Risk Factors for Mortality among Adult HIV/AIDS Patients Following Antiretroviral Therapy in Southwestern Ethiopia: An Assessment through Survival Models
Abstract
:1. Introduction
2. Methods
2.1. Study Setting
2.2. Study Population
2.3. Standard Follow-Up of HIV Patients on ART
2.4. Variables
2.5. Statistical Analysis
2.6. Ethical Approval
3. Results
4. Discussion
4.1. HIV/AIDS Mortality Rate
4.2. Survival Models
4.3. Factors Associated with the Risk of Death
5. Conclusions
Supplementary Materials
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Federal Democratic Republic of Ethiopia (2014). Country progress report on the HIV response. Available online: http://www.unaids.org/sites/default/files/country/documents/ETH_narrative_report_2014.pdf (accessed on 3 September 2014).
- Federal HIV/AIDS Prevention and Control Office, Federal Ministry of Health. Guidelines for management of opportunistic infections and antiretroviral treatment in adolescences and adults in Ethiopia. Available online: http://www.who.int/hiv/pub/guidelines/ethiopia_art.pdf (accessed on 6 May 2008).
- Nakhaee, F.; Law, M. Parametric modelling of survival following HIV and AIDS in the era of highly active antiretroviral therapy: Data from Australia. East. Mediterr. Health J. 2011, 17, 231–237. [Google Scholar] [PubMed]
- Mee, P.; Collinson, M.M.; Madhavan, S.; Kabudula, C.; Gómez-Olivé, F.F.; Kahn, K.; Byass, P. Determinants of the risk of dying of HIV/AIDS in a rural South African community over the period of the decentralised roll-out of antiretroviral therapy: A longitudinal study. Glob. Health Action 2014, 20. [Google Scholar] [CrossRef] [PubMed]
- Brinkhof, M.M.; Boulle, A.; Weigel, R.; Messou, E.; Mathers, C.; Orrell, C. Mortality of HIV-infected patients starting antiretroviral therapy in Sub-Saharan Africa: Comparison with HIV-unrelated mortality. PLoS Med. 2009, 6, e1000066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wajanga, B.B.; Webster, L.L.; Peck, R.R.; Downs, J.J.; Mate, K.; Smart, L.L.; Fitzgerald, D.W. Inpatient mortality of HIV-infected adults in Sub-Saharan Africa and possible interventions: A mixed methods review. BMC Health Serv. Res. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Mageda, K.; Leyna, G.G.; Mmbaga, E.J. High initial HIV/AIDS-related mortality and-its predictors among patients on antiretroviral therapy in the Kagera Region of Tanzania: A five-year retrospective cohort study. AIDS Res. Treat. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Marazzi, M.M.; Liotta, G.; Germano, P.; Guidotti, G.; Altan, A.A.; Ceffa, S.; Palombi, L. Excessive early mortality in the first year of treatment in HIV type 1-infected patients initiating antiretroviral therapy in resource-limited settings. AIDS Res. Hum. Retroviruses 2008, 24, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Kebebew, K.; Wencheko, E. Survival analysis of HIV-infected patients under antiretroviral treatment at the armed forces general teaching hospital, Addis Ababa, Ethiopia. Ethiop. J. Health Dev. 2012, 26, 186–192. [Google Scholar]
- Damtew, B.; Mengistie, B.; Alemayehu, T. Survival and determinants of mortality in adult HIV/AIDS patients initiating antiretroviral therapy in Somali Region, Eastern Ethiopia. Pan Afr. Med. J. 2015, 22. [Google Scholar] [CrossRef] [PubMed]
- Tachbele, E.; Ameni, G. Survival and predictors of mortality among human immunodeficiency virus patients on anti-retroviral treatment at Jinka hospital, South Omo, Ethiopia: A six years retrospective cohort study. Epidemiol. Health 2016, 38, e2016049. [Google Scholar] [PubMed]
- Hambisa, M.M.; Ali, A.; Dessie, Y. Determinants of mortality among HIV positives after initiating antiretroviral therapy in Western Ethiopia: A hospital-based retrospective cohort study. ISRN AIDS 2013. [Google Scholar] [CrossRef] [PubMed]
- Kleinbaum, D.G.; Klein, M. Survival Analysis. Available online: http://www.springer.com/us/book/9780387291505 (accessed on 6 September 2005).
- Prentice, R.R.; Gloeckler, L.A. Regression analysis of grouped survival data with application to breast cancer data. Biometrics 1978, 34, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Royston, P.; Parmar, M.K. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat. Med. 2002, 21, 2175–2197. [Google Scholar] [CrossRef] [PubMed]
- Mulu, A.; Liebert, U.U.; Maier, M. Virological efficacy and immunological recovery among Ethiopian HIV-1 infected adults and children. BMC Infect. Dis. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Federal HIV/AIDS Prevention and Control Office Federal Ministry of Health. Guidelines for HIV counselling and testing in Ethiopia. Available online: http://www.who.int/hiv/topics/vct/ETH_HCT_guidelinesJune26_clean.pdf (accessed on 5 July 2007).
- World Health Organization and Management Sciences for Health. How to investigate adherence to antiretroviral therapy: An indicator based approach. Available online: http://apps.who.int/medicinedocs/documents/s21376en/s21376en.pdf (accessed on 6 September 2011).
- Hosmer, D.W., Jr.; Lemeshow, S. Applied survival analysis: Regression modeling of time to event data (1999). Int. J. Epidemiol. 1999, 21, 561–562. [Google Scholar]
- Kleinbaum, D.D.; Klein, M. Survival analysis: A self-learning text. Available online: https://books.google.com.vn/books?hl=vi&lr=&id=E7hWPJICBv4C&oi=fnd&pg=PA2&dq=21.%09Kleinbaum,+D.D.%3B+Klein,+M.+%282006%29.+Survival+analysis:+a+self-learning+text.+Springer+Science+%26+Business+Media.&ots=VSsnOgNE2-&sig=3q29l2OUY0PHMUDehFj37Q3RDa8&redir_esc=y#v=onepage&q&f=false (accessed on 9 July 2006).
- Tadesse, K.; Haile, F.; Hiruy, N. Predictors of mortality among patients enrolled on antiretroviral therapy in Aksum hospital, Northern Ethiopia: A retrospective cohort study. PloS ONE 2014, 9, e87392. [Google Scholar] [CrossRef] [PubMed]
- Wubshet, M.; Berhane, Y.; Worku, A.; Kebede, Y.; Diro, E. High loss to followup and early mortality create substantial reduction in patient retention at antiretroviral treatment program in North-West Ethiopia. ISRN AIDS 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, A.; Naman, E.; Ngowi, B.B.; Sandvik, L.; Matee, M.M.; Aglen, H.H.; Bruun, J.N. Predictors of mortality in HIV-infected patients starting antiretroviral therapy in a rural hospital in Tanzania. BMC Infect. Dis. 2008, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, H.; Wang, L.; Wang, Q.; Huang, P.; Guo, W.; Peng, Z.; Norris, J. Incidence and risk factors for AIDS-related mortality in HIV patients in China: A cross-sectional study. BMC Public Health 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Mengesha, S.; Belayihun, B.; Kumie, A. Predictors of survival in HIV-infected patient after initiation of HAART in Zewditu memorial hospital, Addis Ababa, Ethiopia. Int. Sch. Res. Notices 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Setegn, T.; Takele, A.; Gizaw, T.; Nigatu, D.; Haile, D. Predictors of mortality among adult antiretroviral therapy users in Southeastern Ethiopia: Retrospective cohort study. AIDS Res. Treat. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Mulissa, Z.; Jerene, D.; Lindtjørn, B. Patients present earlier and survival has improved, but pre-ART attrition is high in a six-year HIV cohort data from Ethiopia. PloS ONE 2010, 5, e13268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yiannoutsos, C.T. Modeling AIDS survival after initiation of antiretroviral treatment by Weibull models with changepoints. J. Int. AIDS Soc. 2009, 12. [Google Scholar] [CrossRef] [PubMed]
- Ayalew, J.; Moges, H.; Worku, A. Identifying factors related to the survival of AIDS patients under the follow-up of antiretroviral therapy (ART): The case of South Wollo. Int. J. Data Envelopment Anal. Oper. Res. 2014, 1, 21–27. [Google Scholar]
- Assefa, T.; Wencheko, E. Survival analysis of patients under chronic HIV-care and antiretroviral treatment at Tikur Anbessa specialized hospital, Addis Ababa, Ethiopia. Ethiop. J. Health Dev. 2012, 26, 22–29. [Google Scholar]
- Banbeta, A.; Seyoum, D.; Belachew, T.; Birlie, B.; Getachew, Y. Modeling time-to-cure from severe acute malnutrition: Application of various parametric frailty models. Arch. Public Health 2015, 73, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.F.; Gange, S.J.; Williams, C.M.; Anastos, K.; Greenblatt, R.M.; Kingsley, L.; Detels, R.; Muñoz, A. Patterns of the hazard of death after AIDS through the evolution of antiretroviral therapy: 1984–2004. AIDS 2005, 19, 2009–2018. [Google Scholar] [CrossRef] [PubMed]
- Veugelers, P.J.; Cornelisse, P.G.A.; Craib, K.J.P.; Marion, S.A.; Hogg, R.S.; Strathdee, S.A.; Montaner, J.S.G.; O’Shaughnessy, M.V.; Schechter, M.T. Models of survival in HIV infection and their use in the quantification of treatment benefits. Am. J. Epidemiol. 1998, 148, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Mgori, N.N.; Mash, R. HIV and/or AIDS-related deaths and modifiable risk factors: A descriptive study of medical admissions at Oshakati intermediate hospital in Northern Namibia. Afr. J. Prim. Health Care Fam. Med. 2015, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Alemu, A.A.; San Sebasti, M. Determinants of survival in adult HIV patients on antiretroviral therapy in Oromiyaa, Ethiopia. Glob. Health Action 2010, 3, 5398. [Google Scholar] [CrossRef] [PubMed]
- Sieleunou, I.; Souleymanou, M.; Schönenberger, A.A.; Menten, J.; Boelaert, M. Determinants of survival in AIDS patients on antiretroviral therapy in a rural centre in the Far-North Province, Cameroon. Trop. Med. Int. Health 2009, 14, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Kim, K.K.; Lee, S.S.; Cho, H.; Chen, D.D.; Chung, J.J.; Cho, G.J. Causes of death and risk factors for mortality among HIV-infected patients receiving antiretroviral therapy in Korea. J. Korean Med. Sci. 2013, 28, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Kigozi, B.B.; Sumba, S.; Mudyope, P.; Namuddu, B.; Kalyango, J.; Karamagi, C.; Ssali, F. The effect of AIDS defining conditions on immunological recovery among patients initiating antiretroviral therapy at Joint Clinical Research Centre, Uganda. AIDS Res. Ther. 2009, 6. [Google Scholar] [CrossRef] [PubMed]
- Teshome, W.; Belayneh, M.; Moges, M.; Endriyas, M.; Mekonnen, E.; Ayele, S.; Kumar, A.M. Who takes the medicine? Adherence to antiretroviral therapy in Southern Ethiopia. Patient Prefer. Adherence 2014, 9, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Yirdaw, K.K.; Hattingh, S. Prevalence and predictors of immunological failure among HIV patients on HAART in Southern Ethiopia. PLoS ONE 2015, 10, e0125826. [Google Scholar] [CrossRef] [PubMed]
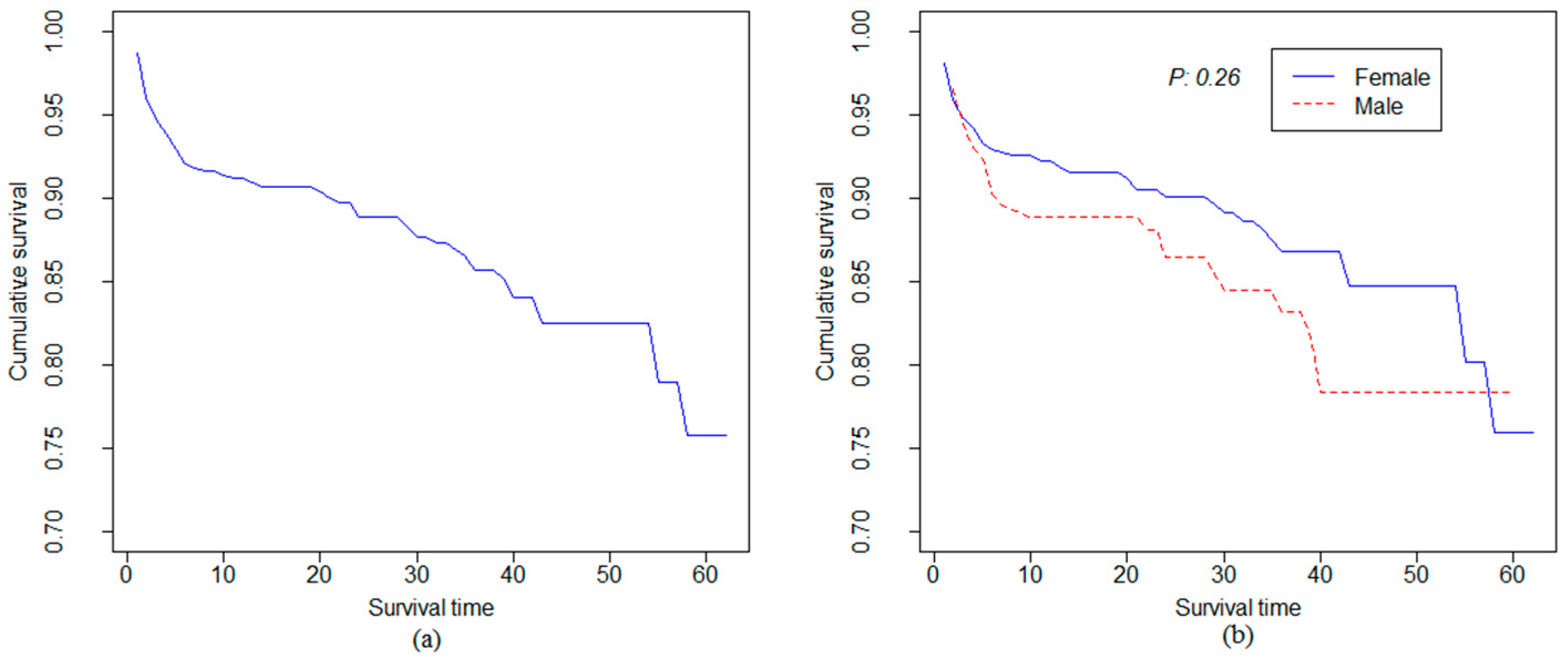
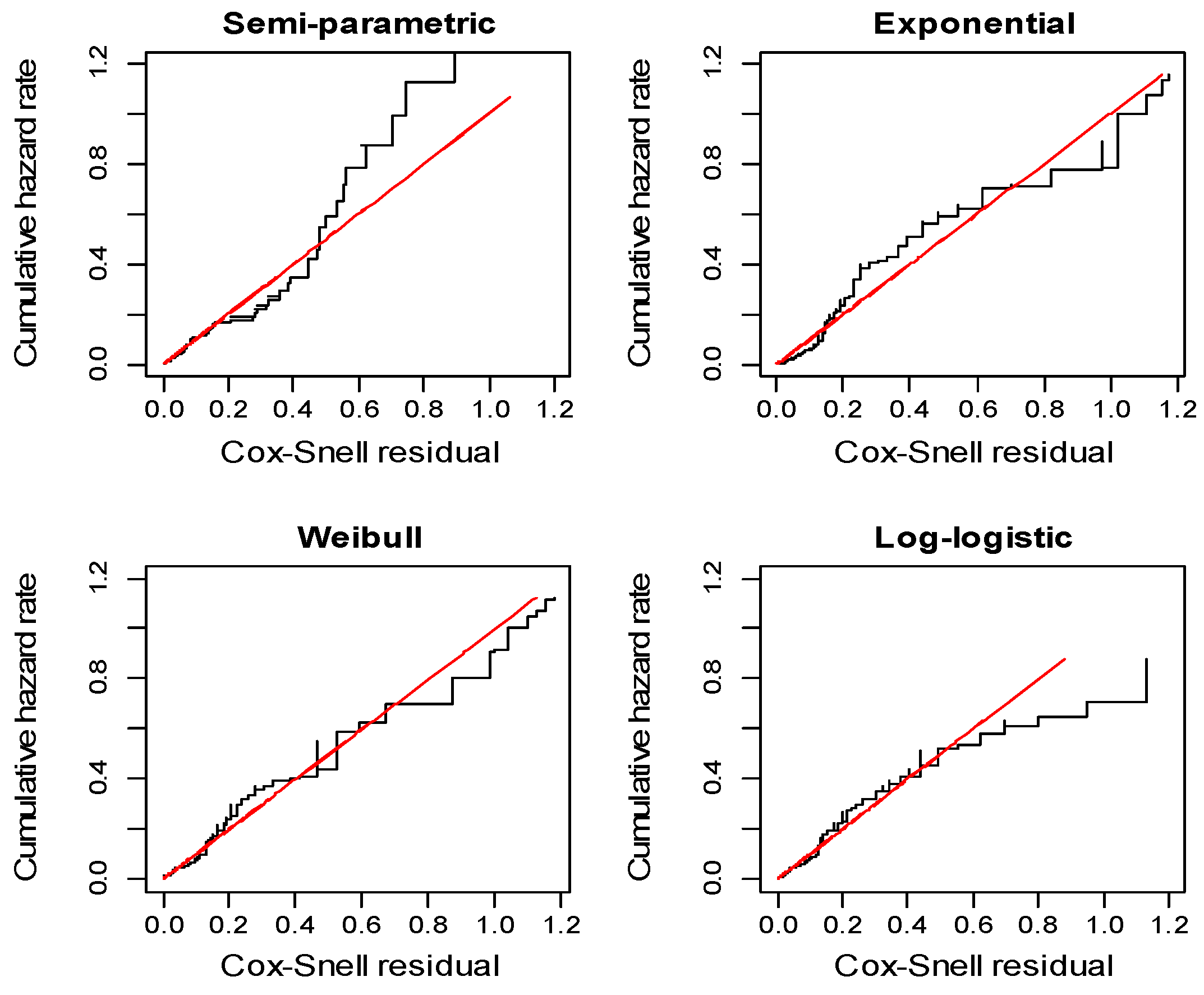
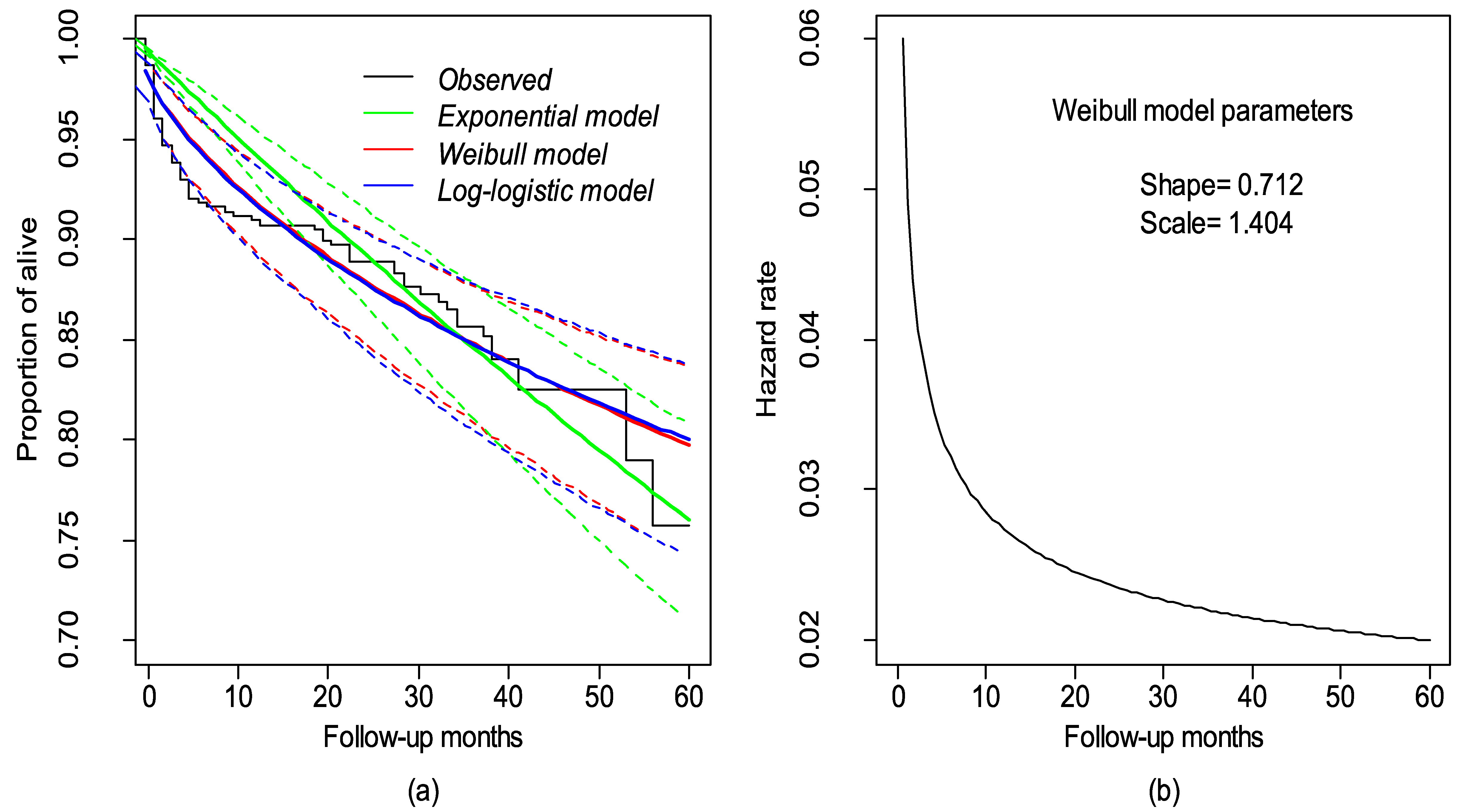
| Covariates | Total | Death N (%) | Censored N (%) | |
|---|---|---|---|---|
| Gender | Female | 312 | 41 (13.1) | 271 (86.9) |
| Male | 144 | 25 (17.4) | 119 (82.6) | |
| Age | <25 | 99 | 7 (7.1) | 92 (92.9) |
| 25–35 | 224 | 29 (12.9) | 195 (87.1) | |
| >35 | 133 | 30 (22.6) | 103 (77.4) | |
| CD4 | ≤200 | 316 | 44 (13.9) | 272 (86.1) |
| >200 | 140 | 22 (15.7) | 118 (84.3) | |
| TB status | Negative | 247 | 27 (10.9) | 220 (89.1) |
| Positive | 209 | 39 (18.7) | 170 (81.3) | |
| POI | No | 255 | 26 (10.2) | 229 (89.8) |
| Yes | 401 | 40 (19.9) | 161 (80.1) | |
| WHO stage | I | 65 | 5 (7.7) | 60 (92.3) |
| II | 149 | 20 (13.4) | 129 (86.6) | |
| III | 188 | 22 (11.7) | 166 (88.3) | |
| IV | 54 | 19 (35.2) | 35 (64.8) | |
| Adherence | Low | 102 | 27 (26.5) | 75 (73.5) |
| High | 354 | 39 (11.0) | 315 (89.0) | |
| Regimen | 1a | 366 | 49 (13.4) | 317 (86.6) |
| 1b | 48 | 9 (18.8) | 39 (81.3) | |
| 1c | 30 | 6 (20.0) | 24 (80.0) | |
| 1d | 12 | 2 (16.7) | 10 (83.3) | |
| Cox | Exponential | Weibull | Log-logistic | |
|---|---|---|---|---|
| AHR (95% C.I.) | AHR (95% C.I.) | AHR (95% C.I.) | (95% C.I.) | |
| Gender | ||||
| Female | 1 | 1 | 1 | 1 |
| Male | 1.42 (0.81–2.49) | 1.59 (0.90–2.80) | 1.46 (0.84–2.58) | 0.63 (0.27–1.41) |
| Age | ||||
| <25 | 1 | 1 | 1 | |
| 25–35 | 1.56 (0.66–3.62) | 1.53 (0.65–3.59) | 1.56 (0.67–3.64) | 0.53 (0.16–1.73) |
| ≥35 | 3.45 * (1.45–8.24) | 4.07 * (1.70–9.77) | 3.81 * (1.60–9.08) | 0.16 * (0.05–0.58) |
| BW | 0.94 * (0.91–0.97) | 0.93 * (0.89–0.95) | 0.93 * (0.90–0.97) | 1.08 * (1.04–1.14) |
| CD4 | ||||
| ≥200 | 1 | 1 | 1 | 1 |
| <200 | 1.58 (0.89–2.79) | 1.88 * (1.05–3.37) | 1.73 (0.98–3.07) | 0.53 (0.24–1.21) |
| TB | ||||
| Negative | 1 | 1 | 1 | 1 |
| Positive | 1.28 (0.74–2.15) | 1.32 (0.76–2.28) | 1.33 (0.77–2.29) | 0.67 (0.31–1.48) |
| OI | ||||
| No | 1 | 1 | 1 | 1 |
| Yes | 1.38 (0.78–2.43) | 1.46 (0.84–2.55) | 1.44 (0.83–2.52) | 0.59 (0.26–1.33) |
| Regimen | ||||
| 1a | 1 | 1 | 1 | 1 |
| 1b | 0.78 (0.36–1.68) | 0.65 (0.29–1.40) | 0.73 (0.34–1.58) | 1.25 (0.39–4.05) |
| 1c | 0.95 (0.38–2.32) | 1.00 (0.41–2.46) | 0.99 (0.41–2.44) | 0.85 (0.22–3.25) |
| 1d | 1.81 (0.42–3.73) | 2.20 (0.50–3.93) | 2.04 (0.49–8.81) | 0.39 (0.11–3.25) |
| WHO stage | ||||
| I | 1 | 1 | 1 | 1 |
| II | 1.75 (0.64–4.79) | 1.68 (0.62–4.57) | 1.67 (0.62–4.52) | 0.44 (0.11–1.84) |
| III | 1.70 (0.63–4.58) | 1.69 (0.63–4.54) | 1.61 (0.61–4.32) | 0.51 (0.02–2.03) |
| IV | 5.68 (2.06–15.71) | 7.1 * (2.56–11.93) | 6.2 * (2.24–14.17) | 0.07 * (0.06–0.35) |
| Adherence | ||||
| High | 1 | 1 | 1 | 1 |
| Low | 3.78 * (2.23–6.42) | 5.03 (2.96–8.55) | 4.16 * (2.45–7.07) | 0.14 * (0.06–0.33) |
| AIC | 696 | 504 | 492 | 497 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seyoum, D.; Degryse, J.-M.; Kifle, Y.G.; Taye, A.; Tadesse, M.; Birlie, B.; Banbeta, A.; Rosas-Aguirre, A.; Duchateau, L.; Speybroeck, N. Risk Factors for Mortality among Adult HIV/AIDS Patients Following Antiretroviral Therapy in Southwestern Ethiopia: An Assessment through Survival Models. Int. J. Environ. Res. Public Health 2017, 14, 296. https://doi.org/10.3390/ijerph14030296
Seyoum D, Degryse J-M, Kifle YG, Taye A, Tadesse M, Birlie B, Banbeta A, Rosas-Aguirre A, Duchateau L, Speybroeck N. Risk Factors for Mortality among Adult HIV/AIDS Patients Following Antiretroviral Therapy in Southwestern Ethiopia: An Assessment through Survival Models. International Journal of Environmental Research and Public Health. 2017; 14(3):296. https://doi.org/10.3390/ijerph14030296
Chicago/Turabian StyleSeyoum, Dinberu, Jean-Marie Degryse, Yehenew Getachew Kifle, Ayele Taye, Mulualem Tadesse, Belay Birlie, Akalu Banbeta, Angel Rosas-Aguirre, Luc Duchateau, and Niko Speybroeck. 2017. "Risk Factors for Mortality among Adult HIV/AIDS Patients Following Antiretroviral Therapy in Southwestern Ethiopia: An Assessment through Survival Models" International Journal of Environmental Research and Public Health 14, no. 3: 296. https://doi.org/10.3390/ijerph14030296





