Meta-Analysis on the Association of ALDH2 Polymorphisms and Type 2 Diabetic Mellitus, Diabetic Retinopathy
Abstract
:1. Introduction
2. Methods
2.1. Literature Search and Inclusion Criteria
2.2. Data Extraction
2.3. Quality Evaluation
2.4. Statistical Methods
3. Results
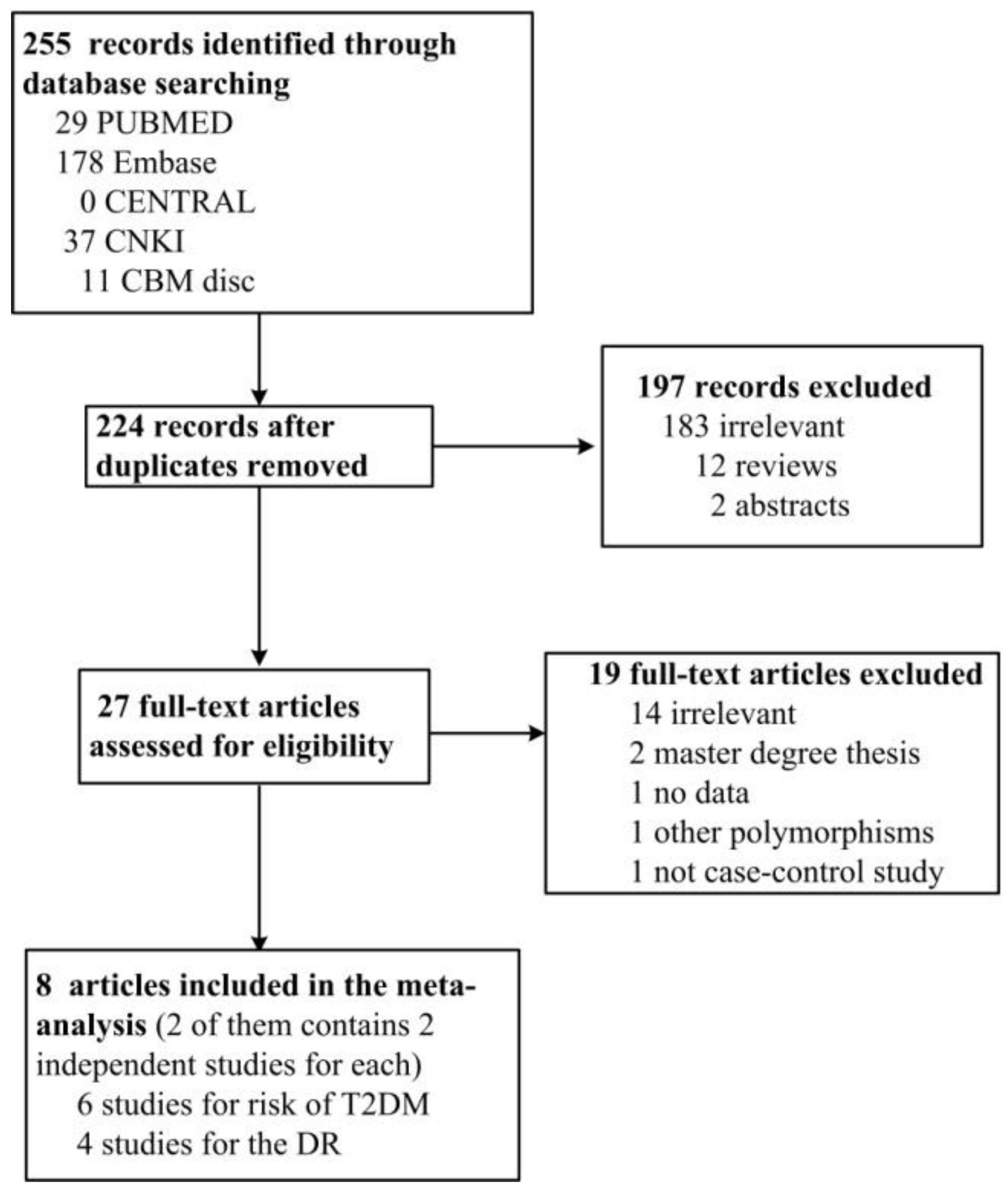
3.1. Literature Search and Characters of Involved Studies
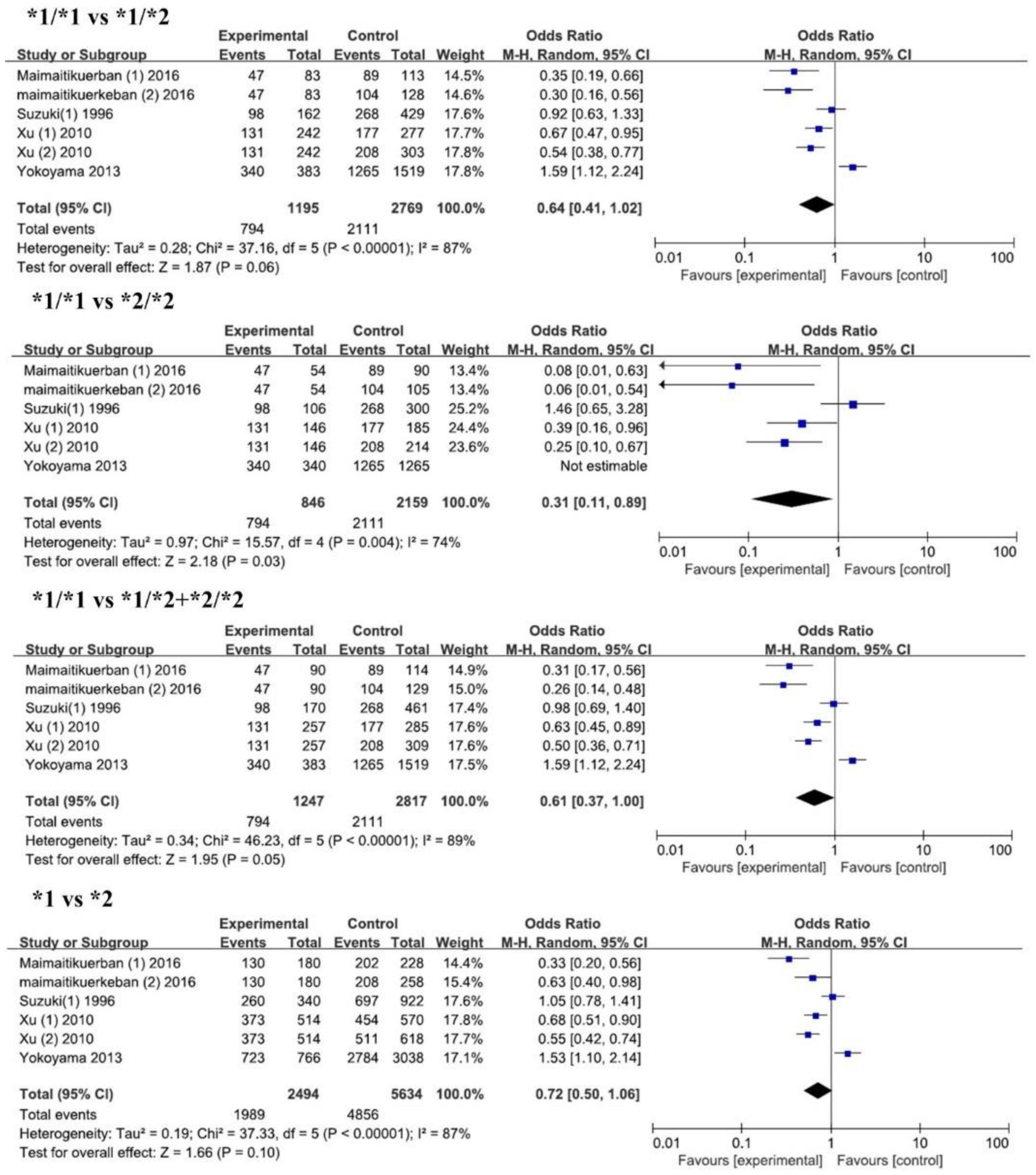
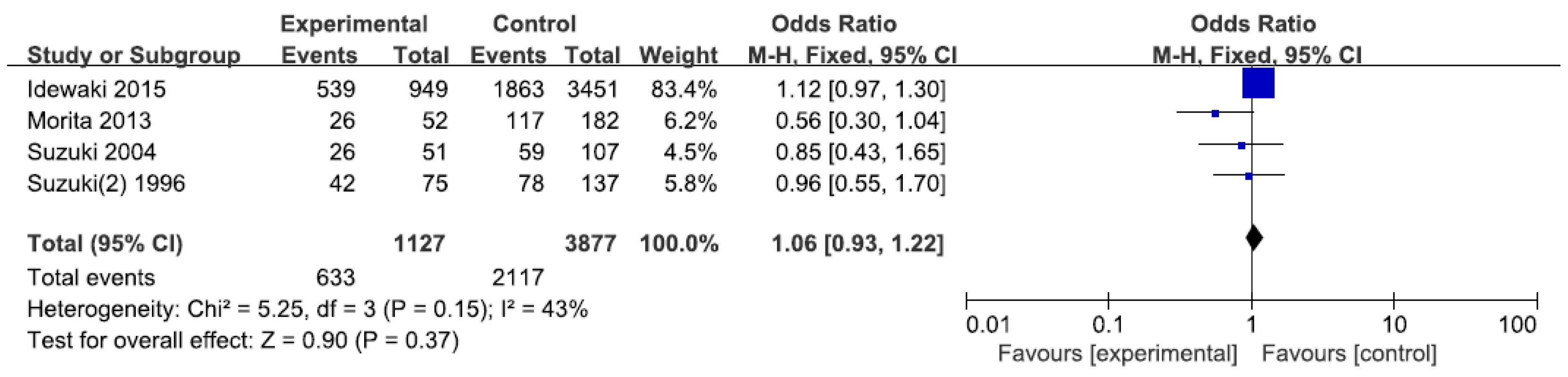
3.2. Results of Meta-Analysis
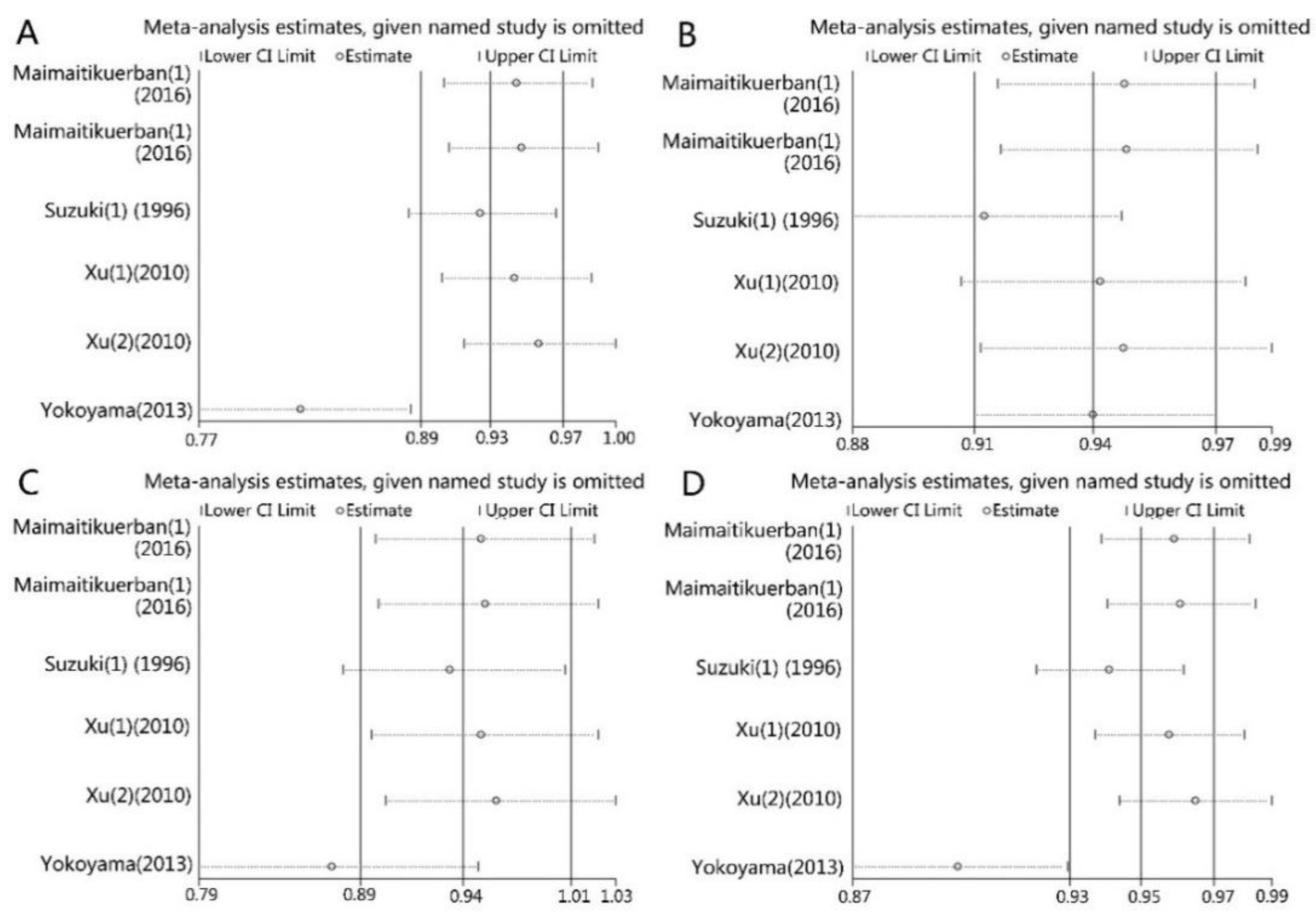
3.3. Sensitivity Analysis
3.4. Publication Bias
3.5. Source of Heterogeneity
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lauruschkat, A.H.; Arnrich, B.; Albert, A.A.; Walter, J.A.; Amann, B.; Rosendahl, U.P.; Alexander, T.; Ennker, J. Prevalence and risks of undiagnosed diabetes mellitus in patients undergoing coronary artery bypass grafting. Circulation 2005, 112, 2397–2402. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Fioretto, P.; Dodson, P.M. Does microvascular disease predict macrovascular events in type 2 diabetes. Atherosclerosis 2011, 218, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Wat, N.; Wong, R.L.; Wong, I.Y. Associations between diabetic retinopathy and systemic risk factors. Hong Kong Med. J. 2016, 22, 589–599. [Google Scholar] [PubMed]
- Munukutla, S.; Pan, G.; Deshpande, M.; Thandavarayan, R.A.; Krishnamurthy, P.; Palaniyandi, S.S. Alcohol toxicity in diabetes and its complications: A double trouble. Alcohol. Clin. Exp. Res. 2016, 40, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Van De Wiel, A. Diabetes mellitus and alcohol. Diabetes Metab. Res. Rev. 2004, 20, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Huang, I.Y.; Ikawa, M. Molecular abnormality of an inactive aldehyde dehydrogenase variant commonly found in Orientals. Proc. Natl. Acad. Sci. USA 1984, 81, 258–261. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.J.; Siraki, A.G.; Shangari, N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit. Rev. Toxicol. 2005, 35, 609–662. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Budas, G.R.; Churchill, E.N.; Disatnik, M.H.; Hurley, T.D.; Mochly-Rosen, D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science 2008, 321, 1493–1495. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Guo, R.; Yu, L.; Zhang, Y.; Ren, J. Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: Role of autophagy paradox and toxic aldehyde. Eur. Heart J. 2011, 32, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Saruwatari, J.; Miyagawa, H.; Uchiyashiki, Y.; Oniki, K.; Sakata, M.; Kajiwara, A.; Yoshida, A.; Jinnouchi, H.; Nakagawa, K. Association between aldehyde dehydrogenase 2 polymorphisms and the incidence of diabetic retinopathy among Japanese subjects with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2013, 12, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Kloner, R.A.; Rezkalla, S.H. To drink or not to drink? That is the question. Circulation 2007, 116, 1306–1317. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Muramatsu, T.; Taniyama, M.; Atsumi, Y.; Kawaguchi, R.; Higuchi, S.; Hosokawa, K.; Asahina, T.; Murata, C.; Matsuoka, K. Association of aldehyde dehydrogenase with inheritance of NIDDM. Diabetologia 1996, 39, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.H.; Luo, H.Q.; Xiang, J.; Tang, L.; Dong, L.P.; Li, G.Y.; Zeng, J.; Li, J.M. Meta-analysis for the association between polymorphisms in interleukin-17A and risk of coronary artery disease. Int. J. Environ. Res. Public Health 2016, 13, 660. [Google Scholar] [CrossRef] [PubMed]
- Attia, J.; Thakkinstian, A.; D’Este, C. Meta-analyses of molecular association studies: Methodologic lessons for genetic epidemiology. J. Clin. Epidemiol. 2003, 56, 297–303. [Google Scholar] [CrossRef]
- Thakkinstian, A.; McEvoy, M.; Minelli, C.; Gibson, P.; Hancox, B.; Duffy, D.; Thompson, J.; Hall, I.; Kaufman, J.; Leung, T.F.; et al. Systematic review and meta-analysis of the association between {beta}2-adrenoceptor polymorphisms and asthma: A HuGE review. Am. J. Epidemiol. 2005, 162, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, J.L.; Sutton, A.J.; Jones, D.R.; Abrams, K.R.; Rushton, L. Comparison of two methods to detect publication bias in meta-analysis. JAMA 2006, 295, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Maimaitikuerban, T.; Abulaiti, P.; Abudula1, Z.; Tuerxunyiming, M.; Mohemaiti, P.; Abudureyimu, S. Correlation between prethrombotic state and gene polymorphism of CYP2C19 and ALDH2 among patients with type 2 diabetes complicated by coronary heart disease and hypertension. Occup. Health 2016, 32, 1379–1383. [Google Scholar]
- Xu, F.; Chen, Y.; Lv, R.; Zhang, H.; Tian, H.; Bian, Y.; Feng, J.; Sun, Y.; Li, R.; Wang, R.; et al. ALDH2 genetic polymorphism and the risk of type II diabetes mellitus in CAD patients. Hypertens. Res. 2010, 33, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, A.; Mizukami, T.; Matsui, T.; Yokoyama, T.; Kimura, M.; Matsushita, S.; Higuchi, S.; Maruyama, K. Genetic polymorphisms of alcohol dehydrogenase-1B and aldehyde dehydrogenase-2 and liver cirrhosis, chronic calcific pancreatitis, diabetes mellitus, and hypertension among Japanese alcoholic men. Alcohol. Clin. Exp. Res. 2013, 37, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Idewaki, Y.; Iwase, M.; Fujii, H.; Ohkuma, T.; Ide, H.; Kaizu, S.; Jodai, T.; Kikuchi, Y.; Hirano, A.; Nakamura, U.; et al. Association of genetically determined aldehyde dehydrogenase 2 activity with diabetic complications in relation to alcohol consumption in Japanese patients with type 2 diabetes mellitus: The Fukuoka Diabetes Registry. PLoS ONE 2015, 10, e0143288. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Taniyama, M.; Muramatsu, T.; Higuchi, S.; Ohta, S.; Atsumi, Y.; Matsuoka, K. ALDH2/ADH2 polymorphism associated with vasculopathy and neuropathy in type 2 diabetes. Alcohol. Clin. Exp. Res. 2004, 28, 111S–116S. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Muramatsu, T.; Taniyama, M.; Atsumi, Y.; Suematsu, M.; Kawaguchi, R.; Higuchi, S.; Asahina, T.; Murata, C.; Handa, M.; et al. Mitochondrial aldehyde dehydrogenase in diabetes associated with mitochondrial tRNA(Leu(UUR)) mutation at position 3243. Diabetes Care 1996, 19, 1423–1425. [Google Scholar] [CrossRef] [PubMed]
- Vasdev, S.; Gill, V.; Singal, P.K. Beneficial effect of low ethanol intake on the cardiovascular system: Possible biochemical mechanisms. Vasc. Health Risk Manag. 2006, 2, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Ihnat, M.A.; Thorpe, J.E. Clinical review 2: The “metabolic memory”: Is more than just tight glucose control necessary to prevent diabetic complications. J. Clin. Endocrinol. Metab. 2009, 94, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Takeuchi, F.; Tabara, Y.; Kelly, T.N.; Go, M.J.; Sim, X.; Tay, W.T.; Chen, C.H.; Zhang, Y.; Yamamoto, K.; et al. Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in east Asians. Nat. Genet. 2011, 43, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Kajiwara, A.; Saruwatari, J.; Hamamoto, A.; Kaku, W.; Oniki, K.; Mihara, S.; Ogata, Y.; Nakagawa, K. The combination of mitochondrial low enzyme-activity aldehyde dehydrogenase 2 allele and superoxide dismutase 2 genotypes increases the risk of hypertension in relation to alcohol consumption. Pharmacogenet. Genom. 2013, 23, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Ildefonso, C.J.; Jaime, H.; Rahman, M.M.; Li, Q.; Boye, S.E.; Hauswirth, W.W.; Lucas, A.R.; McFadden, G.; Levis, A.S. Gene delivery of a viral anti-inflammatory protein to combat ocular inflammation. Hum. Gene Ther. 2015, 26, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Takei, T.; Sakamoto, K.; Nakahara, T.; Ishii, K. 4-Hydroxy-2-nonenal attenuates β2-adrenoceptor-mediated vasodilation of rat retinal arterioles. Naunyn Schmiedebergs Arch. Pharmacol. 2015, 388, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, D.; Jin, W.; Shao, C.; Yan, P.; Xu, C.; Sheng, H.; Liu, Y.; Yu, J.; Xie, Y.; et al. Mitochondrial aldehyde dehydrogenase-2 (ALDH2) Glu504Lys polymorphism contributes to the variation in efficacy of sublingual nitroglycerin. J. Clin. Investig. 2006, 116, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Fan, F.; Li, J.; Fu, M.; Sun, A.; Ge, J. Distributed difference of aldehyde dehydrogenase 2(ALDH2) polymorphism in Shanghai and Shandong Han population. Chin. J. Clin. Med. 2011, 18, 735–737. [Google Scholar]
- Li, H.; Borinskaya, S.; Yoshimura, K.; Kalina, N.; Marusin, A.; Stepanov, V.A.; Qin, Z.; Khaliq, S.; Lee, M.Y.; Yang, Y.; et al. Refined geographic distribution of the oriental ALDH2*504Lys (nee 487Lys) variant. Ann. Hum. Genet. 2009, 73, 335–345. [Google Scholar] [CrossRef] [PubMed]
| ALDH2 rs671 and T2DM | |||||||
| Author | Year | Country | Ethnicity | Genotyping Methods | Sex Ratio (Male %) (Case/Control) | Mean Age (Case/Control) | Quality Score |
| Maimaitikuerban (1) [18] | 2016 | China | Asian | PCR-RFLP | 96.67%/75.21% | 66/60 | 10 |
| Maimaitikuerban (2) [18] | 2016 | China | Asian | PCR-RFLP | 96.67%/64.34% | 66/59 | 10 |
| Suzuki (1) [12] | 1996 | Japan | Asian | PCR-RFLP | 70.6%/51.8% | not mentioned | 9 |
| Xu (1) [19] | 2010 | China | Asian | PCR-sequencing | 70.8%/63.2% | 61.7 ± 10.6/60.9 ± 10.2 | 12 |
| Xu (2) [19] | 2010 | China | Asian | PCR-sequencing | 70.8%/50.8% | 61.7 ± 10.6/61.4 ± 10.0 | 12 |
| Yokoyama [20] | 2013 | Japan | Asian | PCR-RFLP | 100%/100% | 57.7 ± 0.5/56.0 ± 0.2 | 9 |
| ALDH2 rs671 and DR | |||||||
| Author | Year | Country | Ethnicity | Genotyping Methods | Sex Ratio (Male:Female) (Case/Control) | Age (Case/Control) | Quality Score |
| Morita [13] | 2013 | Japan | Asian | Taqman | not mentioned | not mentioned | 10 |
| Idewaki [21] | 2015 | Japan | Asian | PCR-RFLP | not mentioned | not mentioned | 8 |
| Suzuki [22] | 2004 | Japan | Asian | PCR-RFLP | not mentioned | not mentioned | 6 |
| Suzuki (2) [12] | 1996 | Japan | Asian | PCR-RFLP | not mentioned | not mentioned | 7 |
| ALDH2 rs671 and T2DM | |||||||||
| Author | T2DM Patients | Control | HWE of Control | ||||||
| Total | *1/*1 | *1/*2 | *2/*2 | Total | *1/*1 | *1/*2 | *2/*2 | ||
| Maimaitikuerban (1) [18] | 90 | 47 | 36 | 7 | 114 | 89 | 24 | 1 | 0.655 |
| Maimaitikuerban (2) [18] | 90 | 47 | 36 | 7 | 129 | 104 | 24 | 1 | 0.763 |
| Suzuki (1) [12] | 170 | 98 | 64 | 8 | 461 | 268 | 161 | 32 | 0.251 |
| Xu (1) [19] | 257 | 131 | 111 | 15 | 285 | 177 | 100 | 8 | 0.165 |
| Xu (2) [19] | 257 | 131 | 111 | 15 | 309 | 208 | 95 | 6 | 0.195 |
| Yokoyama [20] | 383 | 340 | 43 | 0 | 1519 | 1265 | 254 | 0 | <0.0001 |
| ALDH2 rs671 and DR | |||||||||
| Author | DR Patients | Control | HWE of Control | ||||||
| Total | *1/*1 | *1/*2 + *2/*2 | Total | *1/*1 | *1/*2 + *2/*2 | ||||
| Morita [13] | 52 | 26 | 26 | 182 | 117 | 65 | N/A | ||
| Idewaki [21] | 949 | 539 | 410 | 3451 | 1863 | 1588 | N/A | ||
| Suzuki [22] | 51 | 26 | 25 | 107 | 59 | 48 | N/A | ||
| Suzuki (2) [12] | 75 | 42 | 33 | 137 | 78 | 59 | N/A | ||
| Genetic Model | ALDH2 rs671 with T2DM | |||||
| N | OR (95% CI) | p-Value | I2 (%) | Q Value | ||
| *1/*1 vs. *1/*2 | Overall | 6 | 0.64 (0.41, 1.02) | 0.06 | 87 | 37.16 |
| Control (CAD) | 2 | 0.72 (0.38, 1.36) | 0.31 | 90 | 3.04 | |
| Normal control | 4 | 0.51 (0.28, 0.95) | 0.03 | 67 | 30.02 | |
| Chinese | 4 | 0.48 (0.34, 0.67) | <0.0001 | 53 | 6.41 | |
| Japanese | 2 | 1.21 (0.71, 2.07) | 0.48 | 78 | 4.48 | |
| Large sample | 1 | 1.59 (1.12, 2.24) | 0.009 | N/A | N/A | |
| Small sample | 5 | 0.55 (0.38, 0.78) | 0.0009 | 70 | 13.33 | |
| *1/*1 vs. *2/*2 | Overall | 6 | 0.31 (0.11, 0.89) | 0.03 | 74 | 15.57 |
| Control (CAD) | 2 | 0.23 (0.05, 1.07) | 0.06 | 51 | 2.03 | |
| Normal control | 4 | 0.35 (0.07, 1.83) | 0.21 | 83 | 12.01 | |
| Chinese | 4 | 0.23 (0.13, 0.42) | <0.00001 | 22 | 3.86 | |
| Japanese | 2 | 1.46 (0.65, 3.28) | 0.36 | N/A | N/A | |
| Large sample | 1 | Not estimable | N/A | N/A | N/A | |
| Small sample | 5 | 0.31 (0.11, 0.89) | 0.03 | 74 | 15.57 | |
| *1/*1 vs. *1/*2 + *2/*2 | Overall | 6 | 0.61 (0.37, 1.00) | 0.05 | 89 | 46.23 |
| Control (CAD) | 2 | 0.46 (0.23, 0.93) | 0.03 | 76 | 4.17 | |
| Normal control | 4 | 0.69 (0.35, 1.37) | 0.29 | 92 | 36.64 | |
| Chinese | 4 | 0.43 (0.29, 0.63) | <0.0001 | 65 | 8.58 | |
| Japanese | 2 | 1.25 (0.78, 2.01) | 0.36 | 73 | 3.65 | |
| Large sample | 1 | 1.59 (1.12, 2.24) | 0.009 | N/A | N/A | |
| Small sample | 5 | 0.51 (0.33, 0.77) | 0.002 | 80 | 20.12 | |
| *1 vs. *2 | Overall | 6 | 0.72 (0.50, 1.06) | 0.10 | 87 | 37.33 |
| Control (CAD) | 2 | 0.49 (0.25, 0.98) | 0.04 | 81 | 5.39 | |
| Normal control | 4 | 0.87 (0.54, 1.39) | 0.56 | 88 | 24.50 | |
| Chinese | 4 | 0.57 (0.48, 0.68) | <0.00001 | 46 | 5.59 | |
| Japanese | 2 | 1.26 (0.87, 1.83) | 0.23 | 65 | 2.83 | |
| Large sample | 1 | 1.53 (1.10, 2.14) | 0.01 | N/A | N/A | |
| Small sample | 5 | 0.63 (0.45, 0.87) | 0.005 | 77 | 17.64 | |
| Genetic Model | ALDH2 rs671 with DR | |||||
| N | OR (95% CI) | p-Value | I2 (%) | Q Value | ||
| *1/*1 vs. *1/*2 + *2/*2 | Overall | 4 | 1.06 (0.93, 1.22) | 0.37 | 43 | 5.25 |
| Large sample | 1 | 0.56 (0.30, 1.04) | 0.06 | N/A | N/A | |
| Small sample | 3 | 1.10 (0.96, 1.26) | 0.18 | 0 | 1.73 | |
| Genotype Model | Number of Studies | OR (95% CI) | p-Value | I2 (%) |
|---|---|---|---|---|
| *1/*1 vs. *1/*2 | 5 | 0.55 (0.38, 0.78) | 0.0009 | 70% |
| *1/*1 vs. *2/*2 | 5 | 0.31 (0.11, 0.89) | 0.03 | 74% |
| *1/*1 vs. *1/*2 + *2/*2 | 5 | 0.51 (0.33, 0.77) | 0.002 | 80% |
| *1 vs. *2 | 5 | 1.17 (1.01, 1.35) | 0.04 | 47% |
| Group | ALDH2 rs671 and T2DM (p-Value) | ALDH2 rs671 and DR (p-Value) | |||
|---|---|---|---|---|---|
| *1/*1 vs. *1/*2 | *1/*1 vs. *2/*2 | *1/*1 vs. *1/*2 + *2/*2 | *1 vs. *2 | *1/*1 vs. *1/*2 + *2/*2 | |
| Begg’s test | 0.260 | 0.806 | 0.452 | 0.452 | 0.308 |
| Egger’s test | 0.005 | 0.406 | 0.008 | 0.015 | 0.145 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.-y.; Li, Z.-b.; Li, F.; Dong, L.-p.; Tang, L.; Xiang, J.; Li, J.-m.; Bao, M.-h. Meta-Analysis on the Association of ALDH2 Polymorphisms and Type 2 Diabetic Mellitus, Diabetic Retinopathy. Int. J. Environ. Res. Public Health 2017, 14, 165. https://doi.org/10.3390/ijerph14020165
Li G-y, Li Z-b, Li F, Dong L-p, Tang L, Xiang J, Li J-m, Bao M-h. Meta-Analysis on the Association of ALDH2 Polymorphisms and Type 2 Diabetic Mellitus, Diabetic Retinopathy. International Journal of Environmental Research and Public Health. 2017; 14(2):165. https://doi.org/10.3390/ijerph14020165
Chicago/Turabian StyleLi, Guang-yi, Zi-bo Li, Fang Li, Li-ping Dong, Liang Tang, Ju Xiang, Jian-ming Li, and Mei-hua Bao. 2017. "Meta-Analysis on the Association of ALDH2 Polymorphisms and Type 2 Diabetic Mellitus, Diabetic Retinopathy" International Journal of Environmental Research and Public Health 14, no. 2: 165. https://doi.org/10.3390/ijerph14020165




