Metal Concentrations in Newcomer Women and Environmental Exposures: A Scoping Review
Abstract
:1. Introduction
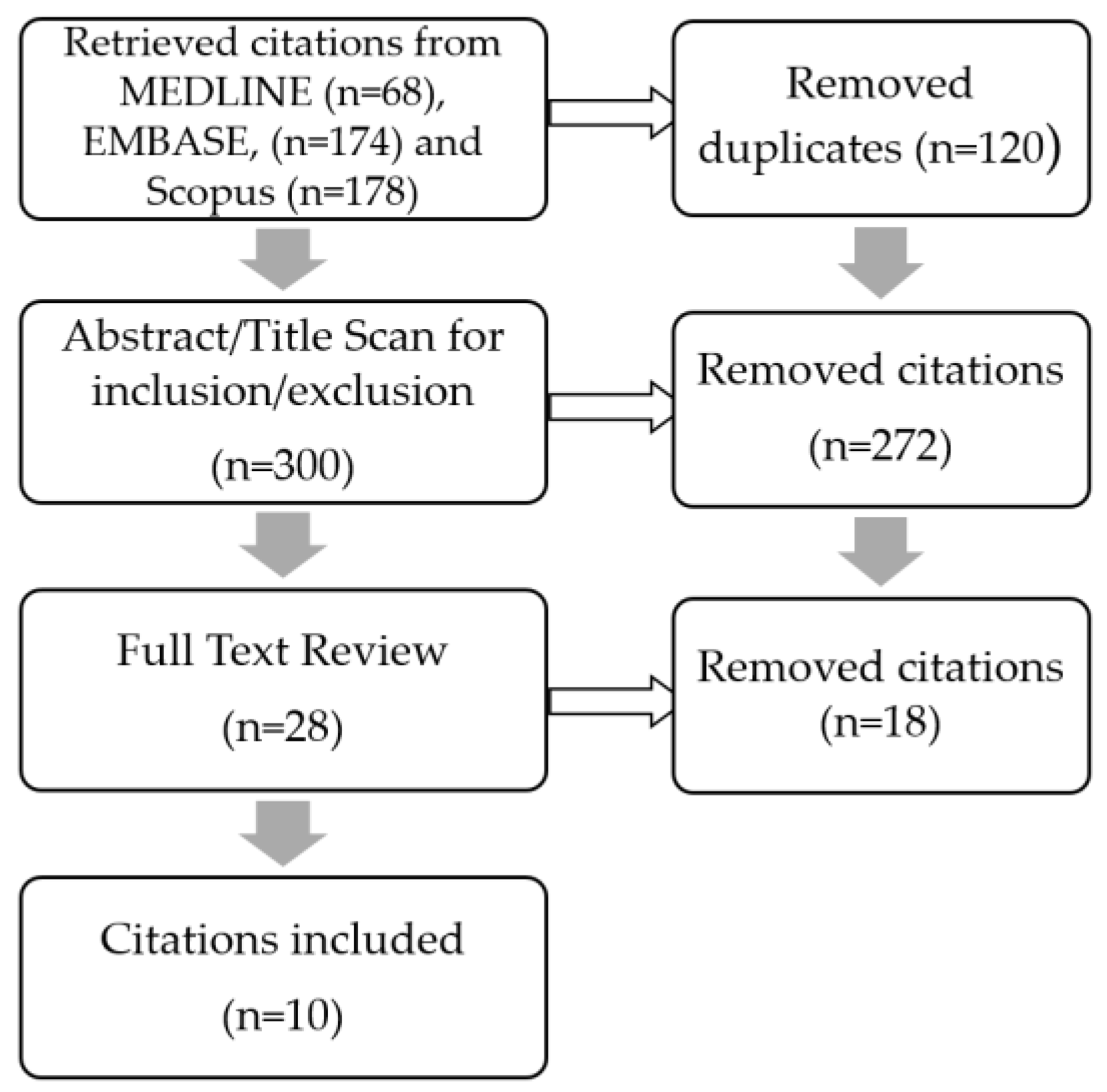
2. Methods
3. Scoping Review Results
3.1. Studies Investigating Pb Concentrations (n = 8)
3.2. Studies Investigating Hg and Cd Concentrations (n = 3)
3.3. Environmental Exposures Identified
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vahter, M.; Åkesson, A.; Lidén, C.; Ceccatelli, S.; Berglund, M. Gender differences in the disposition and toxicity of metals. Environ. Res. 2007, 104, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.V.S. Perspectives in endocrine toxicity of heavy metals—A review. Biol. Trace Elem. Res. 2014, 160, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Klitzman, S.; Sharma, A.; Nicaj, L.; Vitkevich, R. Lead poisoning among pregnant women in New York City: Risk factors and screening practices. J. Urban Health 2002, 79, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Borja-Arbuto, V.H.; Hertz-Picciotto, I.; Lopez, M.R.; Farias, P.; Rios, C.; Blanco, J. Blood lead levels measured prospectively and risk of spontaneous abortion. Am. J. Epidemiol. 1999, 150, 590–597. [Google Scholar] [CrossRef]
- Canfield, R.L.; Henderson, C.R., Jr.; Cory-Slechta, D.A.; Cox, C.; Jusko, T.A.; Lanphear, B.P. Intellectual impairment in children with blood lead concentrations below 10 μg per deciliter. N. Engl. J. Med. 2003, 348, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Ernhart, C.B. Effects of lead on IQ in children. Correspondence. Environ. Health Perspect. 2006, 114, A85–A86. [Google Scholar] [CrossRef] [PubMed]
- Lanphear, B.P.; Hornung, R.; Khoury, J.; Yolton, K.; Baghurst, P.; Bellinger, D.C.; Canfield, R.L.; Dietrich, K.N.; Bornschein, R.; Greene, T.; et al. Low-level environmental lead exposure and children’s intellectual function: An international pooled analysis. Environ. Health Perspect. 2005, 113, 894–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciesielski, T.; Weuve, J.; Bellinger, D.C.; Schwartz, J.; Lanphear, B.; Wright, R.O. Cadmium exposure and neurodevelopmental outcomes in U.S. children. Environ. Health Perspect. 2012, 120, 758–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakravartty, D.; Wiseman, C.L.S.; Cole, D.C. Differential environmental exposure among non-Indigenous Canadians as a function of sex/gender and race/ethnicity variables: A scoping review. Can. J. Public Health 2014, 105, 438–444. [Google Scholar] [CrossRef]
- Martin, D.; Glass, T.A.; Bandee-Roche, K.; Todd, A.C.; Shi, W.; Schwartz, B.S. Association of blood lead and tibia lead with blood pressure and hypertension in a community sample of older adults. Am. J. Epidemiol. 2006, 163, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Nash, D.; Magder, L.; Lustberg, M.; Sherwin, R.W.; Rubin, R.J.; Kaufmann, R.B.; Silbergeld, E.K. Blood lead, blood pressure, and hypertension in perimenopausal and postmenopausal women. JAMA 2003, 289, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Olsson, I.-M.; Bensryd, I.; Lundh, T.; Ottosson, H.; Skerfving, S.; Oskarsson, A. Cadmium in blood and urine—Impact of sex, age, dietary intake, iron status, and former smoking—Association of renal effects. Environ. Health Perspect. 2002, 110, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.M.; Alexander, J.; Brantsæter, A.L.; Borch-Iohnsen, B.; Ellingsen, D.G.; Thomassen, Y.; Holmen, J.; Ydersbond, T.A. The impact of iron status and smoking on blood divalent metal concentrations in Norwegian women in the HUNT2 Study. J. Trace Elem. Med. Biol. 2016, 38, 165–173. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). The Global Prevalence of Anaemia in 2011; World Health Organization: Geneva, Switzerland, 2015; Available online: http://apps.who.int/iris/bitstream/10665/177094/1/9789241564960_eng.pdf?ua=1&ua=1 (accessed on 2 February 2017).
- Buettner, C.; Mukamal, K.; Gardiner, P.; Davis, R.; Phillips, R.; Mittleman, M. Herbal supplement use and blood lead levels of United States adults. J. Gen. Intern. Med. 2009, 24, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, I.; Al-Enazi, S.; Shinwari, N. Assessment of lead in cosmetic products. Reg. Toxicol. Pharm. 2009, 54, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Cristaudo, A.; D’Ilio, S.; Gallinella, B.; Mosca, A.; Majorani, C.; Violante, N.; Senofonte, O.; Morrone, A.; Petrucci, F. Use of potentially harmful skin-lightening products among immigrant women in Rome, Italy: A pilot study. Dermatology 2013, 226, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Caravanos, J.; Gutierrez, L.H.; Ericson, B.; Fuller, R. A comparison of burden of disease from toxic waste sites with other recognized public health threats in India, Indonesia and the Philippines. J. Health Pollut. 2014, 4, 2–13. [Google Scholar] [CrossRef]
- Chatham-Stephens, K.; Caravanos, J.; Ericson, B.; Landrigan, P.; Fuller, R. The pediatric burden of disease from lead exposure at toxic waste sites in low and middle income countries. Environ. Res. 2014, 132, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Janjua, N.Z.; Delzell, E.; Larson, R.R.; Meleth, S.; Kabagambe, E.K.; Kristensen, S.; Sathiakumar, N. Maternal nutritional status during pregnancy and surma use determine cord lead levels in Karachi, Pakistan. Environ. Res. 2008, 108, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.A.; Hwang, J.; Kim, H.; Kim, K.N.; Ha, E.; Park, H.; Ha, M.; Kim, Y.; Hong, Y.; Chang, N. Relationship between maternal sodium intake and blood lead concentration during pregnancy. Br. J. Nutr. 2013, 109, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Liu, P.; Chien, C.; Chou, S.; Han, B. Mercury concentration and fish consumption in Taiwanese pregnant women. BJOG 2006, 114, 81–85. [Google Scholar] [CrossRef] [PubMed]
- You, C.H.; Kim, B.G.; Kim, Y.M.; Lee, S.A.; Kim, R.B.; Seo, J.W.; Hong, Y.S. Relationship between dietary mercury intake and blood mercury level in Korea. J. Korean Med. Sci. 2014, 29, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, B. Strong positive associations between seafood, vegetables, and alcohol with blood mercury and urinary arsenic levels in the Korean adult population. Arch. Environ. Contam. Toxicol. 2013, 64, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Ikeh-Tawari, E.; Anetor, J.; Charles-Davies, M. Cadmium level in pregnancy, influence on neonatal birth weight and possible amelioration by some essential trace elements. Toxicol. Int. 2013, 20, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, J.; Shang, Y.; Sun, B. Health risk assessment of cadmium via dietary intake by adults in China. J. Sci. Food Agric. 2014, 94, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Alba, A.; Carleton, L.; Dinkel, L.; Ruppe, R. Increased lead levels in pregnancy among immigrant women. J. Midwifery Women Health 2012, 57, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.L.; Edwards, S.; Maxson, P.J. Mercury levels in an urban pregnant population in Durham County, North Carolina. Int. J. Environ. Res. Public Health 2011, 8, 698–712. [Google Scholar] [CrossRef] [PubMed]
- Scinicariello, F.; Abadin, H.G.; Murray, H.E. Association of low-level blood lead and blood pressure in NHANES 1999–2006. Environ. Res. 2011, 111, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.; Chun, O.K.; Song, O.W. Determinants of the blood lead level of US women of reproductive age. J. Am. Coll. Nutr. 2005, 109, 853–858. [Google Scholar] [CrossRef]
- Hightower, J.M.; O’Hare, A.; Hernandez, G.T. Blood mercury reporting in NHANES: Identifying Asian, Pacific Islander, Native American, and multiracial groups. Environ. Health Perspect. 2006, 114, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.B. Effect of pregnancy on the levels of blood cadmium, lead and mercury for females aged 17–39 years old: Data from National Health and Nutrition Examination Survey 2003–2010. J. Toxicol. Environ. Health A 2013, 76, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Mahaffey, K.R.; Clickner, R.P.; Bodurow, C.C. Blood organic mercury and dietary mercury intake: National Health and Examination Survey, 1999 and 2000. Environ. Health Perspect. 2004, 117, 1435–1441. [Google Scholar] [CrossRef]
- Mahaffey, K.R.; Clickner, R.P.; Jeffries, R.A. Adult women’s blood mercury concentrations vary regionally in the United States: Association with patterns of fish consumption (NHANES 1999–2004). Environ. Health Perspect. 2009, 112, 562–570. [Google Scholar] [CrossRef]
- Razzaghi, H.; Tinker, S.C.; Crider, K. Blood mercury concentrations in pregnant and nonpregnant women in the United States: National Health and Nutrition Examination Survey 1999–2006. Am. J. Obstet. Gynecol. 2014, 210, 357.e1–357.e9. [Google Scholar] [CrossRef] [PubMed]
- Mijal, R.S.; Holzman, C.B. Blood cadmium levels in women of childbearing age vary by race/ethnicity. Environ. Res. 2010, 110, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Curren, M.S.; Davis, K.; Liang, C.L.; Adlard, B.; Foster, W.G.; Donaldson, S.G.; Kandola, K.; Brewster, J.; Potyrala, M.; Oostdam, J.V. Comparing plasma concentrations of persistent organic pollutants and metals in primiparous women from northern and southern Canada. Sci. Total Environ. 2014, 479–480, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Foster, W.G.; Cheung, A.P.; Davis, K.; Graves, G.; Jarrell, J.; Leblanc, A.; Liang, C.L.; Leech, T.; Walker, M.; Weber, J.P.; et al. Circulating metals and persistent organic pollutant concentrations in Canadian and non-Canadian born primiparous women from five Canadian centres: Results of a pilot biomonitoring study. Sci. Total Environ. 2012, 435–436, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Andersen, B.; Blinder, S. Who Counts as a Migrant? Definitions and Their Consequences; The Migration Observatory at the University of Oxford: Oxford, UK, 2015; Available online: http://www.migrationobservatory.ox.ac.uk/resources/briefings/who-counts-as-a-migrant-definitions-and-their-consequences/ (accessed on 26 September 2016).
- Thihalolipavan, S.; Candalla, B.; Ehrlich, J. Examining pica in NYC pregnant women with elevated blood lead levels. Matern. Child Health J. 2013, 17, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Bakhireva, L.; Rowland, A.; Young, B.; Cano, S.; Phelan, S.T.; Artyushkova, K.; Rayburn, W.F.; Lewis, J. Sources of potential lead exposure among pregnant women in New Mexico. Matern. Child Health J. 2013, 17, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Gulson, B.; Mizon, K.J.; Korsch, M.J.; Taylor, A.J. Low blood lead levels do not appear to be further reduced by dietary supplements. Environ. Health Perspect. 2006, 114, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Nandlike, K.; Fenster, W. Elevated blood lead levels in pregnant women: Identification of high-risk population and interventions. J. Perinat. Med. 2007, 35, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liou, S.; Lin, K.; Liu, T.; Liu, S.; Chen, C.; Sung, F.; Wu, T. Changing blood lead levels and DNA damage (commet assay) among immigrant women in Taiwan. Sci. Total Environ. 2009, 407, 5931–5936. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wu, C.; Lin, Y.; Shen, C.; Liu, T.; Yang, C.; Liou, S.; Wu, T. Changing blood lead levels and oxidative stress with duration of residence among Taiwan immigrants. J. Immigr. Minor. Health 2013, 15, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dewolf, M.; Hammadi, S.; Fris, W.; Noel, E.; Lorenzo, R.; Alexander, S.; The PLOMB 6 Group. Lead levels in umbilical cord blood in Belgium: A cross-sectional study in five maternity units. Int. J. Hyg. Environ. Health 2012, 215, 202–205. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Lead Screening during Pregnancy and Lactation. Committee Opinions. 2012. Available online: http://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Obstetric-Practice/Lead-Screening-During-Pregnancy-and-Lactation (accessed on 10 October 2016).
- Geer, L.A.; Persad, M.D.; Palmer, C.D.; Steuerwald, A.J.; Dalloul, M.; Abulafia, O. Assessment of prenatal mercury exposure in a predominately Caribbean immigrant community in Brooklyn, NY. J. Environ. Monit. 2012, 14, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Soon, R.; Dye, T.D.; Ralston, N.V.; Berry, M.J.; Sauvage, L.M. Seafood consumption and umbilical cord blood mercury concentrations in a multiethnic maternal and child health cohort. BMC Pregnancy Childbirth 2014, 14, 209. [Google Scholar] [CrossRef] [PubMed]
- United States Center for Disease Control and Prevention (U.S. CDC). Fourth National Report on Human Exposure to Environmental Chemicals. 2009. Available online: http://www.cdc.gov/exposurereport/pdf/fourthreport.pdf (accessed on 3 October 2016). [Google Scholar]
- Thomas, M.R.; Boekelheide, K. Multiple environmental chemical exposures to lead, mercury and polychlorinated biphenyls among childbearing-aged women (NHANES 1999–2004): Body burden and risk factors. Environ. Res. 2013, 121, 23–30. [Google Scholar]
| Reference | Metal of Interest | Biomarker Matrix | Study Design | Sample Size | Population | Country of Study | Race/Ethnicity | Mean Metal Level (95% CI) | Comparison Population (If Applicable) | Mean Comparison Metal Level (If Applicable) |
|---|---|---|---|---|---|---|---|---|---|---|
| Gulson et al. 2006 [43] | Pb | Blood | Cross-sectional | n = 39 | Women of child bearing age, including pregnant women | Australia | Former Yugoslavia, former Soviet Union, Poland, Bulgaria, Romania, Albania, China | 2.73 μg/dL | ||
| Rastogi et al. 2007 [44] | Pb | Blood | Chart review | n = 89 | Pregnant women with identified BLL ≥10 μg/dL | USA | Birthplace outside of USA: Mexico, Pakistan, India, Bangladesh, Others | Pre-interevention 16.82 μg/dL (14.82, 18.82) Post-intervention: 11.48 μg/dL | ||
| Bakhireva et al. 2009 [42] | Pb | Blood | Cross-sectional | n = 140 | Pregnant women <20 weeks of gestation | USA | Majority born outside of the U.S. Mainly Latina. | 1.06 μg/dL (0.57, 1.55) (n = 39) | ||
| Wu et al. 2009 [45] | Pb | Blood | Cross-sectional | n = 154 | Immigrant women | Taiwan | Immigrant (Vietnam, Mainland China, Southeast China) | 2.23 μg/dL (1.84, 2.62) (n = 71) | Native-born (non-immigrant) | 1.63 μg/dL (1.41, 1.85) (n = 38) |
| Zhang et al. 2012 [47] | Pb | Umbilical cord blood | Cross-sectional | n = 220 | Mothers who recently delivered in a maternity unit | Belgium | South Mediterranean, Sub-Saharan Africa | South-Mediterranean: 50% ≥20 μg/dL a; Sub-Saharan Africa: 20% ≥20 μg/dL a | Western-European Women, Eastern-European Women | Western-European Women 30% ≥20 μg/L b, Eastern-European Women 0% ≥20 μg/L b |
| Geer et al. 2012 [49] | Hg | Umbilical cord blood/Urine | Cross-sectional | n = 190 | Pregnant women in a predominantly immigrant community | USA | African American, Caribbean or West Indian, African, Latino/Hispanic | Cord blood b: 2.14 μg/L (1.76, 2.60) (n = 78) Maternal urinary mercury b,c: 0.45 μg/L (0.37, 0.55) (n = 183) | ||
| Thihalolipavan et al. 2013 [41] | Pb | Blood | Chart review | n = 491 | Lead poisoned pregnant women | USA | Mexican, Jamaican, Dominican Republic, Other | Pica eaters: 29.5 μg/dL (25.9, 33.1) Non-pica eaters: 23.8 μg/dL (22.9, 24.7) | ||
| Wu et al. 2013 [46] | Pb | Blood | Cross-sectional | n = 239 | Immigrant women | Taiwan | Immigrant | RI d: 2.67 μg/dL (2.45, 2.89) LRI e: 2.40 μg/dL (2.21, 2.59) | Native-born (non-immigrant) | NI f: 2.33 μg/dL (2.17, 2.49) |
| Curren et al. 2014 [37] | Pb, total Hg, Cd | Blood | Cross-sectional | n = 93 | Canadian foreign-born women | Canada | Canadian foreign-born | Pb: 0.78 μg/dL b (0.57, 1.10) (n = 16) Hg: 0.88 μg/L b (0.55, 1.40) (n = 16) Cd: 0.59 μg/L b (0.42, 0.83) (n = 16) | Canadian native-born women | Pb: 0.57 μg/L b (0.53, 0.61) Hg: 0.40 μg/L b (0.32, 0.50) Cd: 0.46 μg/L b (0.38, 0.55) |
| Soon et al. 2014 [50] | MeHg | Umbilical Cord Blood | Secondary analysis of prospective cohort pilot study | n = 100 | Multiethnic maternal and child cohort | USA | Hispanic, Caucasian, Japanese, Chinese, Filipino, Pacific Islander | 5.20 μg/L (4.08, 6.33) % of women with cord blood ≥5 μg/L: Japanese—56.5% Caucasian—42.9% Chinese—34.8% Hispanic—33.3% Filipino—28.1% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.X.; Wiseman, C.L.S.; Chakravartty, D.; Cole, D.C. Metal Concentrations in Newcomer Women and Environmental Exposures: A Scoping Review. Int. J. Environ. Res. Public Health 2017, 14, 277. https://doi.org/10.3390/ijerph14030277
Chen SX, Wiseman CLS, Chakravartty D, Cole DC. Metal Concentrations in Newcomer Women and Environmental Exposures: A Scoping Review. International Journal of Environmental Research and Public Health. 2017; 14(3):277. https://doi.org/10.3390/ijerph14030277
Chicago/Turabian StyleChen, Shirley X., Clare L. S. Wiseman, Dolon Chakravartty, and Donald C. Cole. 2017. "Metal Concentrations in Newcomer Women and Environmental Exposures: A Scoping Review" International Journal of Environmental Research and Public Health 14, no. 3: 277. https://doi.org/10.3390/ijerph14030277





