Stress-Induced Hyperglycemia in Diabetes: A Cross-Sectional Analysis to Explore the Definition Based on the Trauma Registry Data
Abstract
:1. Introduction
2. Methods
2.1. Ethics Statement
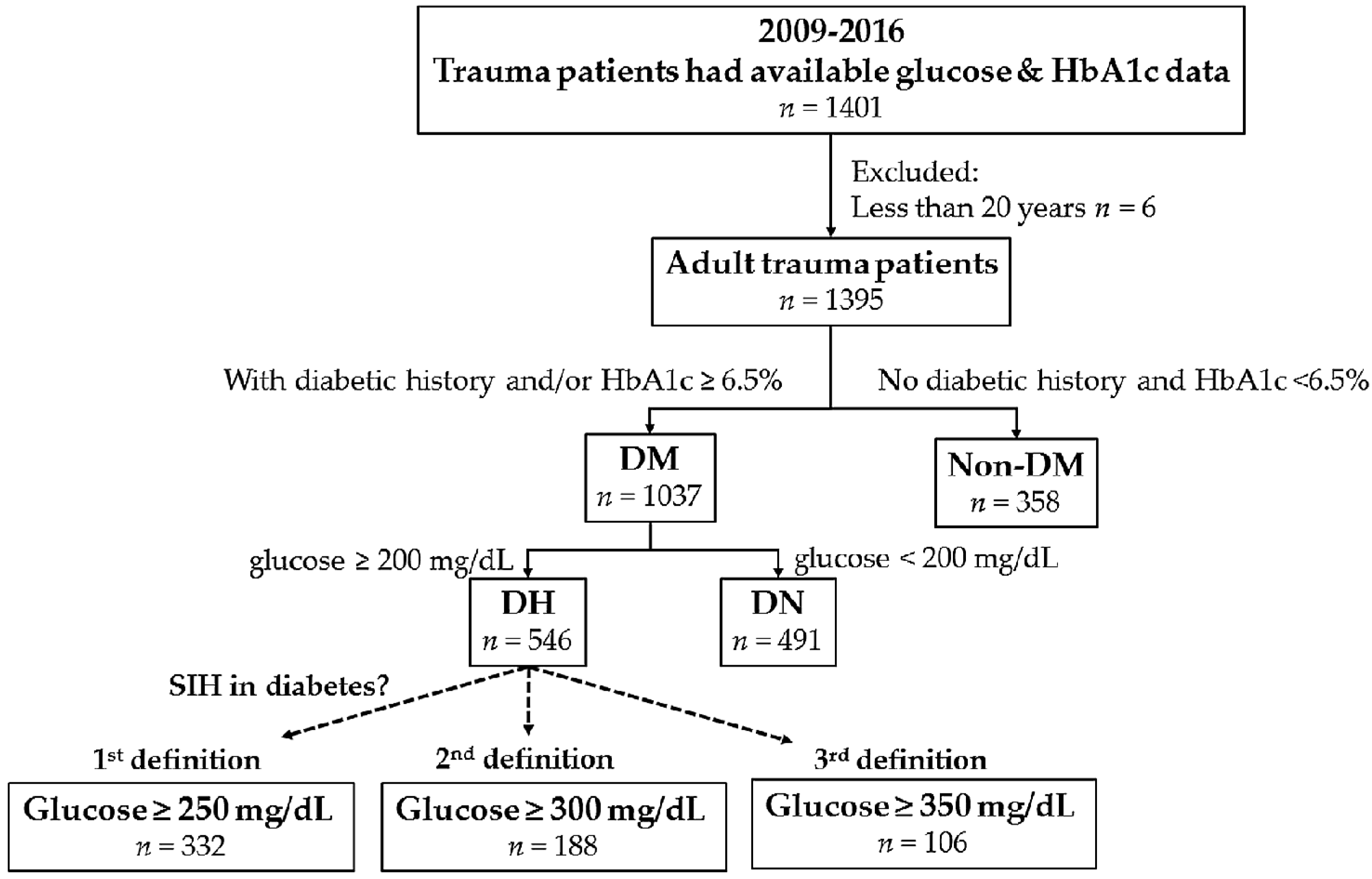
2.2. Data Source and Study Population
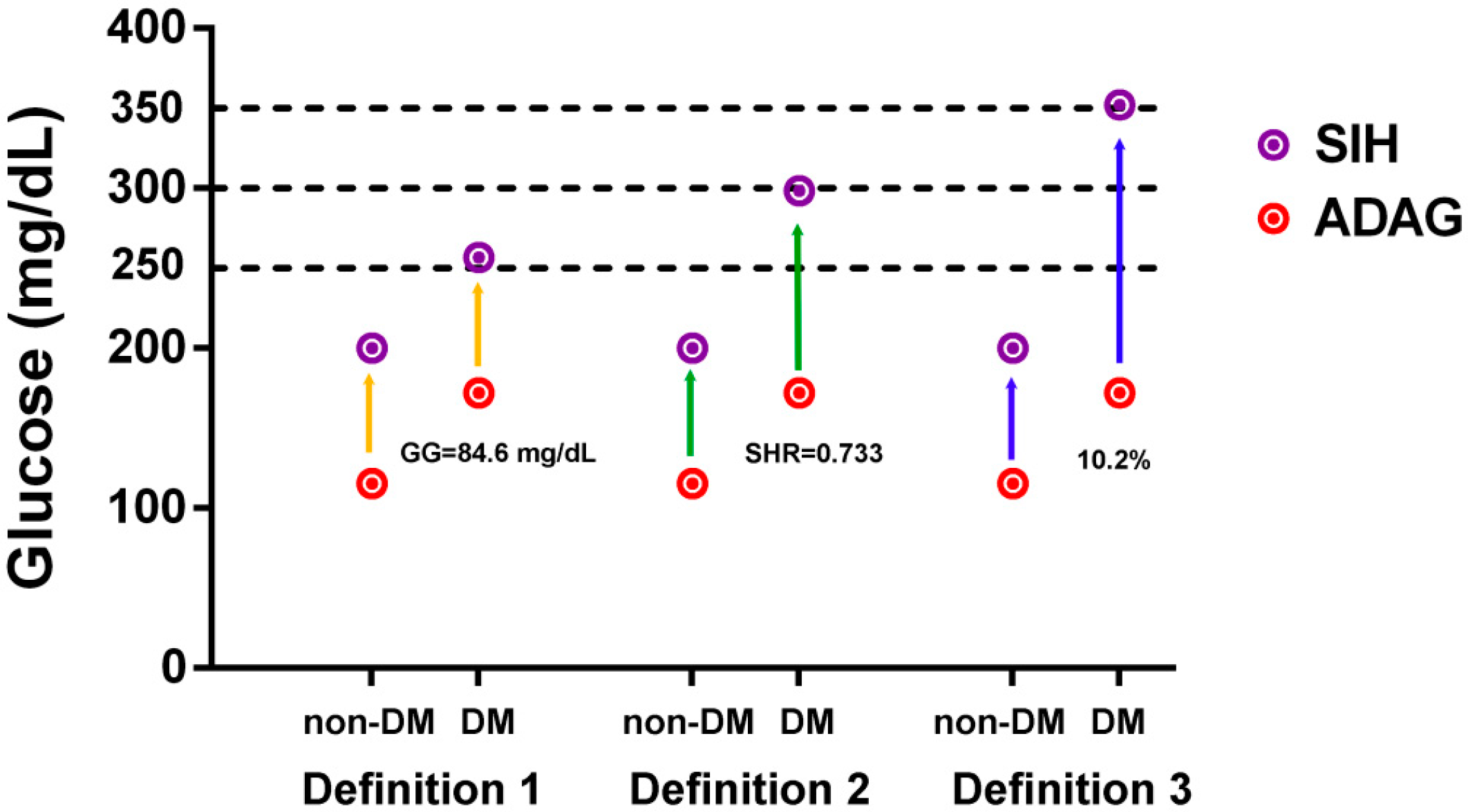
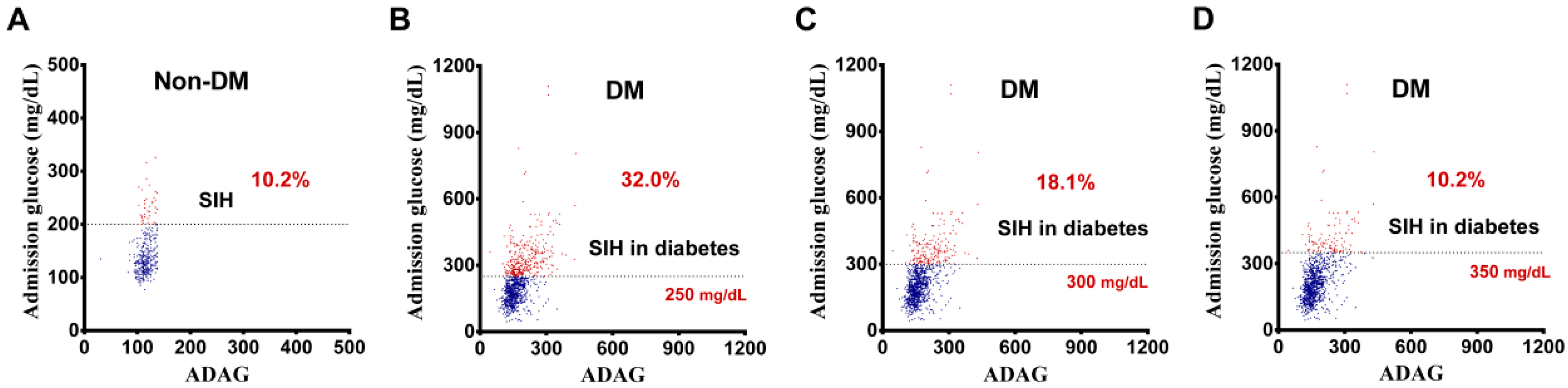
2.3. Definition of SIH in DM
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Patients with and without Diabetes
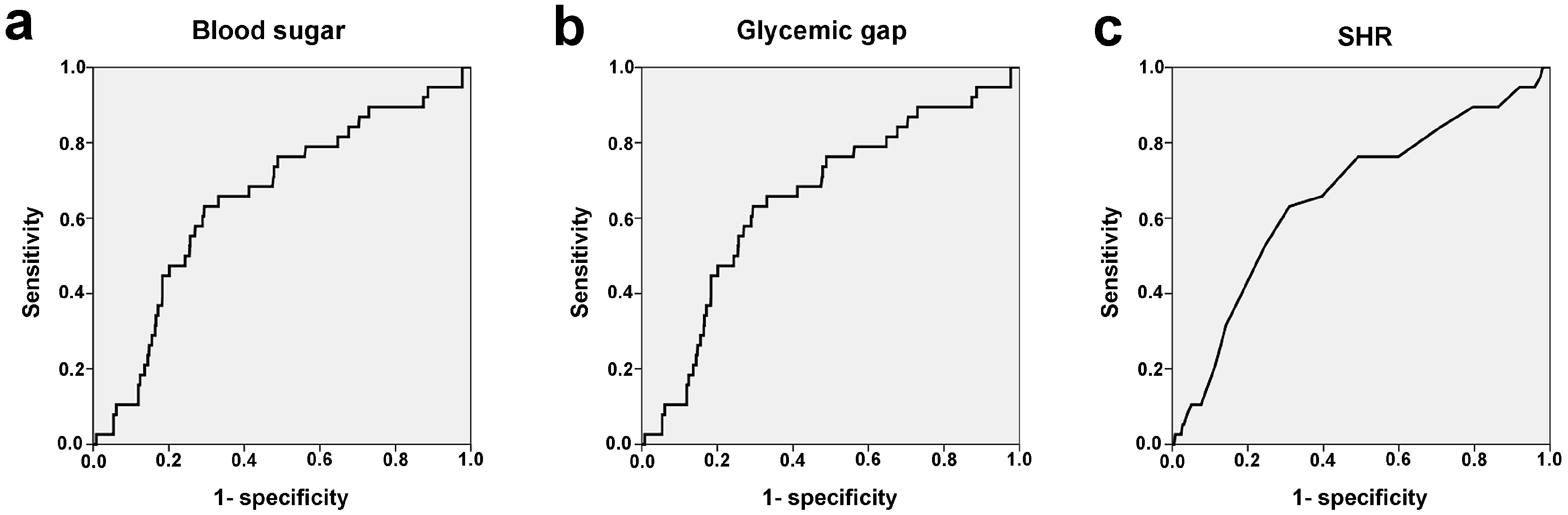
3.2. Definition of SIH in DM
3.3. Patient Outcomes Based on Different Definitions
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Richards, J.E.; Kauffmann, R.M.; Zuckerman, S.L.; Obremskey, W.T.; May, A.K. Relationship of hyperglycemia and surgical-site infection in orthopaedic surgery. J. Bone Joint Surg. Am. 2012, 94, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.E.; Kauffmann, R.M.; Obremskey, W.T.; May, A.K. Stress-induced hyperglycemia as a risk factor for surgical-site infection in nondiabetic orthopedic trauma patients admitted to the intensive care unit. J. Orthop. Trauma 2013, 27, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Mraovic, B.; Suh, D.; Jacovides, C.; Parvizi, J. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J. Diabetes Sci. Technol. 2011, 5, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Leto, R.; Desruelles, D.; Gillet, J.B.; Sabbe, M.B. Admission hyperglycaemia is associated with higher mortality in patients with hip fracture. Eur. J. Emerg. Med. 2015, 22, 99–102. [Google Scholar] [CrossRef] [PubMed]
- McCowen, K.C.; Malhotra, A.; Bistrian, B.R. Stress-induced hyperglycemia. Crit. Care Clin. 2001, 17, 107–124. [Google Scholar] [CrossRef]
- Bosarge, P.L.; Kerby, J.D. Stress-induced hyperglycemia: Is it harmful following trauma? Adv. Surg. 2013, 47, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Kerby, J.D.; Griffin, R.L.; MacLennan, P.; Rue, L.W., III. Stress-induced hyperglycemia, not diabetic hyperglycemia, is associated with higher mortality in trauma. Ann. Surg. 2012, 256, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Rau, C.S.; Wu, S.C.; Chen, Y.C.; Chien, P.C.; Hsieh, H.Y.; Kuo, P.J.; Hsieh, C.H. Higher mortality in trauma patients is associated with stress-induced hyperglycemia, but not diabetic hyperglycemia: A cross-sectional analysis based on a propensity-score matching approach. Int. J. Environ. Res. Public Health 2017, 14, 1161. [Google Scholar] [CrossRef] [PubMed]
- Rau, C.S.; Wu, S.C.; Chen, Y.C.; Chien, P.C.; Hsieh, H.Y.; Kuo, P.J.; Hsieh, C.H. Stress-induced hyperglycemia, but not diabetic hyperglycemia, is associated with higher mortality in patients with isolated moderate and severe traumatic brain injury: Analysis of a propensity score-matched population. Int. J. Environ. Res. Public Health 2017, 14, 1340. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, X.; Meng, K.; Zeng, Z.; Ma, B.; Liu, X.; Qi, B.; Cui, S.; Cao, P.; Yang, Y. Stress-induced hyperglycemia after hip fracture and the increased risk of acute myocardial infarction in nondiabetic patients. Diabetes Care 2013, 36, 3328–3332. [Google Scholar] [CrossRef] [PubMed]
- Dungan, K.M.; Braithwaite, S.S.; Preiser, J.C. Stress hyperglycaemia. Lancet 2009, 373, 1798–1807. [Google Scholar] [CrossRef]
- Brealey, D.; Singer, M. Hyperglycemia in critical illness: A review. J. Diabetes Sci. Technol. 2009, 3, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Egi, M.; Bellomo, R.; Stachowski, E.; French, C.J.; Hart, G.K.; Hegarty, C.; Bailey, M. Blood glucose concentration and outcome of critical illness: The impact of diabetes. Crit. Care Med. 2008, 36, 2249–2255. [Google Scholar] [CrossRef] [PubMed]
- Egi, M.; Bellomo, R.; Stachowski, E.; French, C.J.; Hart, G.K.; Taori, G.; Hegarty, C.; Bailey, M. The interaction of chronic and acute glycemia with mortality in critically ill patients with diabetes. Crit. Care Med. 2011, 39, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.G.; Sheehy, A.M.; Vincent, J.L.; Coursin, D.B. Critical illness-induced dysglycaemia: Diabetes and beyond. Crit. Care 2010, 14, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, W.I.; Wang, J.C.; Chang, W.C.; Hsu, C.W.; Chu, C.M.; Tsai, S.H. Usefulness of glycemic gap to predict ICU mortality in critically ill patients with diabetes. Medicine 2015, 94, e1525. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Liao, W.I.; Wang, Y.C.; Chang, W.C.; Hsu, C.W.; Chen, Y.H.; Tsai, S.H. An elevated glycemic gap is associated with adverse outcomes in diabetic patients with community-acquired pneumonia. Medicine 2015, 94, e1456. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.I.; Lin, C.S.; Lee, C.H.; Wu, Y.C.; Chang, W.C.; Hsu, C.W.; Wang, J.C.; Tsai, S.H. An elevated glycemic gap is associated with adverse outcomes in diabetic patients with acute myocardial infarction. Sci. Rep. 2016, 6, 27770. [Google Scholar] [CrossRef] [PubMed]
- Gillett, M.J. International expert committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009, 32, 1327–1334. [Google Scholar]
- Kilpatrick, E.S.; Bloomgarden, Z.T.; Zimmet, P.Z. International expert committee report on the role of the A1C assay in the diagnosis of diabetes: Response to the international expert committee. Diabetes Care 2009, 32, e160. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Bellomo, R. Stress hyperglycemia: An essential survival response! Crit. Care Med. 2013, 41, e93–e94. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; Kuenen, J.; Borg, R.; Zheng, H.; Schoenfeld, D.; Heine, R.J. Translating the A1C assay into estimated average glucose values. Diabetes Care 2008, 31, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.I.; Sheu, W.H.; Chang, W.C.; Hsu, C.W.; Chen, Y.L.; Tsai, S.H. An elevated gap between admission and A1C-derived average glucose levels is associated with adverse outcomes in diabetic patients with pyogenic liver abscess. PLoS ONE 2013, 8, e64476. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.W.; Quinn, S.J.; Valentine, N.; Alhawassi, T.; O’Dea, H.; Stranks, S.N.; Burt, M.G.; Doogue, M.P. Relative hyperglycemia, a marker of critical illness: Introducing the stress hyperglycemia ratio. J. Clin. Endocrinol. Metab. 2015, 100, 4490–4497. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Hsu, S.Y.; Hsieh, H.Y.; Chen, Y.C. Differences between the sexes in motorcycle-related injuries and fatalities at a Taiwanese level I trauma center. Biomed. J. 2017, 40, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Liu, H.T.; Hsu, S.Y.; Hsieh, H.Y.; Chen, Y.C. Motorcycle-related hospitalizations of the elderly. Biomed. J. 2017, 40, 121–128. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2012, 35 (Suppl. S1), S64–S71. [Google Scholar]
- Finfer, S.; Chittock, D.R.; Su, S.Y.; Blair, D.; Foster, D.; Dhingra, V.; Bellomo, R.; Cook, D.; Dodek, P.; Henderson, W.R.; et al. Intensive versus conventional glucose control in critically ill patients. N. Engl. J. Med. 2009, 360, 1283–1297. [Google Scholar] [PubMed]
- Van den Berghe, G.; Wouters, P.; Weekers, F.; Verwaest, C.; Bruyninckx, F.; Schetz, M.; Vlasselaers, D.; Ferdinande, P.; Lauwers, P.; Bouillon, R. Intensive insulin therapy in critically ill patients. N. Engl. J. Med. 2001, 345, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Cinotti, R.; Ichai, C.; Orban, J.C.; Kalfon, P.; Feuillet, F.; Roquilly, A.; Riou, B.; Blanloeil, Y.; Asehnoune, K.; Rozec, B. Effects of tight computerized glucose control on neurological outcome in severely brain injured patients: A multicenter sub-group analysis of the randomized-controlled open-label CGAO-REA study. Crit. Care 2014, 18, 498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griesdale, D.E.; de Souza, R.J.; van Dam, R.M.; Heyland, D.K.; Cook, D.J.; Malhotra, A.; Dhaliwal, R.; Henderson, W.R.; Chittock, D.R.; Finfer, S.; et al. Intensive insulin therapy and mortality among critically ill patients: A meta-analysis including NICE-SUGAR study data. CMAJ 2009, 180, 821–827. [Google Scholar] [CrossRef] [PubMed]
| Variables | DM | Non-DM |
|---|---|---|
| n = 1037 | n = 358 | |
| Sex | ||
| Female, n (%) | 541 (52.2) | 161 (45.0) |
| Male, n (%) | 496 (47.8) | 197 (55.0) |
| Age (years) | 67.1 ± 12.5 | 64.4 ± 16.8 |
| Comorbidity | ||
| HTN, n (%) | 669 (64.5) | 168 (46.9) |
| CAD, n (%) | 120 (11.6) | 33 (9.2) |
| CHF, n (%) | 30 (2.9) | 11 (3.1) |
| CVA, n (%) | 137 (13.2) | 43 (12.0) |
| ESRD, n (%) | 3 (0.3) | 1 (0.3) |
| GCS | 14.1 ± 2.5 | 13.6 ± 3.2 |
| ISS, median (IQR) | 9 (5–14) | 9 (5–16) |
| <16 | 781 (75.3) | 257 (71.8) |
| 16–24 | 189 (18.2) | 67 (18.7) |
| ≥25 | 67 (6.5) | 34 (9.5) |
| HbA1c (%) | 7.6 ± 1.8 | 5.7 ± 0.4 |
| ADAG | 172.2 ± 51.2 | 115.4 ± 12.0 |
| Blood sugar (mg/dL) | 224.3 ± 103.9 | 144.6 ± 44.5 |
| Glycemic gap (mg/dL) | 52.1 ± 87.9 | 29.2 ± 43.6 |
| SHR | 1.3 ± 0.5 | 1.3 ± 0.4 |
| Mortality, n (%) | 38 (3.7) | 16 (4.5) |
| Variables | (I) | (II) | (III) | (IV) |
|---|---|---|---|---|
| Glucose ≥ 250 | Glucose ≥ 300 | Glucose ≥ 350 | DN | |
| n = 332 | n = 188 | n = 106 | n = 491 | |
| Sex | ||||
| Female, n (%) | 166 (50.0) | 91 (48.4) | 55 (51.9) | 250 (50.9) |
| Male, n (%) | 166 (50.0) | 97 (51.6) | 51 (48.1) | 241 (49.1) |
| Age (years) | 64.6 ± 13.7 | 63.6 ± 13.7 | 63.2 ± 14.1 | 68.5 ± 11.5 |
| Comorbidity | ||||
| HTN, n (%) | 187 (56.3) | 106 (56.4) | 62 (58.5) | 340 (69.2) |
| CAD, n (%) | 33 (9.9) | 16 (8.5) | 7 (6.6) | 64 (13.0) |
| CHF, n (%) | 10 (3.0) | 7 (3.7) | 5 (4.7) | 12 (2.4) |
| CVA, n (%) | 27 (8.1) | 15 (8.0) | 9 (8.5) | 79 (16.1) |
| ESRD, n (%) | 2 (0.6) | 2 (1.1) | 1 (0.9) | 1 (0.2) |
| GCS | 13.6 ± 3.3 | 13.6 ± 3.2 | 13.6 ± 3.2 | 14.4 ± 2.0 |
| ISS, median (IQR) | 9 (9–16) | 9 (6–16) | 9 (9–16) | 9 (5–10) |
| <16 | 223 (67.2) | 124 (66.0) | 70 (66.0) | 389 (79.2) |
| 16–24 | 74 (22.3) | 46 (24.5) | 27 (25.5) | 81 (16.5) |
| ≥25 | 35 (10.5) | 18 (9.6) | 9 (8.5) | 21 (4.3) |
| Mortality, n (%) | 22 (6.6) | 10 (5.3) | 3 (2.8) | 11 (2.2) |
| Adjusted mortality, n (%) | 22 (6.6) | 10 (5.3) | 3 (2.8) | 11 (2.2) |
| Hospital LOS (days) | 13.6 ± 13.5 | 14.2 ± 13.9 | 13.4 ± 11.1 | 11.3 ± 11.2 |
| ICU admission, n (%) | 112 (33.7) | 65 (34.6) | 36 (34.0) | 119 (24.2) |
| Variables | (I) vs. (IV) | (II) vs. (IV) | (III) vs. (IV) |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Sex | 0.796 | 0.558 | 0.856 |
| Female, n (%) | 1.0 (0.73–1.27) | 0.9 (0.65–1.27) | 1.0 (0.68–1.58) |
| Male, n (%) | 1.0 (0.79–1.37) | 1.1 (0.79–1.55) | 1.0 (0.63–1.46) |
| Comorbidity | |||
| HTN, n (%) | 0.6 (0.43–0.77) | 0.6 (0.41–0.81) | 0.6 (0.41–0.96) |
| CAD, n (%) | 0.7 (0.47–1.15) | 0.6 (0.35–1.10) | 0.5 (0.21–1.06) |
| CHF, n (%) | 1.2 (0.53–2.90) | 1.5 (0.60–3.98) | 2.0 (0.68–5.73) |
| CVA, n (%) | 0.5 (0.29–0.73) | 0.5 (0.25–0.81) | 0.5 (0.24–1.00) |
| ESRD, n (%) | 3.0 (0.27–32.88) | 5.3 (0.48–58.45) | 4.7 (0.29–75.21) |
| ISS | |||
| <16 | 0.5 (0.39–0.74) | 0.5 (0.35–0.74) | 0.5 (0.32–0.81) |
| 16–24 | 1.5 (1.02–2.06) | 1.6 (1.09–2.47) | 1.7 (1.05–2.85) |
| ≥25 | 2.6 (1.51–4.62) | 2.4 (1.23–4.56) | 2.1 (0.92–4.67) |
| Mortality, n (%) | 3.1 (1.48–6.48) | 2.5 (1.02–5.87) | 1.3 (0.35–4.64) |
| Adjusted mortality, n (%) | 3.5 (1.61–7.46) | 3.0 (1.11–8.03) | 1.8 (0.44–7.14) |
| ICU admission, n (%) | 1.6 (1.17–2.16) | 1.7 (1.15–2.38) | 1.6 (1.02–2.53) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rau, C.-S.; Wu, S.-C.; Chen, Y.-C.; Chien, P.-C.; Hsieh, H.-Y.; Kuo, P.-J.; Hsieh, C.-H. Stress-Induced Hyperglycemia in Diabetes: A Cross-Sectional Analysis to Explore the Definition Based on the Trauma Registry Data. Int. J. Environ. Res. Public Health 2017, 14, 1527. https://doi.org/10.3390/ijerph14121527
Rau C-S, Wu S-C, Chen Y-C, Chien P-C, Hsieh H-Y, Kuo P-J, Hsieh C-H. Stress-Induced Hyperglycemia in Diabetes: A Cross-Sectional Analysis to Explore the Definition Based on the Trauma Registry Data. International Journal of Environmental Research and Public Health. 2017; 14(12):1527. https://doi.org/10.3390/ijerph14121527
Chicago/Turabian StyleRau, Cheng-Shyuan, Shao-Chun Wu, Yi-Chun Chen, Peng-Chen Chien, Hsiao-Yun Hsieh, Pao-Jen Kuo, and Ching-Hua Hsieh. 2017. "Stress-Induced Hyperglycemia in Diabetes: A Cross-Sectional Analysis to Explore the Definition Based on the Trauma Registry Data" International Journal of Environmental Research and Public Health 14, no. 12: 1527. https://doi.org/10.3390/ijerph14121527






