Increased Risk of Stroke in Patients of Concussion: A Nationwide Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Ethic Concern
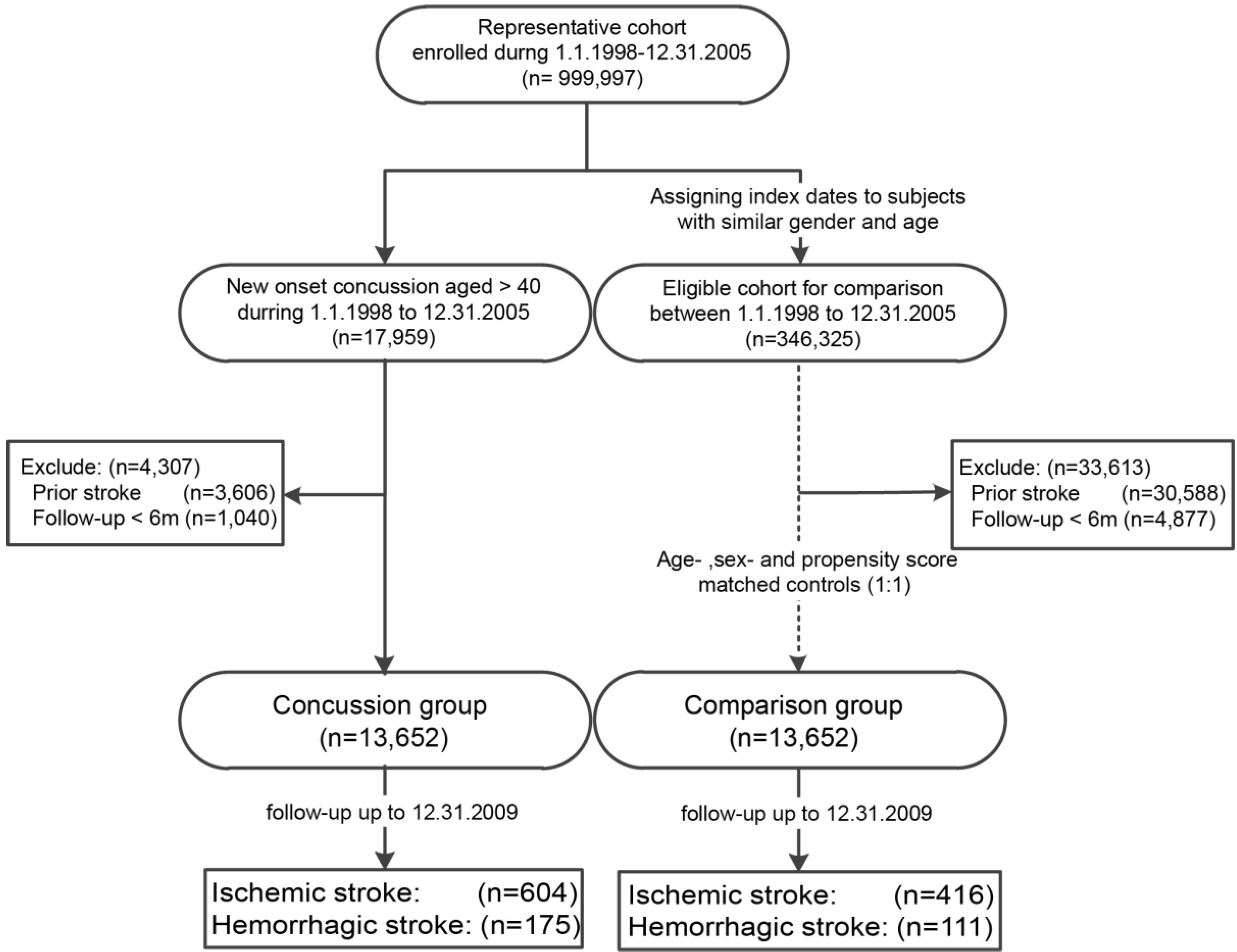
2.2. Study Cohort and Study Population
2.3. Covariates and Study Endpoint
2.4. Statistical Analysis
2.5. Quantitative Bias Analysis
3. Results
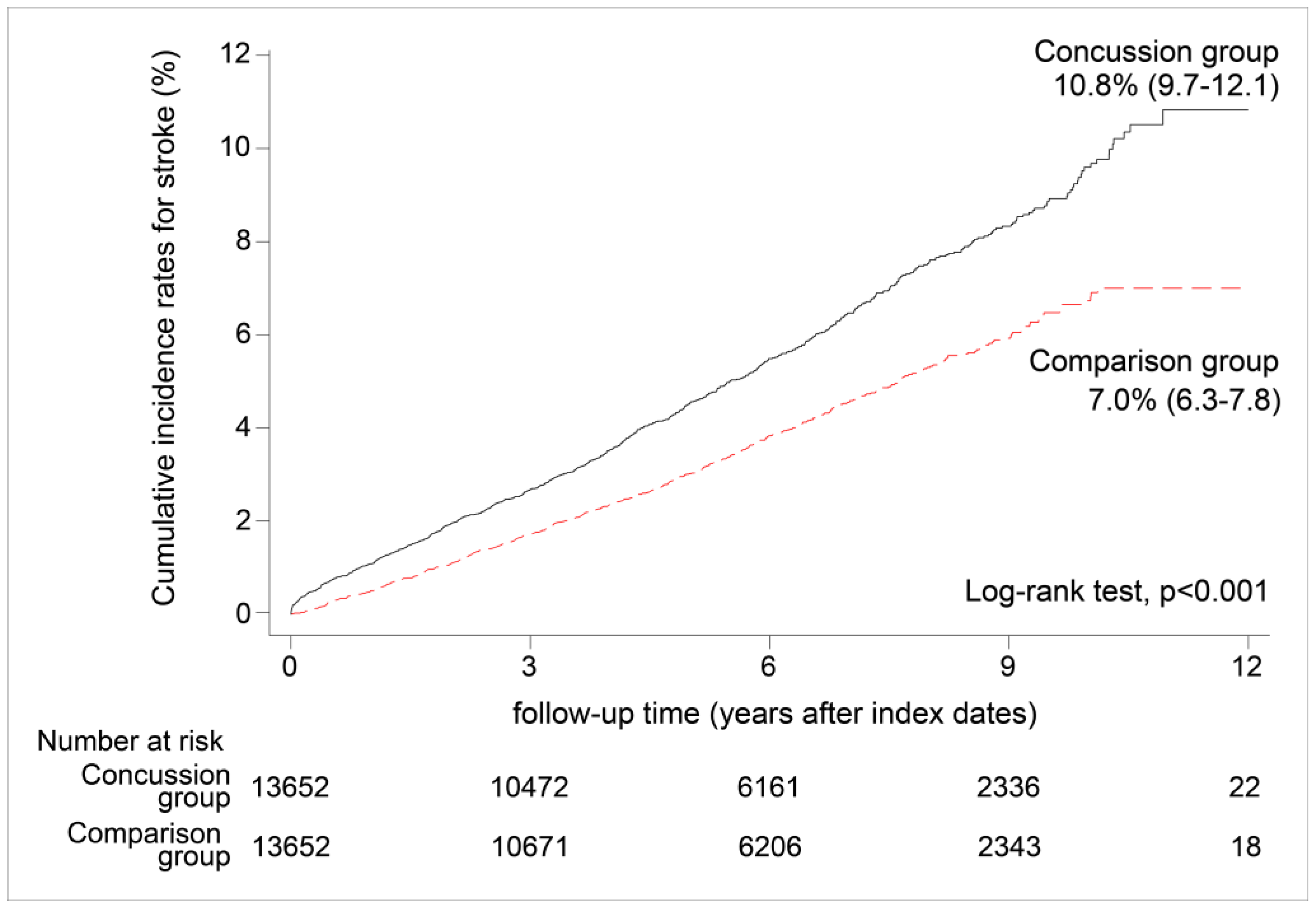
3.1. Incidence of All Strokes
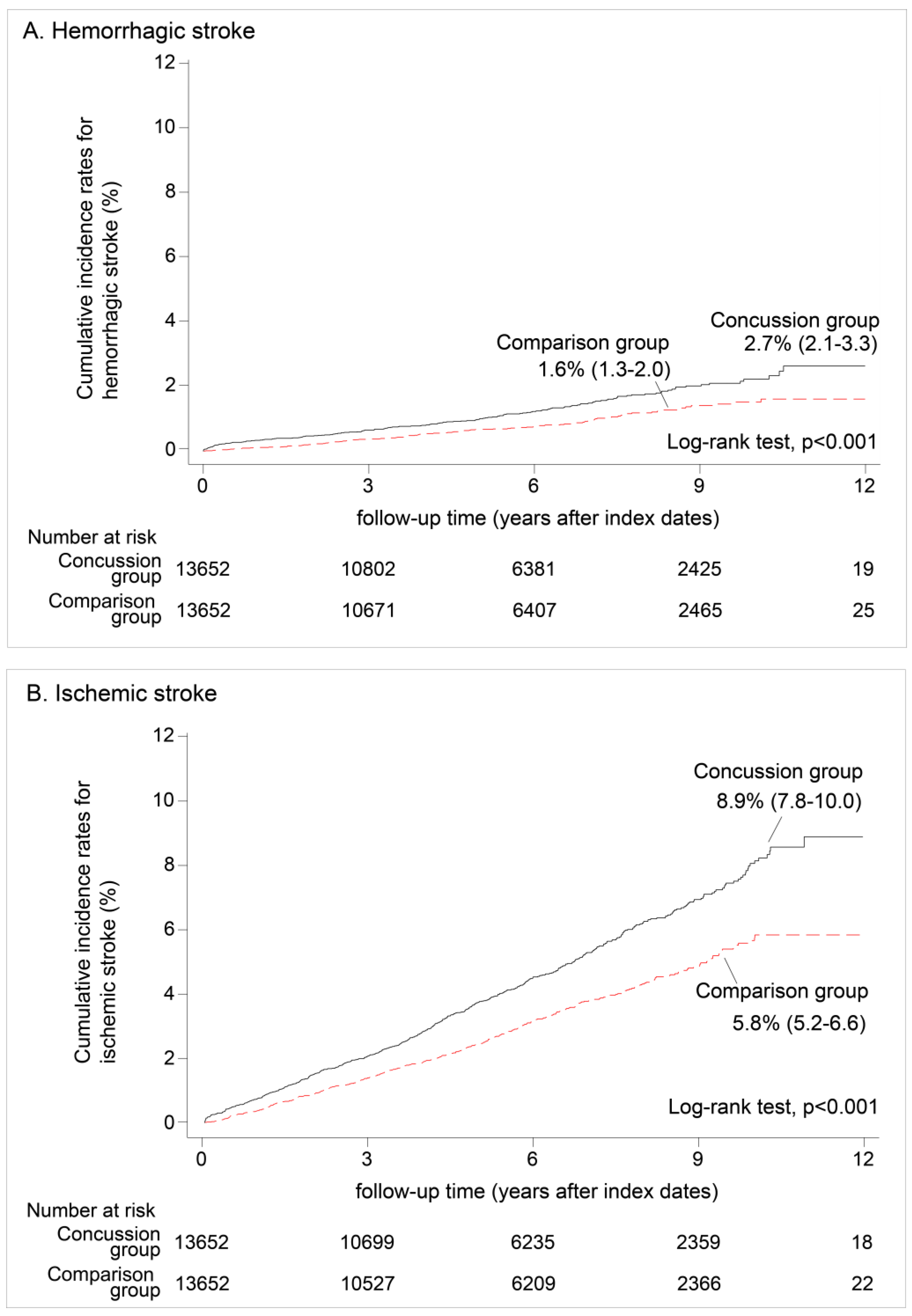
3.2. Hemorrhagic Stroke vs. Ischemic Stroke
3.3. Probabilistic Sensitivity Analysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rutland-Brown, W.; Langlois, J.A.; Thomas, K.E.; Xi, Y.L. Incidence of traumatic brain injury in the United States, 2003. J. Head Trauma Rehabil. 2006, 21, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Bazarian, J.J.; McClung, J.; Shah, M.N.; Cheng, Y.T.; Flesher, W.; Kraus, J. Mild traumatic brain injury in the United States, 1998–2000. Brain Inj. 2005, 19, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Till, C.; Colella, B.; Verwegen, J.; Green, R.E. Postrecovery cognitive decline in adults with traumatic brain injury. Arch. Phys. Med. Rehabil. 2008, 89, S25–S34. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Hou, S.W.; Lee, C.C.; Hsu, C.Y.; Huang, Y.S.; Su, Y.C. Increased risk of dementia in patients with mild traumatic brain injury: A nationwide cohort study. PLoS ONE 2013, 8, e62422. [Google Scholar] [CrossRef] [PubMed]
- McCrory, P.; Zazryn, T.; Cameron, P. The evidence for chronic traumatic encephalopathy in boxing. Sports Med. 2007, 37, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.H.; Maraganore, D.M.; Peterson, B.J.; McDonnell, S.K.; Ahlskog, J.E.; Rocca, W.A. Head trauma preceding Pd: A case-control study. Neurology 2003, 60, 1610–1615. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.J.; Kreitschmann-Andermahr, I.; Ghigo, E.; Stalla, G.K.; Agha, A. Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: A systematic review. JAMA J. Am. Med. Assoc. 2007, 298, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Fleminger, S. Long-term psychiatric disorders after traumatic brain injury. Eur. J. Anaesthesiol. 2008, 25 (Suppl. 42), 123–130. [Google Scholar] [CrossRef] [PubMed]
- Agha, A.; Thompson, C.J. Anterior pituitary dysfunction following traumatic brain injury (TBI). Clin. Endocrinol. 2006, 64, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Aquilani, R.; Iadarola, P.; Boschi, F.; Pistarini, C.; Arcidiaco, P.; Contardi, A. Reduced plasma levels of tyrosine, precursor of brain catecholamines, and of essential amino acids in patients with severe traumatic brain injury after rehabilitation. Arch. Phys. Med. Rehabil. 2003, 84, 1258–1265. [Google Scholar] [CrossRef]
- Borsheim, E.; Bui, Q.U.; Wolfe, R.R. Plasma amino acid concentrations during late rehabilitation in patients with traumatic brain injury. Arch. Phys. Med. Rehabil. 2007, 88, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.F.; Stulc, J.L.; Skolarus, L.E.; Sears, E.D.; Zahuranec, D.B.; Morgenstern, L.B. Traumatic brain injury may be an independent risk factor for stroke. Neurology 2013, 81, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Kang, J.H.; Lin, H.C. Patients with traumatic brain injury: Population-based study suggests increased risk of stroke. Stroke J. Cereb. Circ. 2011, 42, 2733–2739. [Google Scholar] [CrossRef] [PubMed]
- Dashnaw, M.L.; Petraglia, A.L.; Bailes, J.E. An overview of the basic science of concussion and subconcussion: Where we are and where we are going. Neurosurg. Focus 2012, 33, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ellemberg, D.; Henry, L.C.; Macciocchi, S.N.; Guskiewicz, K.M.; Broglio, S.P. Advances in sport concussion assessment: From behavioral to brain imaging measures. J. Neurotrauma 2009, 26, 2365–2382. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.B. Estimating causal effects from large data sets using propensity scores. Ann. Intern. Med. 1997, 127, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Lash, T.L.; Fox, M.P.; MacLehose, R.F.; Maldonado, G.; McCandless, L.C.; Greenland, S. Good practices for quantitative bias analysis. Int. J. Epidemiol. 2014, 43, 1969–1985. [Google Scholar] [CrossRef] [PubMed]
- Falkstedt, D.; Wolff, V.; Allebeck, P.; Hemmingsson, T.; Danielsson, A.K. Cannabis, tobacco, alcohol use, and the risk of early Stroke: A population-based cohort study of 45,000 Swedish men. Stroke 2017, 48, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.; Hallett, M.; Slobounov, S. Follow-up evaluation of oculomotor performance with FMRI in the subacute phase of concussion. Neurology 2015, 85, 1163–1166. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.; Zhang, K.; Hallett, M.; Slobounov, S. Functional neuroimaging of acute oculomotor deficits in concussed athletes. Brain Imaging Behav. 2015, 9, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, J.D.; Carroll, L.J.; Peloso, P.M.; Borg, J.; von Holst, H.; Holm, L.; Kraus, J.; Coronado, V.G. Incidence, risk factors and prevention of mild traumatic brain injury: Results of the WHO collaborating centre task force on mild traumatic brain injury. J. Rehabil. Med. Off. J. UEMS Eur. Board Phys. Rehabil. Med. 2004, 36, 28–60. [Google Scholar] [CrossRef]
- Langlois, J.A.; Rutland-Brown, W.; Wald, M.M. The epidemiology and impact of traumatic brain injury: A brief overview. J. Head Trauma Rehabil. 2006, 21, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Mihalik, J.P.; Stump, J.E.; Collins, M.W.; Lovell, M.R.; Field, M.; Maroon, J.C. Posttraumatic migraine characteristics in athletes following sports-related concussion. J. Neurosurg. 2005, 102, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.F.; Thomas, B.; Sudlow, C.L. Epidemiology of stroke and its subtypes in Chinese vs. white populations: A systematic review. Neurology 2013, 81, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.C.; Chou, Y.C.; Yeh, C.C.; Hu, C.J.; Chiu, W.T.; Chen, T.L. Stroke risk and outcomes in patients with traumatic brain injury: 2 nationwide studies. Mayo Clin. Proc. 2014, 89, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Lee, C.W.; Huang, M.Y.; Hsu, C.Y.; Su, Y.C. Increased risk of ischemic stroke in patients with mild traumatic brain injury: A nationwide cohort study. Scand. J. Trauma Resusc. Emerg. Med. 2014, 22, 66. [Google Scholar] [CrossRef] [PubMed]
- McCrory, P.; Meeuwisse, W.; Johnston, K.; Dvorak, J.; Aubry, M.; Molloy, M.; Cantu, R. Consensus statement on concussion in sport. Clin. J. Sport Med. 2009, 19, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Len, T.K.; Neary, J.P. Cerebrovascular pathophysiology following mild traumatic brain injury. Clin. Physiol. Funct. Imaging 2011, 31, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Junger, E.C.; Newell, D.W.; Grant, G.A.; Avellino, A.M.; Ghatan, S.; Douville, C.M.; Lam, A.M.; Aaslid, R.; Winn, H.R. Cerebral autoregulation following minor head injury. J. Neurosurg. 1997, 86, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Strebel, S.; Lam, A.M.; Matta, B.F.; Newell, D.W. Impaired cerebral autoregulation after mild brain injury. Surg. Neurol. 1997, 47, 128–131. [Google Scholar] [CrossRef]
- Laroche, M.; Kutcher, M.E.; Huang, M.C.; Cohen, M.J.; Manley, G.T. Coagulopathy after traumatic brain injury. Neurosurgery 2012, 70, 1334–1345. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.C.; Graham, D.I.; Chen, X.H.; Smith, D.H. Association between intravascular microthrombosis and cerebral ischemia in traumatic brain injury. Neurosurgery 2004, 54, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Mahmood, A.; Goussev, A.; Qu, C.; Zhang, Z.G.; Chopp, M. Delayed thrombosis after traumatic brain injury in rats. J. Neurotrauma 2004, 21, 1756–1766. [Google Scholar] [CrossRef] [PubMed]
- Schwarzmaier, S.M.; Kim, S.W.; Trabold, R.; Plesnila, N. Temporal profile of thrombogenesis in the cerebral microcirculation after traumatic brain injury in mice. J. Neurotrauma 2010, 27, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Kieslich, M.; Fiedler, A.; Heller, C.; Kreuz, W.; Jacobi, G. Minor head injury as cause and co-factor in the aetiology of stroke in childhood: A report of eight cases. J. Neurol. Neurosurg. Psychiat. 2002, 73, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Landi, A.; Marotta, N.; Mancarella, C.; Marruzzo, D.; Salvati, M.; Delfini, R. Basal ganglia stroke due to mild head trauma in pediatric age—Clinical and therapeutic management: A case report and 10 year literature review. Ital. J Pediatr. 2011, 37, 2. [Google Scholar] [CrossRef] [PubMed]
- Nabika, S.; Kiya, K.; Satoh, H.; Mizoue, T.; Oshita, J.; Kondo, H. Ischemia of the internal capsule due to mild head injury in a child. Pediat. Neurosurg. 2007, 43, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, L.; Rich, P.M.; Pohl, K.R.; Ganesan, V. Can mild head injury cause ischaemic stroke? Arch. Dis. Child. 2003, 88, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Buompadre, M.C.; Arroyo, H.A.; Stroke, G. Basal ganglia and internal capsule stroke in childhood—Risk factors, neuroimaging, and outcome in a series of 28 patients: A tertiary hospital experience. J. Child Neurol. 2009, 24, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.L.; Kao, Y.H.; Lin, S.J.; Lee, C.H.; Lai, M.L. Validation of the national health insurance research database with ischemic stroke cases in Taiwan. Pharmacoepidemiol. Drug Saf. 2011, 20, 236–242. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | Comparison Group | Concussion Group | p-Value | ||||
|---|---|---|---|---|---|---|---|
| n = 13,652 | (%) | n = 13,652 | (%) | ||||
| Age at index dates | |||||||
| Mean, (SD) | 56.2 | (12.0) | 56.3 | (12.1) | 0.755 | ||
| Sex | |||||||
| Female | 7373 | (54.0) | 7385 | (54.1) | 0.894 | ||
| Male | 6279 | (46.0) | 6267 | (45.9) | |||
| Comorbidities | |||||||
| Hypertension | 0.808 | ||||||
| Yes | 2689 | (19.7) | 2705 | (19.8) | |||
| No | 10,963 | (80.3) | 10,947 | (80.2) | |||
| Diabetes | 0.518 | ||||||
| Yes | 1249 | (9.1) | 1280 | (9.4) | |||
| No | 12,403 | (90.9) | 12,372 | (90.6) | |||
| Arrhythmia | 0.292 | ||||||
| Yes | 492 | (3.6) | 525 | (3.8) | |||
| No | 13,160 | (96.4) | 13,127 | (96.2) | |||
| Coronary heart disease | 0.983 | ||||||
| Yes | 1145 | (8.4) | 1143 | (8.4) | |||
| No | 12,507 | (91.6) | 12,509 | (91.6) | |||
| Exposure to medications | |||||||
| Exposed to Aspirin | 0.836 | ||||||
| Yes | 1290 | (9.4) | 1300 | (9.5) | |||
| No | 12,362 | (90.6) | 12,352 | (90.5) | |||
| Exposed to anti-coagulants | 1.000 | ||||||
| Yes | 1181 | (8.7) | 1181 | (8.7) | |||
| No | 12,471 | (91.3) | 12,471 | (91.3) | |||
| Exposed to lipid-lowering drugs | 0.564 | ||||||
| Yes | 1288 | (9.4) | 1316 | (9.6) | |||
| No | 12,364 | (90.6) | 12,336 | (90.4) | |||
| Exposed to Nitrates | 0.720 | ||||||
| Yes | 803 | (5.9) | 817 | (6.0) | |||
| No | 12,849 | (94.1) | 12,835 | (94.0) | |||
| Propensity score | |||||||
| Mean, (SD) | 0.05 | (0.01) | 0.05 | (0.01) | 1.000 | ||
| Stroke during Follow-Up | Total Sample | Comparison Group | Concussion Group | (95% CI) Sig. 1 |
|---|---|---|---|---|
| Incidence of stroke (per 1000 person-years) | 8.07 | 6.52 | 9.63 | |
| Number of occurrences | 1248 | 507 | 741 | |
| Observed person-years | 154,657.3 | 77,725.9 | 76,931.4 | |
| Crude hazard ratio (95% CI) 2 | 1.00 | 1.48 | (1.32–1.66) *** | |
| Adjusted hazard ratio (95% CI) | 1.00 | 1.65 | (1.47–1.85) *** |
| Comparison Group (95% CI) | Concussion Group (95% CI) | p-Value | Sig. 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hemorrhagic stroke 1 | |||||||||
| Crude HR | 1.00 | 1.58 | (1.24 | −2.02) | <0.001 | *** | |||
| Adjusted HR | 1.00 | 1.73 | (1.36 | −2.20) | 0.002 | ** | |||
| Ischemic stroke 1 | |||||||||
| Crude HR | 1.00 | 1.46 | (1.29 | −1.66) | <0.001 | *** | |||
| Adjusted HR | 1.00 | 1.62 | (1.43 | −1.84) | <0.001 | *** | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.-W.; Huang, L.-C.; Chung, W.-F.; Chang, H.-K.; Wu, J.-C.; Chen, L.-F.; Chen, Y.-C.; Huang, W.-C.; Cheng, H.; Lo, S.-S. Increased Risk of Stroke in Patients of Concussion: A Nationwide Cohort Study. Int. J. Environ. Res. Public Health 2017, 14, 230. https://doi.org/10.3390/ijerph14030230
Liu S-W, Huang L-C, Chung W-F, Chang H-K, Wu J-C, Chen L-F, Chen Y-C, Huang W-C, Cheng H, Lo S-S. Increased Risk of Stroke in Patients of Concussion: A Nationwide Cohort Study. International Journal of Environmental Research and Public Health. 2017; 14(3):230. https://doi.org/10.3390/ijerph14030230
Chicago/Turabian StyleLiu, Shih-Wei, Liang-Chung Huang, Wu-Fu Chung, Hsuan-Kan Chang, Jau-Ching Wu, Li-Fu Chen, Yu-Chun Chen, Wen-Cheng Huang, Henrich Cheng, and Su-Shun Lo. 2017. "Increased Risk of Stroke in Patients of Concussion: A Nationwide Cohort Study" International Journal of Environmental Research and Public Health 14, no. 3: 230. https://doi.org/10.3390/ijerph14030230






