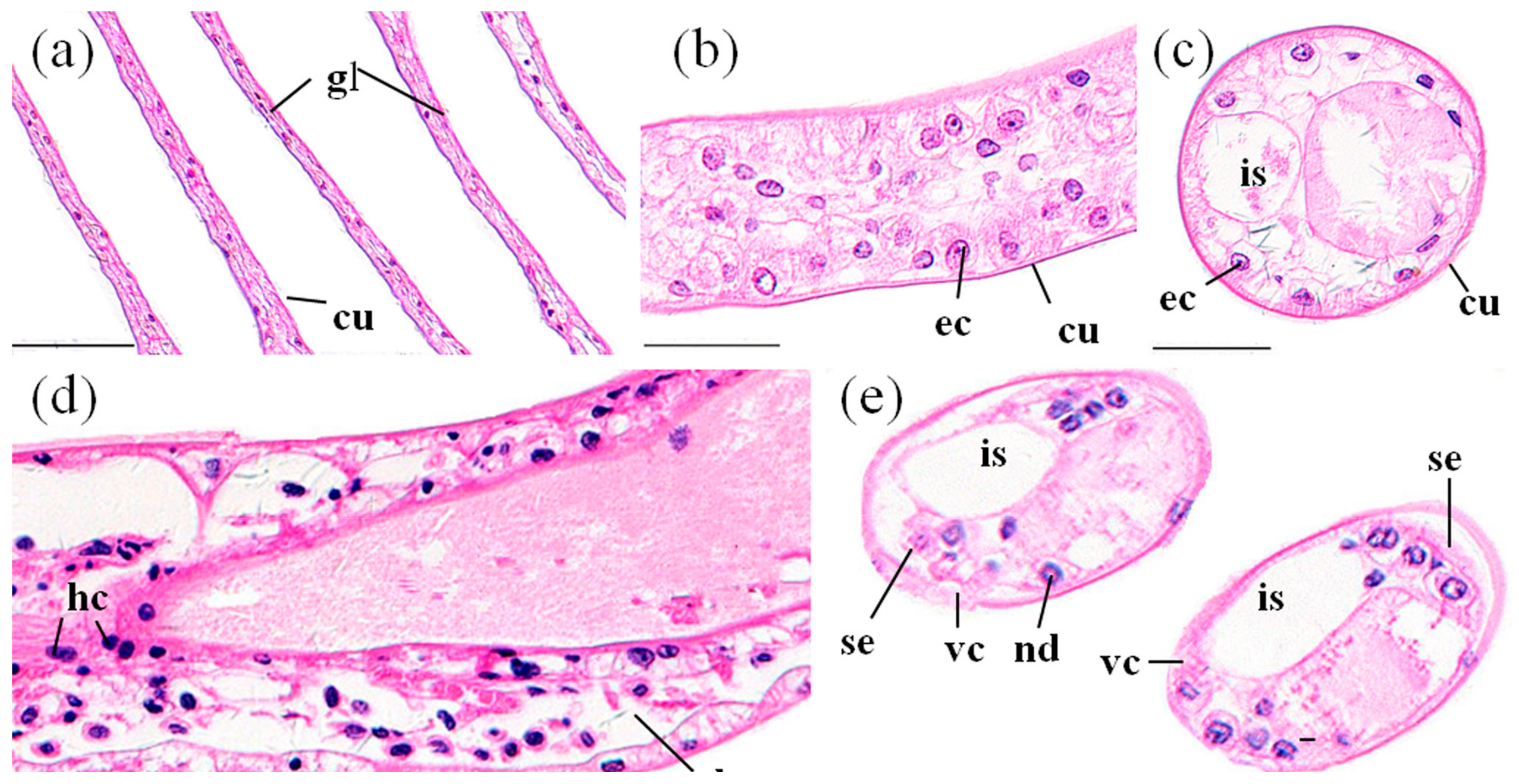
3.3.1. Gills
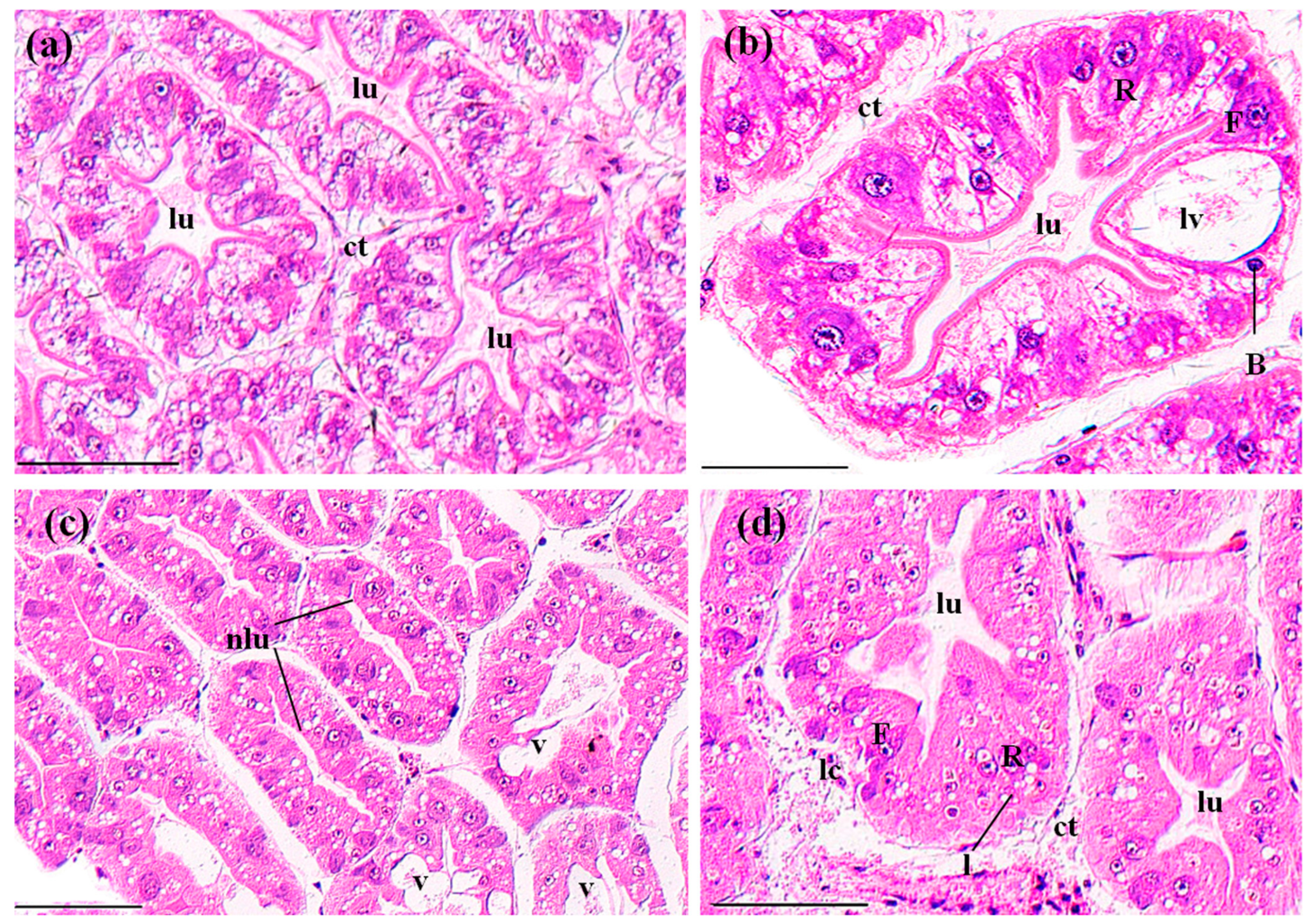
3.3.2. Perigastric Organs
3.3.3. Hearts
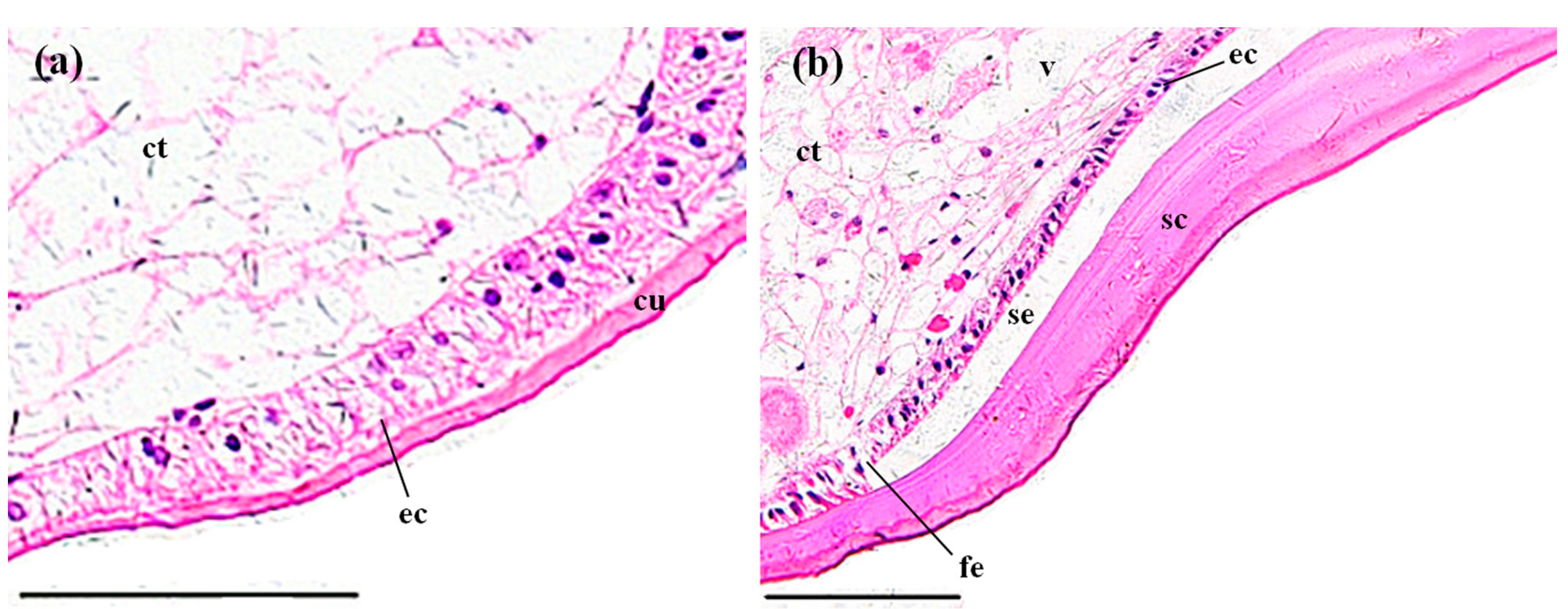
3.3.4. Stomachs
3.3.5. Midguts
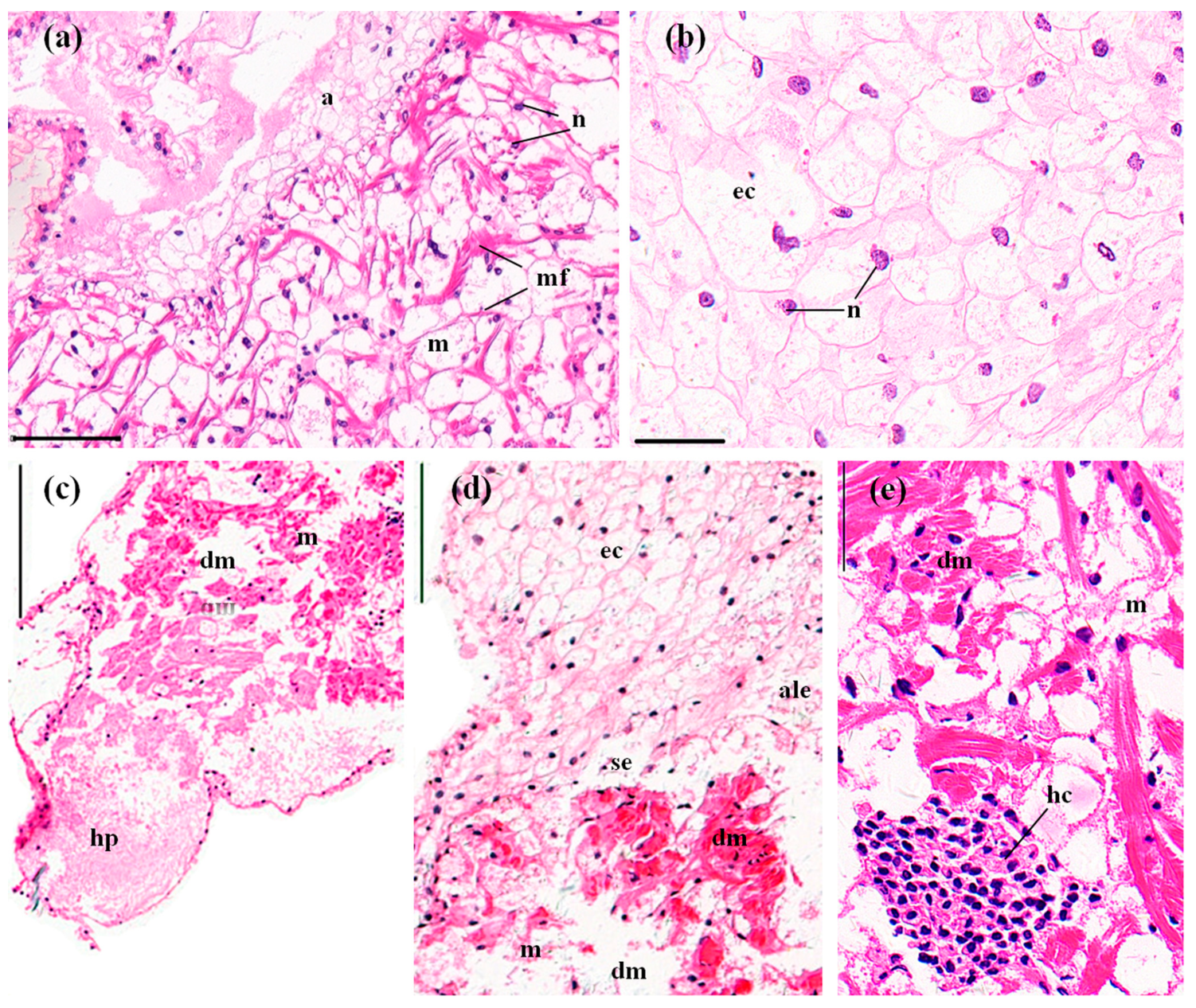
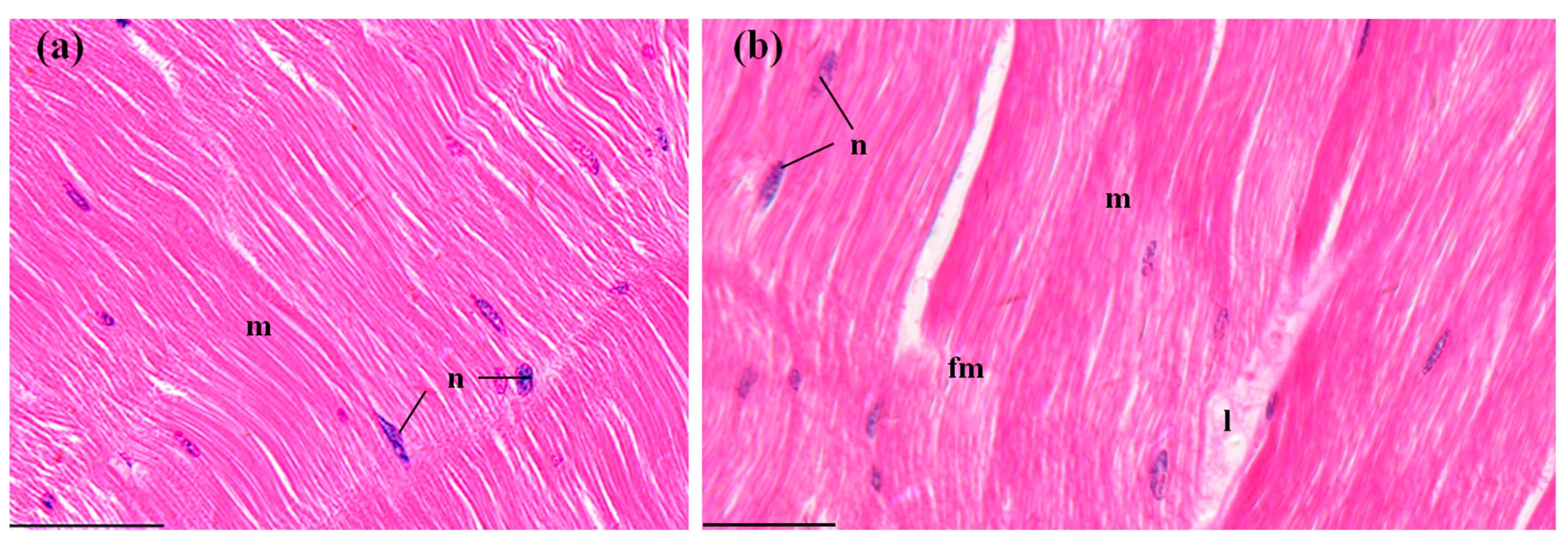
3.3.6. Abdominal Muscles
4. Discussion
4.1. Behavioral and Morphological Effects of MBA
4.2. Comparison of MBA 96 h LC50 with Other Herbicides
4.3. Histopathological Effects of MBA
4.4. Environmental Risk of MBA in RCIS
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Desouky, M.M.A.; Abdel-Gawad, H.; Hegazi, B. Distribution, fate and histopathological effects of ethion insecticide on selected organs of the crayfish, Procambarus clarkii. Food Chem. Toxicol. 2013, 52, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.H.; Wang, Y.Y.; Zhang, L.; Cai, Y.J.; Chen, Y.W. Bioaccumulation and tissue distribution of organochlorine pesticides (OCPs) in freshwater fishes: A case study performed in Poyang Lake, China’s largest lake. Environ. Sci. Pollut. Res. 2014, 21, 8740–8749. [Google Scholar] [CrossRef] [PubMed]
- Benli, A.C.K. The influence of etofenprox on narrow clawed crayfish (Astacus leptodactylus Eschscholtz, 1823): Acute toxicity and sublethal effects on histology, hemolymph parameters, and total hemocyte counts. Environ. Toxicol. 2015, 30, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Rempel, M.A.; Schlenk, D. Effects of environmental estrogens and antiandrogens on endocrine function, gene regulation, and health in fish. Int. Rev. Cell Mol. Biol. 2008, 267, 207–252. [Google Scholar] [PubMed]
- Stará, A.; Kouba, A.; Velíšek, J. Effect of chronic exposure to prometryne on oxidative stress and antioxidant response in red swamp crayfish (Procambarus clarkii). BioMed Res. Int. 2014, 2014, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.C. Study on the acute toxicity and genetics toxicity of bensulfuron-methyl on Danio rerio. J. Anhui Agric. Sci. 2009, 37, 15879–15881. (In Chinese) [Google Scholar]
- Okamoto, Y.; Fisher, R.L.; Armbrust, K.L.; Peter, C.J. Surface water monitoring survey for bensulfuron methyl applied in paddy fields. J. Pestic. Sci. 1998, 23, 235–240. [Google Scholar] [CrossRef]
- Parveen, S.; Kohguchi, T.; Biswas, M.; Nakagoshi, N. Predicting herbicides concentrations in paddy water and runoff to the river basin. J. Environ. Sci. 2005, 17, 631–636. [Google Scholar]
- Hu, C.L.; Yu, X.W.; Zhao, Z.K.; Yuan, J.L.; Yang, X. Study on embryo toxicity of bensulfuron-methyl in zebrafish. J. Public Health Prev. Med. 2011, 22, 1–4. (In Chinese) [Google Scholar]
- Wei, L.P.; Yu, H.X.; Sun, Y.; Fen, J.F.; Wang, L.S. The effects of three sulfonylurea herbicides and their degradation products on the green algae Chlorella pyrenoidosa. Chemosphere 1998, 37, 747–751. [Google Scholar] [CrossRef]
- Sabater, C.; Cuesta, A.; Carrasco, R. Effects of bensulfuron-methyl and cinosulfuron on growth of four freshwater species of phytoplankton. Chemosphere 2002, 46, 953–960. [Google Scholar] [CrossRef]
- Li, W.; Zha, J.M.; Li, Z.L.; Yang, L.H.; Wang, Z.J. Effects of exposure to acetochlor on the expression of thyroid hormone related genes in larval and adult rare minnow (Gobiocypris rarus). Aquat. Toxicol. 2009, 94, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Boyd, R.A. Herbicides and herbicide degradates in shallow groundwater and the Cedar River near a municipal well field, Cedar Rapids, Iowa. Sci. Total Environ. 2000, 248, 241–253. [Google Scholar] [CrossRef]
- Scribner, E.A.; Battaglin, W.A.; Goolsby, D.A.; Thurman, E.M. Changes in herbicide concentrations in midwestern streams in relation to changes in use, 1989–1998. Sci. Total Environ. 2000, 248, 255–263. [Google Scholar] [CrossRef]
- Sun, X.Y.; Zhou, Q.X.; Ren, W.J.; Li, X.H.; Ren, L.P. Spatial and temporal distribution of acetochlor in sediments and riparian soils of the Songhua River Basin in northeastern China. J. Environ. Sci. 2011, 23, 1684–1690. [Google Scholar] [CrossRef]
- Wang, Z.J.; Lv, Y.B.; Wang, Y.; Ma, M. Assessing the ecological risk of substituted benzenes in Huaihe River, China. Acta Sci. Circumst. 2002, 22, 300–304. (In Chinese) [Google Scholar]
- Yang, M.; Hu, J.J.; Li, S.Y.; Ma, Y.N.; Gui, W.J.; Zhu, G.N. Thyroid endocrine disruption of acetochlor on zebrafish (Danio rerio) larvae. J. Appl. Toxicol. 2016, 36, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.M.; Liu, J.H.; Huang, D.J. The role of reactive oxygen species in the herbicide acetochlor-induced DNA damage on Bufo raddei tadpole liver. Aquat. Toxicol. 2006, 78, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.; Linderman, R.; Hodgson, E.; Rose, R.L. Comparative metabolism of chloroacetamide herbicides and selected metabolites in human and rat liver microsomes. Environ. Health Perspect. 2000, 108, 1151–1157. [Google Scholar] [PubMed]
- Lin, C.Y. The application and development of Bensulfuron-methyl in China. Pesticide 2000, 39, 11–12. (In Chinese) [Google Scholar]
- Green, R.M.; Abdelghani, A.A. Toxicity of a mixture of 2,4-Dichlorophenoxyacetic acid and mono-soduim methanearsonate to the red swamp crawfish, Procambarus clarkii. Int. J. Environ Res. Public Health 2004, 1, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Fornstrom, C.B.; Landrum, P.F.; Weisskopf, C.P.; La Point, T.W. Effects of terbufos on juvenile red swamp crayfish (Procambarus clarkii): Differential routes of exposure. Environ. Toxicol. Chem. 1997, 16, 2514–2520. [Google Scholar] [CrossRef]
- Gherardi, F. Towards a sustainable human use of freshwater crayfish (Crustacea, Decapoda, Astacidea). Knowl. Manag. Aquat. Ecosyst. 2011, 401, 2. [Google Scholar] [CrossRef]
- Schlenk, D.; Huggett, D.B.; Allgood, J.; Bennett, E.; Rimoldi, J.; Beeler, A.B.; Block, D.; Holder, A.W.; Hovinga, R.; Bedient, P. Toxicity of fipronil and its degradation products to Procambarus sp.: Field and laboratory studies. Arch. Environ. Contam. Toxicol. 2001, 41, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Biever, R.C.; Hoberg, J.R.; Jacobson, B.; Dionne, E.; Sulaiman, M.; McCahon, P. ICON® rice seed treatment toxicity to crayfish (Procambarus clarkii) in experimental rice paddies. Environ. Toxicol. Chem. 2003, 22, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Wei, H.; Shen, H.; Wu, T.T.; Guo, M. Acute toxic effects of deltamethrin on red swamp crayfish, Procambarus clarkii (Decapoda, Cambaridae). Crustaceana 2012, 85, 993–1005. [Google Scholar] [CrossRef]
- Cheah, M.L.; Avault, J.W., Jr.; Graves, J.B. Acute toxicity of selected rice pesticides to crayfish Procambarus clarkii. Prog. Fish Cult. 1980, 42, 169–172. [Google Scholar] [CrossRef]
- Naqvi, S.M.; Hawkins, R.; Naqvi, N.H. Mortality response and LC50 values for juvenile and adult crayfish, Procambarus clarkii exposed to Thiodan® (insecticide), Treflan®, MSMA, Oust® (herbicides) and Cutrine-Plus® (algicide). Environ. Pollut. 1987, 48, 275–283. [Google Scholar] [CrossRef]
- Hebel, D.K.; Jones, M.B.; Depledge, M.H. Responses of crustaceans to contaminant exposure: A holistic approach. Estuar. Coast. Shelf Sci. 1997, 44, 177–184. [Google Scholar] [CrossRef]
- Velmurugan, B.; Selvanayagam, M.; Cengiz, E.I.; Unlu, E. The effects of fenvalerate on different tissues of freshwater fish Cirrhinus mrigala. J. Environ. Sci. Health B 2007, 42, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.F.; Xue, H.; Wang, X.F.; Tang, J.Q. Acute toxicity of chlorpyrifos (CPF) to crayfish (Procambarus clarkii) and the histopathological observation. J. Ecol. Rural Environ. 2012, 28, 462–467. (In Chinese) [Google Scholar]
- Xu, Y.; Liu, Q.G.; Hu, Z.J.; Shen, H.; Cao, J.Y.; Tong, L.Q. Acute toxicity of ten pesticides to larval red swamp crayfish Procambarus clarkii. Asian J. Ecotoxicol. 2010, 5, 50–56. (In Chinese) [Google Scholar]
- Qiu, W.J.; Chen, M.D.; Song, Y.Z.; Zhou, J.Y.; Shan, Z.J. Acute toxicity of two pesticides to Macrobrachium nipponense. J. Ecol. Rural Environ. 2013, 29, 676–680. (In Chinese) [Google Scholar]
- Li, Z.H.; Zlabek, V.; Velisek, J.; Grabic, R.; Machova, J.; Kolarova, J.; Li, P.; Randak, T. Acute toxicity of carbamazepine to juvenile rainbow trout (Oncorhynchus mykiss): Effects on antioxidant responses, hematological parameters and hepatic erod. Ecotoxicol. Environ. Saf. 2011, 74, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.M.; Leung, T.S. Trifluralin and oryzalin herbicides toxicities to juvenile crawfish (Procambarus clarkii) and mosquitofish (Gambusia affinis). Bull. Environ. Contam. Toxicol. 1983, 31, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.S.; Naqvi, S.M.; Naqvi, N.Z. Paraquat toxicity to Louisiana crayfish (Procambarus clarkii). Bull. Environ. Contam. Toxicol. 1980, 25, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Sommer, T.R. Laboratory and field studies on the toxic effects of thiobencarb (Bolero) to the crawfish Procambarus clarkii. J. World Maric. Soc. 1983, 14, 434–440. [Google Scholar] [CrossRef]
- Randall, D.J.; Connell, D.W.; Yang, R.; Wu, S.S. Concentrations of persistent lipophilic compounds in fish are determined by exchange across the gills, not through the food chain. Chemosphere 1998, 37, 1263–1270. [Google Scholar] [CrossRef]
- Chang, C.C.; Lee, P.P.; Hsu, J.P.; Yeh, S.P.; Cheng, W. Survival, and biochemical, physiological, and histopathological responses of the giant freshwater prawn, Macrobrachium rosenbergii, to short-term trichlorfon exposure. Aquaculture 2006, 253, 653–666. [Google Scholar] [CrossRef]
- Xing, D.L.; Wang, H.; Wang, L.; Wang, W.; Lei, Y.Z. Accumulation of metsulfuron-methyl residues in Nile tilapia Oreochromis niloticus. J. Dalian Ocean Univ. 2011, 26, 12–16. (In Chinese) [Google Scholar]
- Lignot, J.H.; Trilles, J.P.; Charmantier, G. Effect of an organophosphorus insecticide, fenitrothion, on survival and osmoregulation of various developmental stages of the shrimp Penaeus japonicus (Crustacea: Decapoda). Mar. Biol. 1997, 128, 307–316. [Google Scholar] [CrossRef]
- Vogt, G.; Quinitio, E.T. Accumulation and excretion of metal granules in the prawn, Penaeus monodon, exposed to water-borne copper, lead, iron and calcium. Aquat. Toxicol. 1994, 28, 223–241. [Google Scholar] [CrossRef]
- Bhavan, P.S.; Geraldine, P. Histopathology of the hepatopancreas and gills of the prawn Macrobrachium malcolmsonii exposed to endosulfan. Aquat. Toxicol. 2000, 50, 331–339. [Google Scholar] [CrossRef]
- Sousa, L.G.; Petriella, A.M. Functional morphology of the hepatopancreas of Palaemonetes argentinus (Crustacea: Decapoda): Influence of environmental pollution. Rev. Biol. Trop. 2007, 55, 79–86. [Google Scholar]
- Ashby, J.; Kier, L.; Wilson, A.G.E.; Green, T.; Lefevre, P.A.; Tinwell, H.; Willis, G.A.; Heydens, W.F.; Clapp, M.J.L. Evaluation of the potential carcinogenicity and genetic toxicity to humans of the herbicide acetochlor. Hum. Exp. Toxicol. 1996, 15, 702–735. [Google Scholar] [CrossRef] [PubMed]
- Heiba, F.N. Effect of the insecticide diazinon on the hepatopanceas of the freshwater crayfish, Procambarus clarkii. Egypt. J. Aquat. Biol. Fish. 1999, 3, 197–213. [Google Scholar]
- Shi, X.J.; Du, Y.B.; Lam, P.K.S.; WU, R.S.S.; Zhou, B.S. Developmental toxicity and alteration of gene expression in zebrafish embryos exposed to PFOS. Toxicol. Appl. Pharm. 2008, 230, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hao, X.L.; Kou, Z.Y.; Wang, R.J. Influences of the herbicide acetochlor solution on Bufo gargarizans heart activities. Sichuan J. Zool. 2010, 29, 27–30. (In Chinese) [Google Scholar]
- Suvarchala, G.; Philip, G.H. Toxicity of 3, 5, 6-trichloro-2-pyridinol tested at multiple stages of zebrafish (Danio rerio) development. Environ. Sci. Pollut. Res. 2016, 23, 15515–15523. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y. Toxic Effects of Triazophos on Penaeus monodon and Two Bivalves. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2013. (In Chinese). [Google Scholar]
- Matthiessen, P.; Fox, P.J.; Douthwaite, R.J.; Wood, A.B. Accumulation of endosulfan residues in fish and their predators after aerial spraying for the control of tsetse fly in Botswana. Pestic. Manag. Sci. 1982, 13, 39–48. [Google Scholar] [CrossRef]
- Ballesteros, M.L.; Gonzalez, M.; Wunderlin, D.A.; Bistoni, M.A.; Miglioranza, K.S.B. Uptake, tissue distribution and metabolism of the insecticide endosulfan in Jenynsia multidentata (Anablepidae, Cyprinodontiformes). Environ. Pollut. 2011, 159, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Szegletes, T.; Bálint, T.; Szegletes, Z.; Nemcsók, J. Changes caused by methidathion in activity and distribution of molecular forms of carp (Cyprinus carpio L.) AChE. Pestic. Biochem. Phys. 1995, 52, 71–79. [Google Scholar] [CrossRef]
- Green, R.M.; Abdelghani, A.A. Effects of long-term exposure of the red swamp crawfish Procambarus clarkii to a mixture of two herbicides, 2,4-Dichloro-phenoxyacetic acid and monosodium methanearsonate, and associated human health risks. Int. J. Environ. Res. Public Health 2004, 1, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.H.; Zhou, X.; Zhou, D.Y.; Wang, S.J. Ecological and efficient model and technology of crayfish-rice co-culture. China Fish. 2013, 7, 68–70. (In Chinese) [Google Scholar]

| Time (h) | Regression Equation | R2 | LC50 (mg/L) | 95% Confidence Limits (mg/L) |
|---|---|---|---|---|
| 24 | P = −22.79 + 9.99C | 0.925 | 191.25 | 179.37–215.75 |
| 48 | P = −23.83 + 10.72C | 0.941 | 166.81 | 159.49–176.55 |
| 72 | P = −28.01 + 12.80C | 0.900 | 154.30 | 148.36–160.59 |
| 96 | P = −25.87 + 11.96C | 0.919 | 145.24 | 138.94–151.27 |
| Herbicides | 96 h LC50 (mg/L) | 95% Confidence Interval (mg/L) | US EPA Toxicity Category | Reference |
|---|---|---|---|---|
| Sulfometuron | 12,174 | 11,890–12,360 | Practically non-toxic | [28] |
| MBA | 145.24 | 138.9–151.3 | Practically non-toxic | This study |
| 2,4-D | 185 | 75–343 | Practically non-toxic | [21] |
| Molinate | 14 | 11–16 | Slightly toxic | [27] |
| Trifluralin | 12 | 11–13 | Slightly toxic | [35] |
| Propanil | 7.9 | 6.8–8.6 | Moderately toxic | [27] |
| Paraquat | 1.4 | 0.5–3 | Moderately toxic | [36] |
| Thiobencarb (for juveniles) | 0.47 | 0.36–0.58 | Highly toxic | [37] |
| Thiobencarb (for adults) | 0.20 | 0.09–0.31 | Highly toxic | [37] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Xu, E.G.; Ren, Y.; Jin, S.; Zhang, T.; Liu, J.; Li, Z. Mixture Toxicity of Bensulfuron-Methyl and Acetochlor to Red Swamp Crayfish (Procambarus clarkii): Behavioral, Morphological and Histological Effects. Int. J. Environ. Res. Public Health 2017, 14, 1466. https://doi.org/10.3390/ijerph14121466
Yu J, Xu EG, Ren Y, Jin S, Zhang T, Liu J, Li Z. Mixture Toxicity of Bensulfuron-Methyl and Acetochlor to Red Swamp Crayfish (Procambarus clarkii): Behavioral, Morphological and Histological Effects. International Journal of Environmental Research and Public Health. 2017; 14(12):1466. https://doi.org/10.3390/ijerph14121466
Chicago/Turabian StyleYu, Jixin, Elvis Genbo Xu, Yan Ren, Shiyu Jin, Tanglin Zhang, Jiashou Liu, and Zhongjie Li. 2017. "Mixture Toxicity of Bensulfuron-Methyl and Acetochlor to Red Swamp Crayfish (Procambarus clarkii): Behavioral, Morphological and Histological Effects" International Journal of Environmental Research and Public Health 14, no. 12: 1466. https://doi.org/10.3390/ijerph14121466




