Do Cervical Cancer Patients Diagnosed with Opportunistic Screening Live Longer? An Arkhangelsk Cancer Registry Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setting, Design, and Sample Size
2.2. Data Collection
2.3. Data Analysis
2.4. Ethical Considerations
3. Results
4. Discussion
4.1. Main Findings
4.2. Data Interpretation and Comparisons with Previous Studies
4.3. Limitations and Strengths
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bruni, L.; Barrionuevo-Rosas, L.; Albero, G.; Serrano, B.; Mena, M.; Gómez, D.; Muñoz, J.; Bosch, F.X.; de Sanjosé, S. Human Papillomavirus and Related Diseases in the World; Summary Report 2015-04-08; ICO Information Centre on HPV and Cancer (HPV Information Centre): Barcelona, Spain; Available online: http://www.hpvcentre.net/statistics/reports/XWX.pdf (accessed on 15 January 2017).
- World Health Organisation. Media Centre. Human Papillomavirus (HPV) and Cervical Cancer. Fact sheet No. 380. Reviewed March 2015. Available online: http://www.who.int/mediacentre/factsheets/fs380/en/ (accessed on 15 January 2017).
- Bruni, L.; Barrionuevo-Rosas, L.; Albero, G.; Serrano, B.; Mena, M.; Gómez, D.; Muñoz, J.; Bosch, F.X.; de Sanjosé, S. Human Papillomavirus and Related Diseases in Russian Federation; Summary Report 2017-07-27; ICO Information Centre on HPV and Cancer (HPV Information Centre): Barcelona, Spain; Available online: http://www.hpvcentre.net/statistics/reports/RUS.pdf (accessed on 18 September 2017).
- Petrova, G.V.; Kaprin, A.D.; Gretzova, O.P.; Starisky, V.V. Malignant Neoplasms in Russia. Overview of Statistical Data 2015; Moscow Research Oncological Institute named after P.A. Herzen: Moscow, Russia, 2016; Available online: http://www.oncology.ru/service/statistics/malignant_tumors/2015.pdf (accessed on 18 September 2017). (In Russian)
- Territorial Office of the Federal State Statistics Service of the Arkhangelsk Region. Available online: http://arhangelskstat.gks.ru/wps/wcm/connect/rosstat_ts/arhangelskstat/ru/statistics/population/ (accessed on 10 January 2017).
- Hakama, M.; Räsänen-Virtanen, U. Effect of a mass screening program on the risk of cervical cancer. Am. J. Epidemiol. 1976, 103, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Laärä, E.; Day, N.; Hakama, M. Trends in mortality from cervical cancer in the Nordic countries: Association with organised screening programmes. Lancet 1987, 329, 1247–1249. [Google Scholar] [CrossRef]
- Klint, A.; Tryggvadottir, L.; Bray, F.; Gislum, M.; Hakulinen, T.; Storm, H.H.; Engholm, G. Trends in the survival of patients diagnosed with cancer in female genital organs in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol. 2010, 49, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Lynge, E.; Madsen, M.; Engholm, G. Effect of organised screening on incidence and mortality of cervical cancer in Denmark. Cancer Res. 1989, 49, 2157–2160. [Google Scholar] [PubMed]
- Nieminen, P.; Kallio, M.; Anttila, A.; Hakama, M. Organised versus spontaneous Pap-smear screening for cervical cancer: A case control study. Int. J. Cancer 1999, 83, 55–58. [Google Scholar] [CrossRef]
- Coleman, D.; Day, N.E.; Douglas, G.; Farmery, E.; Lynge, E.; Philip, J.; Segnan, N. European guidelines for quality assurance in cervical cancer screening. Eur. J. Cancer 1993, 29A (Suppl. 4), S1–S38. [Google Scholar] [PubMed]
- Seidel, D.; Becker, N.; Rohrmann, S.; Nimptsch, K.; Linseisen, J. Socio-demographic characteristics of participation in the opportunistic German cervical cancer screening programme: Results from the EPIC-Heidelberg cohort. J. Cancer Res. Clin. Oncol. 2009, 135, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Ronco, G.; Senore, C.; Giordano, L.; Quadrino, S.; Ponti, A.; Segnan, N. Who does Pap-test? The effect of one call program on coverage and determinants of compliance. Epidemiol. Prev. 1994, 18, 218–223. [Google Scholar] [PubMed]
- Hansen, B.T.; Hukkelberg, S.S.; Haldorsen, T.; Eriksen, T.; Skare, G.B.; Nygard, M. Factors associated with non-attendance, opportunistic attendance and reminded attendance to cervical screening in an organized screening program: A cross-sectional study of 12,058 Norwegian women. BMC Public Health 2011, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.M.; Nieminen, P.; Paavonen, J.; Lehtinen, M. Cervical cancer incidence can increase despite HPV vaccination. Correspondence. Lancet Infect. Dis. 2010, 10, 594–595. [Google Scholar] [CrossRef]
- Van der Aa, M.A.; Pukkala, E.; Coebergh, J.W.; Anttila, A.; Siesling, S. Mass screening programmes and trends in cervical cancer in Finland and The Netherlands. Int. J. Cancer 2008, 122, 1854–1858. [Google Scholar] [CrossRef] [PubMed]
- Moser, K.; Patnick, J.; Beral, V. Inequalities in reported use of breast and cervical screening in Great Britain: Analysis of cross sectional survey data. Br. Med. J. 2009, 338, b2025. [Google Scholar] [CrossRef] [PubMed]
- Azerkan, F.; Widmark, C.; Sparen, P.; Weiderpass, E.; Tillgren, P.; Faxelid, E. When Life Got in the Way: How Danish and Norwegian Immigrant Women in Sweden Reason about Cervical Screening and Why They Postpone Attendance. PLoS ONE 2015, 10, e0107624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, R.; Richardson, J.; Esmail, A.; Pickles, A. Uptake for cervical screening by ethnicity and place-of-birth: A population-based cross-sectional study. J. Public Health 2004, 26, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Canadian Cancer Society. Screening for Cervical Cancer. Available online: http://www.cancer.ca/en/prevention-and-screening/early-detection-and-screening/screening/screening-for-cervical-cancer/?region=on (accessed on 10 November 2017).
- Saraiya, M.; Steben, M.; Watson, M.; Markowitz, L. Evolution of cervical cancer screening and prevention in United States and Canada: Implications for public health practitioners and clinicians. Prev. Med. 2013, 57, 426–433. [Google Scholar] [CrossRef] [PubMed]
- The Council of the European Union Council recommendation of 2 December 2003 on cancer screening. Off. J. Eur. Union 2003, 878, 34–38. Available online: https://ec.europa.eu/jrc/sites/jrcsh/files/2_December_2003%20cancer%20screening.pdf (accessed on 18 September 2017).
- World Health Organization. Guidelines for Screening and Treatment of Precancerous Lesions for Cervical Cancer Prevention. 2013. Available online: http://apps.who.int/iris/bitstream/10665/94830/1/9789241548694_eng.pdf (accessed on 10 November 2017).
- Hinkula, M.; Pukkala, E.; Kyyronen, P.; Laukkanen, P.; Koskela, P.; Paavonen, J.; Lehtinen, M.; Kauppila, A. A population based study on the risk of cervical cancer and cervical intraepithelial neoplasia among grand multiparious women in Finland. Br. J. Cancer 2004, 90, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Petrova, G.V.; Kaprin, A.D.; Gretzova, O.P.; Starisky, V.V. Malignant Neoplasms in Russia. Overview of Statistical Data 1993–2013; Moscow Research Oncological Institute named after P.A. Herzen: Moscow, Russia, 2015. (In Russian) [Google Scholar]
- Novik, V.I. Screening for cervical cancer. J. Pract Oncol. 2010, 11, 66–72. (In Russian) [Google Scholar]
- Order of the Ministry of Health of the Russian Federation of 10.02.2003 No. 50. On the Improvement of Obstetric and Gynecological Care in Outpatient Clinics. Available online: http://www.med-pravo.ru/PRICMZ/PricMZ2003/50/50_2–1.html (accessed on 18 September 2017).
- Order of the Ministry of Health of the Russian Federation of November 1, 2012 No. 572n. On the Approval of the Procedure for the Provision of Medical Assistance in the Field of Obstetrics and Gynecology. Available online: https://www.rosminzdrav.ru/documents/9154-prikaz-ministerstva-zdravoohraneniya-rossiyskoy-federatsii-ot-1-noyabrya-2012-g-572n-ob-utverzhdenii-poryadka-okazaniya-meditsinskoy-pomoschi-po-profilyu-akusherstvo-i-ginekologiya-za-isklyucheniem-ispolzovaniya-vspomogatelnyh-reproduktivnyh-tehnologiy (accessed on 18 September 2017).
- National Cancer Control Programs. Policies and Managerial Guidelines; WHO: Geneva, Switzerland, 2002; Available online: http://www.who.int/cancer/media/en/408.pdf (accessed on 10 January 2017).
- Vaktskjold, A.; Lebedintseva, J.A.; Korotov, D.S.; Tkatsjov, A.V.; Podjakova, T.S.; Lund, E. Cancer incidence in Arkhangelskaja Oblast in northwestern Russia. The Arkhangelsk Cancer Registry. BMC Cancer 2005, 5, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Cancer Institute. The International Cancer Survival Standard (ICSS) Weights for Cervical Cancer. Available online: https://seer.cancer.gov/stdpopulations/survival.html (accessed on 18 September 2017).
- Sasieni, P.; Castanon, A.; Cuzick, J. Effectiveness of cervical screening with age: Population based case-control study of prospectively recorded data. BMJ 2009, 339, b2968. [Google Scholar] [CrossRef] [PubMed]
- Hellman, K.; Hellstrom, A.C.; Pettersson, B.F. Uterine cervix cancer treatment at Radiumhemmet: 90 years’ experience. Time trends of age, stage, and histopathology distribution. Cancer Med. 2014, 3, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, A.; Cybulski, M.; Buda, I.; Janosz, I.; Olszak-Wasik, K.; Bodzek, P.; Sliwczynski, A.; Teter, Z.; Olejek, A.; Baranowski, W. Cervical Cancer Histology, Staging and Survival before and after Implementation of Organised Cervical Screening Programme in Poland. PLoS ONE 2016, 11, e0155849. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on Cervical Cancer Screening. Screening for squamous cervical cancer—The duration of low risk following negative results in cervical cytology test: Introduction. Br. Med. J. 1986, 292, 659–664. [Google Scholar]
- Landy, R.; Birke, H.; Castanon, A.; Sasieni, P. Benefits and harms of cervical screening from age 20 years compared with screening from age 25 years. Br. J. Cancer 2014, 110, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, S.S.; Thompson, T.D.; Hall, H.I.; Logan, P.; Uhler, R.J. Breast and cervical carcinoma screening practices among women in rural and nonrural areas of the United States, 1998–1999. Cancer 2002, 94, 2801–2812. [Google Scholar] [CrossRef] [PubMed]
- Liff, J.M.; Chow, W.H.; Greenberg, R.S. Rural-urban differences in stage at diagnosis. Possible relationship to cancer screening. Cancer 1991, 67, 1454–1459. [Google Scholar] [CrossRef]
- Wang, F.; McLafferty, S.; Escamilla, V.; Luo, L. Late-Stage Breast Cancer Diagnosis and Health Care Access in Illinois. Prof. Geogr. 2008, 60, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Ward, E.; Wu, X.; Martin, H.J.; McLaughlin, C.C.; Thun, M.J. Geographic patterns of prostate cancer mortality and variations in access to medical care in the United States. Cancer Epidemiol. Prev. Biomark. 2005, 14, 590–595. [Google Scholar]
- Elnicki, D.M.; Morris, D.K.; Shockcor, W.T. Patient-perceived barriers to preventive health care among indigent, rural Appalachian patients. Arch. Intern. Med. 1995, 155, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Fazio, L.; Cotterchio, M.; Manno, M.; McLaughlin, J.; Gallinger, S. Association between colonic screening, subject characteristics, and stage of colorectal cancer. Am. J. Gastroenterol. 2005, 100, 2531–2539. [Google Scholar] [CrossRef] [PubMed]
- Blair, S.L.; Sadler, G.R.; Bristol, R.; Summers, C.; Tahar, Z.; Saltzstein, S.L. Early cancer detection among rural and urban Californians. BMC Public Health 2006, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Shugarman, L.R.; Sorbero, M.E.; Tian, H.; Jain, A.K.; Ashwood, J.S. An exploration of urban and rural differences in lung cancer survival among medicare beneficiaries. Am. J. Public Health 2008, 98, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Accounts Chamber of Russian Federation. Press-Centre Release, 13 April 2015. Available online: http://www.ach.gov.ru/press_center/news/21297 (accessed on 17 January 2017). (In Russian)
- Bakhlaev, I.E.; Kovchur, P.I.; Mikhetko, A.A.; Kurmyshkina, O.V.; Nilva, S.E. Cervical cancer in Karelia. Vestnik ROINC Imeni Blokhina 2011, 22, 22–29. (In Russian) [Google Scholar]
- Bakhlaev, I.E.; Kovchur, P.I. Prevention of cancer of the cervix uteri at an antenatal clinic. Tumors Womens Reprod. Syst. 2009, 3–4, 94–98. (In Russian) [Google Scholar]
- Bakhlaev, I.E.; Kovchur, P.I.; Mikhetko, A.A.; Nilva, S.E. Epidemiologic features and cytological screening of cervical cancer in Karelia. Vliyanie Ecologii na Vnutrennie Bolezni 2010, 1, 1757–1761. (In Russian) [Google Scholar]
- Smith, H.O.; Tiffany, M.F.; Qualls, C.R.; Key, C.R. The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States—A 24-year population-based study. Gynecol. Oncol. 2000, 78, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Vinh-Hung, V.; Bourgain, C.; Vlastos, G.; Cserni, G.; De Ridder, M.; Storme, G.; Vlastos, A.T. Prognostic value of histopathology and trends in cervical cancer: A SEER population study. BMC Cancer 2007, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Vizcaino, A.P.; Moreno, V.; Bosch, F.X.; Munoz, N.; Barros-Dios, X.M.; Borras, J.; Parkin, D.M. International trends in incidence of cervical cancer: II. Squamous-cell carcinoma. Int. J. Cancer 2000, 86, 429–435. [Google Scholar] [CrossRef]
- Mathew, A.; George, P.S. Trends in incidence and mortality rates of squamous cell carcinoma and adenocarcinoma of cervix-worldwide. Asian Pac. J. Cancer Prev. 2009, 10, 645–650. [Google Scholar] [PubMed]
- U.S. Preventive Services Task Force. Screening for cervical cancer: Recommendations and rationale. Am. J. Nurs. 2003, 103, 101–109. [Google Scholar]
- OECD Health Statistics. Available online: http://www.oecd.org/els/health-systems/health-data.htm (accessed on 10 January 2017).
- American Cancer Society. Cancer Facts and Figures; American Cancer Society: Atlanta, GA, USA, 2010; Available online: https://www.cancer.org/cancer/cervical-cancer/detection-diagnosis-staging/survival.html (accessed on 10 January 2017).
- Kosary, C.L. FIGO stage, histology, histologic grade, age and race as prognostic factors in determining survival for cancers of the female gynecological system: An analysis of 1973–87 SEER cases of cancers of the endometrium, cervix, ovary, vulva, and vagina. Semin. Surg. Oncol. 1994, 10, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.A.; Grigsby, P.W.; Nene, S.M.; Camel, H.M.; Galakatos, A.; Kao, M.S.; Lockett, M.A. Effect of tumor size on the prognosis of carcinoma of the uterine cervix treated with irradiation alone. Cancer 1992, 69, 2796–2806. [Google Scholar] [CrossRef]
- Dickman, P.W.; Hakulinen, T.; Luostarinen, T.; Pukkala, E.; Sankila, R.; Sodermam, B.; Teppo, L. Survival of cancer patients in Finland 1955–1994. Acta Oncol. 1999, 38, 1–103. [Google Scholar] [CrossRef] [PubMed]
- Kramer, B.S.; Croswell, J.M. Cancer screening: The clash of science and intuition. Annu. Rev. Med. 2009, 60, 125–137. [Google Scholar] [CrossRef] [PubMed]

| Variables | N (%) | No Screening | Screening | Р 1 |
|---|---|---|---|---|
| N = 1285 | N = 263 | |||
| Age at Diagnosis (years) 2: Mean (SD) | 48.5 (15.2) | 48.1 (15.1) | 50.6 (15.7) | 0.013 |
| Residence: | 0.145 | |||
| urban | 1122 (72.5) | 941 (73.2) | 181 (68.8) | |
| rural | 426 (27.5) | 344 (26.8) | 82 (31.2) | |
| Stages: | <0.001 | |||
| I | 606 (39.1) | 471 (36.7) | 135 (51.3) | |
| II | 404 (26.1) | 349 (27.2) | 55 (20.9) | |
| III | 352 (22.7) | 314 (24.4) | 38 (14.4) | |
| IV | 186 (12.0) | 151 (11.7) | 35 (13.4) | |
| Histological tumor type: | 0.829 | |||
| squamous cell | 1292 (83.5) | 1072 (83.4) | 220 (83.7) | |
| carcinoma | 141 (9.1) | 116 (9.0) | 15 (9.5) | |
| adenocarcinoma unspecified/other | 94 (6.1) | 78 (6.1) | 16 (6.1) | |
| uknown | 21 (1.4) | 19 (1.5) | 2 (0.7) | |
| Vital status: | <0.001 | |||
| alive | 969 (62.6) | 773 (60.2) | 196 (74.5) | |
| died from cervical cancer | 514 (33.2) | 455 (35.4) | 59 (22.4) | |
| died from other causes | 65 (4.2) | 57 (4.4) | 8 (3.0) | |
| Age of death (years) 2: Mean (SD) | 54.2 (16.5) | 53.5 (16.3) | 60.3 (17.3) | 0.002 |
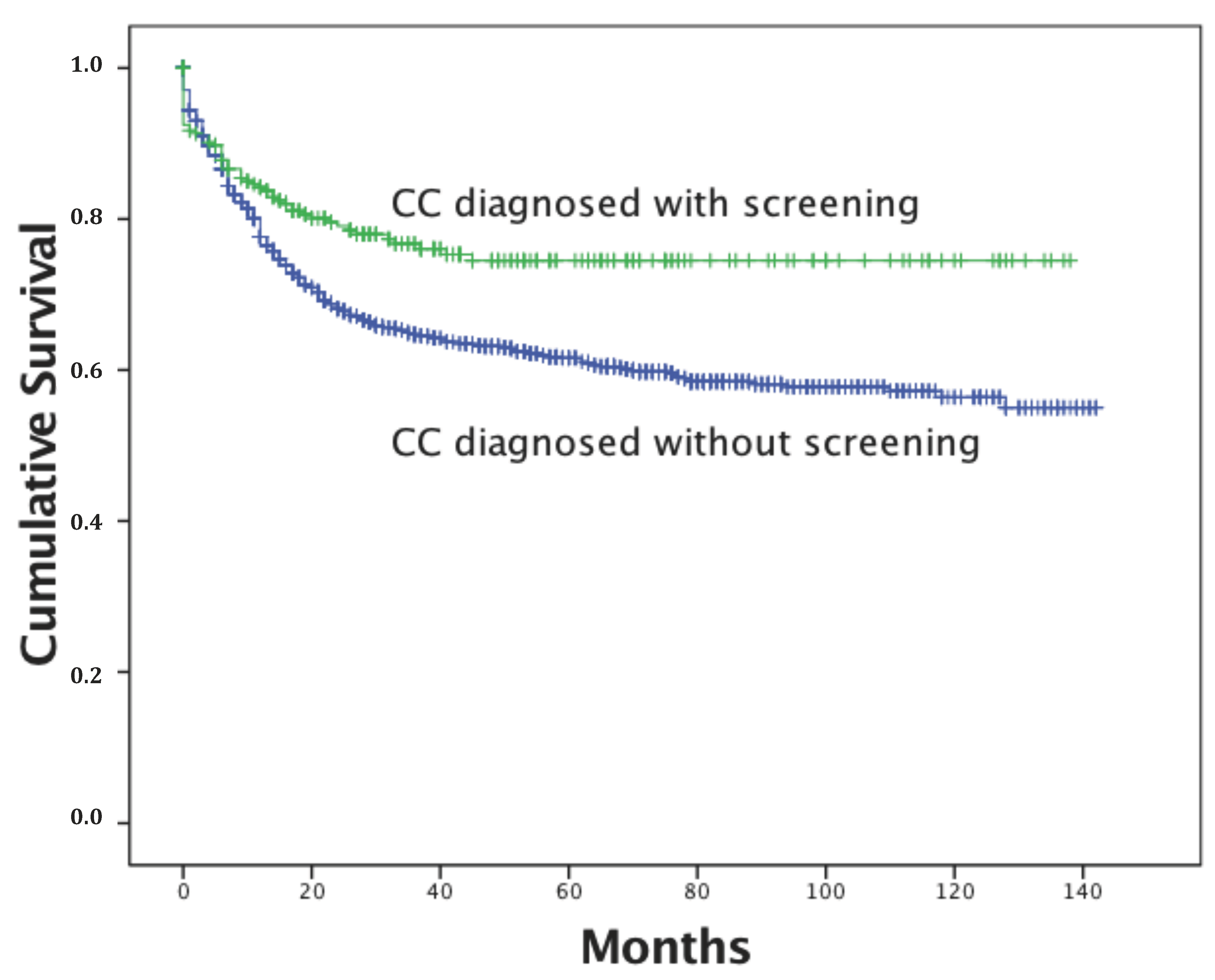
| N | Deaths | Mean Survival Time (Months) | Log-Rank p-Value | |||
|---|---|---|---|---|---|---|
| Mean | 95% LL | 95% UL | ||||
| Cervical cancer (CC) diagnosed by screening: | 0.001 | |||||
| no | 1285 | 455 | 89.7 | 85.9 | 93.4 | |
| yes | 263 | 59 | 105.8 | 98.5 | 113.0 | |
| Age in years: | <0.001 | |||||
| 15–44 | 746 | 202 | 101.7 | 97.0 | 106.4 | |
| 44–54 | 314 | 99 | 94.1 | 86.5 | 101.7 | |
| 55–64 | 244 | 93 | 80.6 | 71.2 | 90.0 | |
| 65–74 | 158 | 60 | 82.8 | 72.2 | 93.1 | |
| 75 and more | 107 | 60 | 53.4 | 40.7 | 66.0 | |
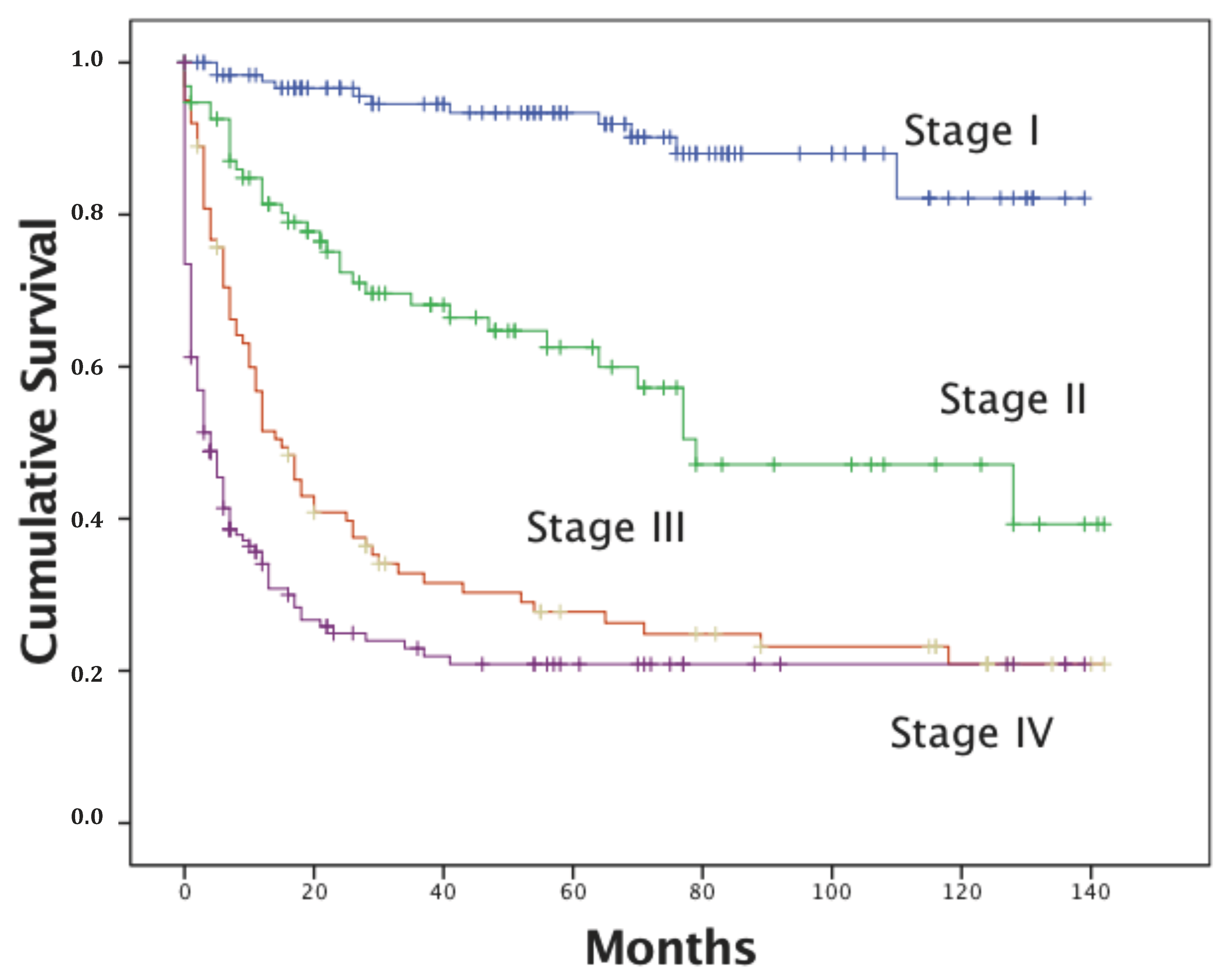
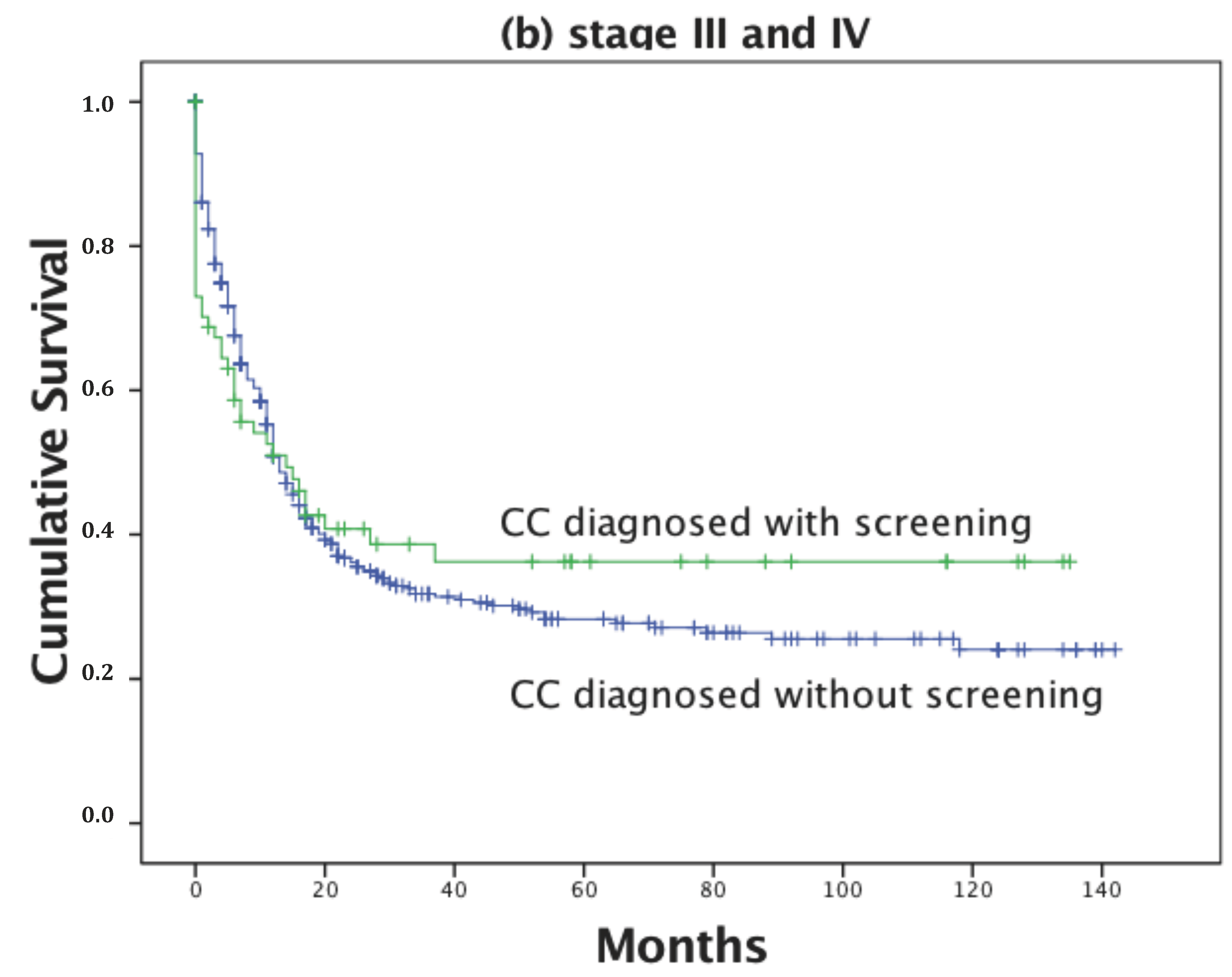
| Stage: | <0.001 | |||||
| I | 606 | 36 | 132.5 | 129.5 | 135.5 | |
| II | 404 | 135 | 92.2 | 85.6 | 98.9 | |
| III | 352 | 203 | 55.7 | 48.5 | 62.9 | |
| IV | 186 | 140 | 30.7 | 22.4 | 39.0 | |
| Residence: | 0.018 | |||||
| urban | 1122 | 358 | 95.0 | 91.0 | 98.9 | |
| rural | 426 | 156 | 85.9 | 78.9 | 92.8 | |
| Histological tumor type: | <0.001 | |||||
| squamous cell carcinoma | 1292 | 390 | 97.1 | 93.4 | 100.7 | |
| adenocarcinoma | 141 | 66 | 67.7 | 55.2 | 80.2 | |
| unspecified/other | 94 | 42 | 73.6 | 59.8 | 87.5 | |
| unknown | 21 | 16 | 31.6 | 11.3 | 51.8 | |
| Crude | Adjusted 1 | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Level | HR | 95% CI | p-Level | |
| Cervical cancer diagnosed by screening: | ||||||
| no | 1.61 | 1.22–2.10 | 0.001 | 1.37 | 1.04–1.80 | 0.027 |
| yes | 1.00 | 1.00 | ||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roik, E.E.; Nieboer, E.; Kharkova, O.A.; Grjibovski, A.M.; Postoev, V.A.; Odland, J.Ø. Do Cervical Cancer Patients Diagnosed with Opportunistic Screening Live Longer? An Arkhangelsk Cancer Registry Study. Int. J. Environ. Res. Public Health 2017, 14, 1500. https://doi.org/10.3390/ijerph14121500
Roik EE, Nieboer E, Kharkova OA, Grjibovski AM, Postoev VA, Odland JØ. Do Cervical Cancer Patients Diagnosed with Opportunistic Screening Live Longer? An Arkhangelsk Cancer Registry Study. International Journal of Environmental Research and Public Health. 2017; 14(12):1500. https://doi.org/10.3390/ijerph14121500
Chicago/Turabian StyleRoik, Elena E., Evert Nieboer, Olga A. Kharkova, Andrej M. Grjibovski, Vitaly A. Postoev, and Jon Ø. Odland. 2017. "Do Cervical Cancer Patients Diagnosed with Opportunistic Screening Live Longer? An Arkhangelsk Cancer Registry Study" International Journal of Environmental Research and Public Health 14, no. 12: 1500. https://doi.org/10.3390/ijerph14121500





