Pharmacokinetic and Pharmacodynamic Responses to Clopidogrel: Evidences and Perspectives
Abstract
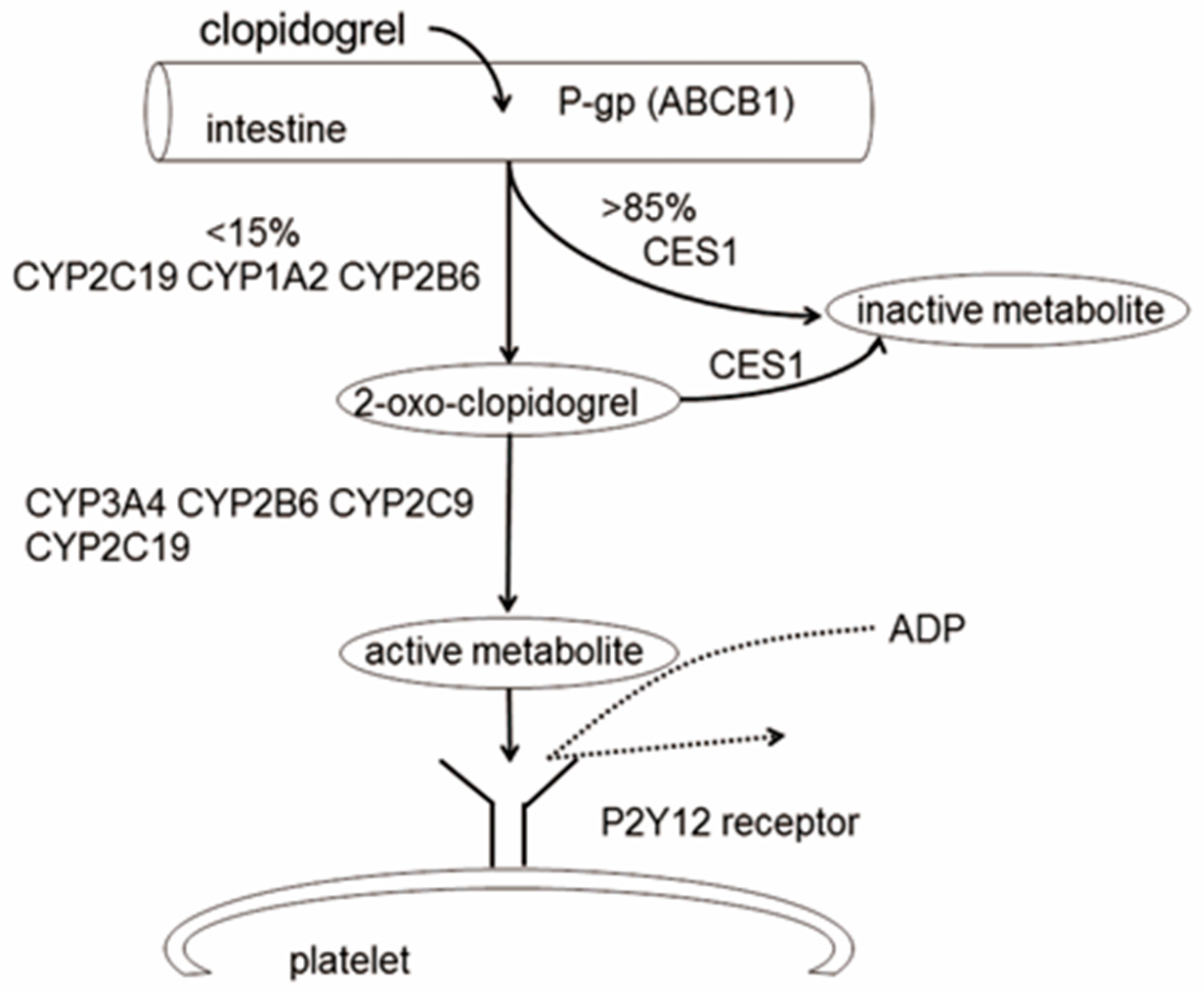
:1. Introduction
2. Genetic Polymorphisms in Drug Disposition and Drug Targets
2.1. ABCB1 Polymorphisms
2.2. CES1 Polymorphisms
2.3. PON1 Polymorphisms
2.4. CYP2C19 Polymorphisms
2.5. CYP3A4/5 Polymorphisms
2.6. P2RY12 Polymorphisms
2.7. PEAR1 Polymorphisms
3. Epigenetics Influencing Clopidogrel Response
3.1. MicroRNAs
3.2. DNA Methylation
4. Non-Genetic Factors Influencing Clopidogrel Response
4.1. Demographic Characteristics
4.2. Complications
4.2.1. Diabetes Mellitus
4.2.2. Chronic Kidney Disease
4.3. Drug Interactions
4.3.1. Proton Pump Inhibitor
4.3.2. Calcium Channel Blocker
4.3.3. Statin
4.3.4. Morphine
4.3.5. Caffeine
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yusuf, S.; Zhao, F.; Mehta, S.R.; Chrolavicius, S.; Tognoni, G.; Fox, K.K. Clopidogrel in unstable angina to prevent recurrent events trial investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N. Engl. J. Med. 2001, 345, 494–502. [Google Scholar] [PubMed]
- Price, M.J.; Angiolillo, D.J.; Teirstein, P.S.; Lillie, E.; Manoukian, S.V.; Berger, P.B.; Tanguay, J.F.; Cannon, C.P.; Topol, E.J. Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention: A time-dependent analysis of the gauging responsiveness with a VerifyNow P2Y12 assay: Impact on Thrombosis and Safety (GRAVITAS) trial. Circulation 2011, 124, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Witzenbichler, B.; Weisz, G.; Rinaldi, M.J.; Neumann, F.-J.; Metzger, D.C.; Henry, T.D.; Cox, D.A.; Duffy, P.L.; Mazzaferri, E.; et al. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): A prospective multicentre registry study. Lancet 2013, 382, 614–623. [Google Scholar] [CrossRef]
- Taubert, D.; von Beckerath, N.; Grimberg, G.; Lazar, A.; Jung, N.; Goeser, T.; Kastrati, A.; Schomig, A.; Schomig, E. Impact of P-glycoprotein on clopidogrel absorption. Clin. Pharmacol. Ther. 2006, 80, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Verstuyft, C.; Mary-Krause, M.; Quteineh, L.; Méneveau, N.; Danchin, G.S.N.; Becquemont, L. Genetic determinants of response to clopidogrel and cardiovascular events. N. Engl. J. Med. 2009, 360, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Park, M.W.; Her, S.H.; Kim, C.J.; SunCho, J.; Park, G.M.; Kim, T.S.; Choi, Y.S.; Park, C.S.; Koh, Y.S.; Park, H.J.; et al. Evaluation of the incremental prognostic value of the combination of CYP2C19 poor metabolizer status and ABCB1 3435 TT polymorphism over conventional risk factors for cardiovascular events after drug-eluting stent implantation in East Asians. Genet. Med. 2016, 18, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Xu, J.; Li, X.; Zhang, H.; Hu, J.; Fang, R.; Chen, X. ABCB1 C3435T polymorphism and response to clopidogrel treatment in coronary artery disease (CAD) patients: A meta-analysis. PLoS ONE 2012, 7, e46366. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Shen, C.L.; Wang, B.N.; Huang, X.H.; Hu, Z.L.; Li, J. Genetic polymorphisms of CYP2C19 2 and ABCB1 C3435T affect the pharmacokinetic and pharmacodynamic responses to clopidogrel in 401 patients with acute coronary syndrome. Gene 2015, 558, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Stokanovic, D.; Nikolic, V.N.; Konstantinovic, S.S.; Zvezdanovic, J.B.; Lilic, J.; Apostolovic, S.R.; Pavlovic, M.; Zivkovic, V.S.; Jevtovic-Stoimenov, T.; Jankovic, S.M. P-glycoprotein polymorphism C3435T is associated with dose-adjusted clopidogrel and 2-oxo-clopidogrel concentration. Pharmacology 2016, 97, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Karazniewicz-Lada, M.; Danielak, D.; Rubis, B.; Burchardt, P.; Komosa, A.; Lesiak, M.; Glowka, F. Impact of common ABCB1 polymorphism on pharmacokinetics and pharmacodynamics of clopidogrel and its metabolites. J. Clin. Pharm. Ther. 2015, 40, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Li, J.; Xu, X.; Sun, X.; Sheng, W. ABCB1 C3435T polymorphism and risk of adverse clinical events in clopidogrel treated patients: A meta-analysis. Thromb. Res. 2012, 129, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Jaitner, J.; Morath, T.; Byrne, R.A.; Braun, S.; Gebhard, D.; Bernlochner, I.; Schulz, S.; Mehilli, J.; Schomig, A.; Koch, W.; et al. No association of ABCB1 C3435T genotype with clopidogrel response or risk of stent thrombosis in patients undergoing coronary stenting. Circ. Cardiovasc. Interv. 2012, 5, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, X.; Sun, S.; Mei, S.; Ma, N.; Miao, Z.; Zhao, M.; Peng, S. Impact of genetic polymorphisms related to clopidogrel or acetylsalicylic acid pharmacology on clinical outcome in Chinese patients with symptomatic extracranial or intracranial stenosis. Eur. J. Clin. Pharmacol. 2016, 72, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.P.; Horenstein, R.B.; Ryan, K.; O’Connell, J.R.; Gibson, Q.; Mitchell, B.D.; Tanner, K.; Chai, S.; Bliden, K.P.; Tantry, U.S.; et al. The functional G143E svariant of carboxylesterase 1 is associated with increased clopidogrel active metabolite levels and greater clopidogrel response. Pharmacogenet. Genom. 2013, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tarkiainen, E.K.; Holmberg, M.T.; Tornio, A.; Neuvonen, M.; Neuvonen, P.J.; Backman, J.T.; Niemi, M. Carboxylesterase 1 c.428G>A single nucleotide variation increases the antiplatelet effects of clopidogrel by reducing its hydrolysis in humans. Clin. Pharmacol. Ther. 2015, 97, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Ding, X.; Gao, J.; Wang, H.; Hang, Y.; Zhang, H.; Zhang, J.; Jiang, B.; Miao, L. The effects of CES1A2 A (-816) C and CYP2C19 loss-of-function polymorphisms on clopidogrel response variability among Chinese patients with coronary heart disease. Pharmacogenet. Genom. 2014, 24, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Bouman, H.J.; Schomig, E.; van Werkum, J.W.; Velder, J.; Hackeng, C.M.; Hirschhauser, C.; Waldmann, C.; Schmalz, H.G.; ten Berg, J.M.; Taubert, D. Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nat. Med. 2011, 17, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Cuisset, T.; Morange, P.E.; Quilici, J.; Bonnet, J.L.; Gachet, C.; Alessi, M.C. Paraoxonase-1 and clopidogrel efficacy. Nat. Med. 2011, 17, 1039, author reply 1042–1044. [Google Scholar] [CrossRef] [PubMed]
- Mega, J.L.; Close, S.L.; Wiviott, S.D.; Man, M.; Duvvuru, S.; Walker, J.R.; Sundseth, S.S.; Collet, J.P.; Delaney, J.T.; Hulot, J.S.; et al. PON1 Q192R genetic variant and response to clopidogrel and prasugrel: Pharmacokinetics, pharmacodynamics, and a meta-analysis of clinical outcomes. J. Thromb. Thrombolysis 2016, 41, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Hulot, J.S.; Bura, A.; Villard, E.; Azizi, M.; Remones, V.; Goyenvalle, C.; Aiach, M.; Lechat, P.; Gaussem, P. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood 2006, 108, 2244–2247. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.Q.; Wang, D.G.; Yang, H.; Cao, H. Cytochrome P450 CYP 2C19*2 associated with adverse 1-year cardiovascular events in patients with acute coronary syndrome. PLoS ONE 2015, 10, e0132561. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Pare, G.; Eikelboom, J.W.; Simonsen, K.L.; Emison, E.S.; Fox, K.A.; Steg, P.G.; Montalescot, G.; Bhakta, N.; Hacke, W.; et al. The relationship between CYP2C19 polymorphisms and ischaemic and bleeding outcomes in stable outpatients: The CHARISMA genetics study. Eur. Heart J. 2012, 33, 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- Shuldiner, A.R.; O’Connell, J.R.; Bliden, K.P.; Gandhi, A.; Ryan, K.; Horenstein, R.B.; Damcott, C.M.; Pakyz, R.; Tantry, U.S.; Gibson, Q.; et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA 2009, 302, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Mega, J.L.; Close, S.L.; Wiviott, S.D.; Shen, L.; Hockett, R.D.; Brandt, J.T.; Walker, J.R.; Antman, E.M.; Macias, W.; Braunwald, E.; et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N. Engl. J. Med. 2009, 360, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Hulot, J.S.; Pena, A.; Villard, E.; Esteve, J.B.; Silvain, J.; Payot, L.; Brugier, D.; Cayla, G.; Beygui, F.; et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: A cohort study. Lancet 2009, 373, 309–317. [Google Scholar] [CrossRef]
- Lin, R.; Zhang, L.; Zhang, P.; Zhou, L.; Liu, T.; Li, Y.; Zhang, W.; Wang, W.; Zhang, J. Influence of CYP2C19 loss-of-function variants on the metabolism of clopidogrel in patients from north-western China. J. Clin. Pharm. Ther. 2015, 40, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Sibbing, D.; Koch, W.; Gebhard, D.; Schuster, T.; Braun, S.; Stegherr, J.; Morath, T.; Schomig, A.; von Beckerath, N.; Kastrati, A. Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Circulation 2010, 121, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Harmsze, A.M.; van Werkum, J.W.; Hackeng, C.M.; Ruven, H.J.; Kelder, J.C.; Bouman, H.J.; Breet, N.J.; Ten Berg, J.M.; Klungel, O.H.; de Boer, A.; et al. The influence of CYP2C19*2 and *17 on on-treatment platelet reactivity and bleeding events in patients undergoing elective coronary stenting. Pharmacogenet. Genom. 2012, 22, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.J.; Hou, J.K.; Zhang, W.; Chang, Y.Z.; Li, Z.S.; Wang, Z.Y.; Du, Y.Y.; Ma, X.J.; Zhang, L.R.; Kan, Q.C.; et al. CYP3A4 * 1G genetic polymorphism influences metabolism of fentanyl in human liver microsomes in Chinese patients. Pharmacology 2015, 96, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Hokimoto, S.; Chitose, T.; Mizobe, M.; Akasaka, T.; Arima, Y.; Kaikita, K.; Iwashita, S.; Morita, K.; Miyazaki, H.; Oniki, K.; et al. Impact of CYP3A5 polymorphism on platelet reactivity at percutaneous coronary intervention and after 9 months of aspirin and clopidogrel therapy in Japanese patients with coronary artery disease. Eur. J. Clin. Pharmacol. 2014, 70, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Nakkam, N.; Tiamkao, S.; Kanjanawart, S.; Tiamkao, S.; Vannaprasaht, S.; Tassaneeyakul, W.; Tassaneeyakul, W. The impact of genetic polymorphisms of drug metabolizing enzymes on the pharmacodynamics of clopidogrel under steady state conditions. Drug Metab. Pharmacokinet. 2015, 30, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Park, K.W.; Kang, J.; Park, J.J.; Yang, H.M.; Lee, H.Y.; Kang, H.J.; Koo, B.K.; Oh, B.H.; Park, Y.B.; Kim, H.S. Amlodipine, clopidogrel and CYP3A5 genetic variability: Effects on platelet reactivity and clinical outcomes after percutaneous coronary intervention. Heart 2012, 98, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Fontana, P.; Dupont, A.; Gandrille, S.; Bachelot-Loza, C.; Reny, J.L.; Aiach, M.; Gaussem, P. Adenosine diphosphate-induced platelet aggregation is associated with P2Y12 gene sequence variations in healthy subjects. Circulation 2003, 108, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Rudez, G.; Bouman, H.J.; van Werkum, J.W.; Leebeek, F.W.; Kruit, A.; Ruven, H.J.; ten Berg, J.M.; de Maat, M.P.; Hackeng, C.M. Common variation in the platelet receptor P2RY12 gene is associated with residual on-clopidogrel platelet reactivity in patients undergoing elective percutaneous coronary interventions. Circ. Cardiovasc. Genet. 2009, 2, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Cuisset, T.; Frere, C.; Quilici, J.; Morange, P.E.; Saut, N.; Lambert, M.; Camoin, L.; Vague, I.J.; Bonnet, J.L.; Alessi, M.C. Role of the T744C polymorphism of the P2Y12 gene on platelet response to a 600-mg loading dose of clopidogrel in 597 patients with non-ST-segment elevation acute coronary syndrome. Thromb. Res. 2007, 120, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Suktitipat, B.; Yanek, L.R.; Faraday, N.; Wilson, A.F.; Becker, D.M.; Becker, L.C.; Mathias, R.A. Targeted deep resequencing identifies coding variants in the PEAR1 gene that play a role in platelet aggregation. PLoS ONE 2013, 8, e64179. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Tang, X.F.; Zhang, J.H.; He, C.; Ma, Y.L.; Xu, J.J.; Song, Y.; Liu, R.; Meng, X.M.; Song, L.; et al. Association of PEAR1 genetic variants with platelet reactivity in response to dual antiplatelet therapy with aspirin and clopidogrel in the Chinese patient population after percutaneous coronary intervention. Thromb. Res. 2016, 141, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Galeano, J.E.; Becker, D.M.; Wilson, A.F.; Yanek, L.R.; Bray, P.; Vaidya, D.; Faraday, N.; Becker, L.C. A novel variant in the platelet endothelial aggregation receptor-1 gene is associated with increased platelet aggregability. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.I.; Bray, S.; Garner, S.F.; Stephens, J.; de Bono, B.; Angenent, W.G.; Bentley, D.; Burns, P.; Coffey, A.; Deloukas, P.; et al. A functional genomics approach reveals novel quantitative trait loci associated with platelet signaling pathways. Blood 2009, 114, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.P.; Ryan, K.; O’Connell, J.R.; Horenstein, R.B.; Damcott, C.M.; Gibson, Q.; Pollin, T.I.; Mitchell, B.D.; Beitelshees, A.L.; Pakzy, R.; et al. Genetic variation in PEAR1 is associated with platelet aggregation and cardiovascular outcomes. Circ. Cardiovasc. Genet. 2013, 6, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Mukundan, M.; Yang, J.; Charpentier, N.; LeCluyse, E.L.; Black, C.; Yang, D.; Shi, D.; Yan, B. Antiplatelet agents aspirin and clopidogrel are hydrolyzed by distinct carboxylesterases, and clopidogrel is transesterificated in the presence of ethyl alcohol. J. Pharmacol. Exp. Ther. 2006, 319, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.J.; Patrick, K.S.; Yuan, H.J.; Wang, J.S.; Donovan, J.L.; DeVane, C.L.; Malcolm, R.; Johnson, J.A.; Youngblood, G.L.; Sweet, D.H.; et al. Two CES1 gene mutations lead to dysfunctional carboxylesterase 1 activity in man: Clinical significance and molecular basis. Am. J. Hum. Genet. 2008, 82, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Dansette, P.M.; Rosi, J.; Bertho, G.; Mansuy, D. Cytochromes P450 catalyze both steps of the major pathway of clopidogrel bioactivation, whereas paraoxonase catalyzes the formation of a minor thiol metabolite isomer. Chem. Res. Toxicol. 2012, 25, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Mega, J.L.; Simon, T.; Collet, J.P.; Anderson, J.L.; Antman, E.M.; Bliden, K.; Cannon, C.P.; Danchin, N.; Giusti, B.; Gurbel, P.; et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: A meta-analysis. JAMA 2010, 304, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.S.; Cho, K.I.; Jin, H.Y.; Seo, J.S.; Yang, T.H.; Kim, D.K.; Kim, D.S.; Seol, S.H.; Kim, D.I.; Kim, B.H.; et al. Meta-analysis of cytochrome P450 2C19 polymorphism and risk of adverse clinical outcomes among coronary artery disease patients of different ethnic groups treated with clopidogrel. Am. J. Cardiol. 2012, 110, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Djebli, N.; Fabre, D.; Boulenc, X.; Fabre, G.; Sultan, E.; Hurbin, F. Physiologically based pharmacokinetic modeling for sequential metabolism: Effect of CYP2C19 genetic polymorphism on clopidogrel and clopidogrel active metabolite pharmacokinetics. Drug Metab. Dispos. 2015, 43, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R., Jr.; Dehmer, G.J.; Kaul, S.; Leifer, D.; O’Gara, P.T.; Stein, C.M. ACCF/AHA clopidogrel clinical alert: Approaches to the FDA “boxed warning”: A report of the American College of Cardiology Foundation Task Force on clinical expert consensus documents and the American Heart Association. Circulation 2010, 122, 537–557. [Google Scholar] [PubMed]
- Sim, S.; Risinger, C.; Dahl, M.; Aklillu, E.; Christensen, M.; Bertilsson, L.; Ingelmansundberg, M. A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin. Pharmacol. Ther. 2006, 79, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.A.; Sangkuhl, K.; Stein, C.M.; Hulot, J.S.; Mega, J.L.; Roden, D.M.; Klein, T.E.; Sabatine, M.S.; Johnson, J.A.; Shuldiner, A.R. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 Update. Clin. Pharmacol. Ther. 2013, 94, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T.A.; Waskell, L.A. The metabolism of clopidogrel is catalyzed by human cytochrome P450 3A and is inhibited by atorvastatin. Drug Metab. Dispos. 2003, 31, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Danielak, D.; Karazniewicz-Lada, M.; Wisniewska, K.; Bergus, P.; Burchardt, P.; Komosa, A.; Glowka, F. Impact of CYP3A4*1G allele on clinical pharmacokinetics and pharmacodynamics of clopidogrel. Eur. J. Drug Metab. Pharmacokinet. 2016, 42, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, P.; Zhang, J.; Lin, Y.; Lamba, J.; Assem, M.; Schuetz, J.; Watkins, P.B.; Daly, A.; Wrighton, S.A.; Hall, S.D.; et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat. Genet. 2001, 27, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V. MicroRNA pathways in flies and worms: Growth, death, fat, stress, and timing. Cell 2003, 113, 673–676. [Google Scholar] [CrossRef]
- Carrington, J.C.; Ambros, V. Role of microRNAs in plant and animal development. Science 2003, 301, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Nagalla, S.; Shaw, C.; Kong, X.; Kondkar, A.A.; Edelstein, L.C.; Ma, L.; Chen, J.; McKnight, G.S.; López, J.A.; Yang, L.; et al. Platelet microRNA-mRNAcoexpression profiles correlate with platelet reactivity. Blood 2011, 117, 5189–5197. [Google Scholar] [CrossRef] [PubMed]
- Kondkar, A.A.; Bray, M.S.; Leal, S.M.; Nagalla, S.; Liu, D.J.; Jin, Y.; Dong, J.F.; Ren, Q.; Whiteheart, S.W.; Shaw, C.; et al. VAMP8/endobrevin is overexpressed in hyperreactive human platelets: Suggested role for platelet microRNA. J. Thromb. Haemost. 2010, 8, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Girardot, M.; Pecquet, C.; Boukour, S.; Knoops, L.; Ferrant, A.; Vainchenker, W.; Giraudier, S.; Constantinescu, S.N. miR-28 is a thrombopoietin receptor targeting microRNA detected in a fraction of myeloproliferative neoplasm patient platelets. Blood 2010, 116, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Willeit, P.; Zampetaki, A.; Dudek, K.; Kaudewitz, D.; King, A.; Kirkby, N.S.; Crosby-Nwaobi, R.; Prokopi, M.; Drozdov, I.; Langley, S.R.; et al. Circulating microRNAs as novel biomarkers for platelet activation. Circ. Res. 2013, 112, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Landry, P.; Plante, I.; Ouellet, D.L.; Perron, M.P.; Rousseau, G.; Provost, P. Existence of a microRNA pathway in anucleate platelets. Nat. Struct. Mol. Biol. 2009, 16, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Ge, L.; Zhou, X.; Ji, W.J.; Lu, R.Y.; Zhang, Y.Y.; Zeng, S.; Liu, X.; Zhao, J.H.; Zhang, W.C.; et al. Decreased platelet miR-223 expression is associated with high on-clopidogrel platelet reactivity. Thromb. Res. 2013, 131, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Zhou, X.; Ji, W.J.; Shi, R.; Lu, R.Y.; Li, J.L.; Yang, G.H.; Luo, T.; Zhang, J.Q.; Zhao, J.H.; et al. Decreased circulating microRNA-223 level predicts high on-treatment platelet reactivity in patients with troponin-negative non-ST elevation acute coronary syndrome. J. Thromb. Thrombolysis 2014, 38, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Qi, X.; Chen, H.; Li, M.; Gu, J.; Liu, C.; Xue, H.; Wang, L.; Geng, Y.; Qi, P.; et al. Expression of miRNA-26a in platelets is associated with clopidogrel resistance following coronary stenting. Exp. Ther. Med. 2016, 12, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Jjingo, D.; Conley, A.B.; Yi, S.V.; Lunyak, V.V.; Jordan, I.K. On the presence and role of human gene-body DNA methylation. Oncotarget 2012, 3, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Tomiyasu, H.; Goto-Koshino, Y.; Fujino, Y.; Ohno, K.; Tsujimoto, H. Epigenetic regulation of the ABCB1 gene in drug-sensitive and drug-resistant lymphoid tumour cell lines obtained from canine patients. Vet. J. 2014, 199, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Oberstadt, M.C.; Bien-Moller, S.; Weitmann, K.; Herzog, S.; Hentschel, K.; Rimmbach, C.; Vogelgesang, S.; Balz, E.; Fink, M.; Michael, H.; et al. Epigenetic modulation of the drug resistance genes MGMT, ABCB1 and ABCG2 in glioblastoma multiforme. BMC Cancer 2013, 13, 617. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xue, X.; Wang, F.; An, Y.; Tang, D.; Xu, Y.; Wang, H.; Yuan, Z.; Gao, W.; Wei, J.; et al. Expression and promoter methylation analysis of ATP-binding cassette genes in pancreatic cancer. Oncol. Rep. 2012, 27, 265–269. [Google Scholar] [PubMed]
- Yang, J.; Zhou, J.S.; Zhao, Y.X.; Yang, Z.H.; Zhao, H.D.; Zhang, Y.D.; Zou, J.J. ABCB1 hypomethylation is associated with decreased antiplatelet effects of clopidogrel in Chinese ischemic stroke patients. Pharmazie 2015, 70, 97–102. [Google Scholar] [PubMed]
- Luchessi, A.D.; Silbiger, V.N.; Cerda, A.; Hirata, R.D.; Carracedo, A.; Brion, M.; Iniguez, A.; Bravo, M.; Bastos, G.; Sousa, A.G.; et al. Increased clopidogrel response is associated with ABCC3 expression: A pilot study. Clin. Chim. Acta 2012, 413, 417–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, J.J.; Fan, H.W.; Chen, S.L.; Tan, J.; He, B.S.; Xie, H.G. Efffect of the ABCC3-211C/T polymorphism on clopidogrel responsiveness in patients with percutaneous coronary intervention. Clin. Exp. Pharmacol. Physiol. 2013, 40, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Jie, Y.; Jun-Shan, Z.; Ying-Dong, Z.; You-Yong, T.; Jian-Jun, Z. The association of ABCC3 promoter methylation with clopidogrel response in Chinese ischemic stroke patients. Pharmazie 2014, 69, 764–768. [Google Scholar]
- Su, J.; Li, X.; Yu, Q.; Liu, Y.; Wang, Y.; Song, H.; Cui, H.; Du, W.; Fei, X.; Liu, J.; et al. Association of P2Y12 gene promoter DNA methylation with the risk of clopidogrel resistance in coronary artery disease patients. Biomed. Res. Int. 2014, 2014, 450814. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Fabrega, C.; Carrera, C.; Reny, J.L.; Fontana, P.; Slowik, A.; Pera, J.; Pezzini, A.; Serrano-Heras, G.; Segura, T.; Marti-Fabregas, J.; et al. TRAF3 epigenetic regulation is associated with vascular recurrence in patients with ischemic stroke. Stroke 2016, 47, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Jin, R.; Yu, S.; Rivet, J.J.; Smyth, S.S.; Nanda, A.; Granger, D.N.; Li, G. CD40 is essential in the upregulation of TRAF proteins and NF-kappaB-dependent proinflammatory gene expression after arterial injury. PLoS ONE 2011, 6, e23239. [Google Scholar] [CrossRef] [PubMed]
- Kuijpers, M.J.; Mattheij, N.J.; Cipolla, L.; van Geffen, J.P.; Lawrence, T.; Donners, M.M.; Boon, L.; Lievens, D.; Torti, M.; Noels, H.; et al. Platelet CD40L modulates thrombus growth via phosphatidylinositol 3-kinase beta, and not via CD40 and IkappaB kinase alpha. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Cuisset, T.; Frere, C.; Quilici, J.; Morange, P.E.; Camoin, L.; Bali, L.; Lambert, M.; Juhan-Vague, I.; Alessi, M.C.; Bonnet, J.L. Relationship between aspirin and clopidogrel responses in acute coronary syndrome and clinical predictors of non response. Thromb. Res. 2009, 123, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Khalil, B.M.; Shahin, M.H.; Solayman, M.H.; Langaee, T.; Schaalan, M.F.; Gong, Y.; Hammad, L.N.; Al-Mesallamy, H.O.; Hamdy, N.M.; El-Hammady, W.A.; et al. Genetic and nongenetic factors affecting clopidogrel response in the Egyptian population. Clin. Transl. Sci. 2016, 9, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Verdoia, M.; Rolla, P.P.R.; Nardin, M.; Schaffer, A.; Barbieri, L.; Marino, P.; Suryapranata, G.B.H.; de Luca, G. Advanced age and high-residual platelet reactivity in patients receiving dual antiplatelet therapy with clopidogrel or ticagrelor. J. Thromb. Hamost. 2015, 14, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Gremmel, T.; Steiner, S.; Seidinger, D.; Koppensteiner, R.; Panzer, S.; Kopp, C.W. Obesity is associated with poor response to clopidogrel and an increased susceptibility to protease activated receptor-1 mediated platelet activation. Transl. Res. 2013, 161, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Gurbel, P.A.; Bliden, K.P.; Logan, D.K.; Kereiakes, D.J.; Lasseter, K.C.; White, A.; Angiolillo, D.J.; Nolin, T.D.; Maa, J.F.; Bailey, W.L.; et al. The influence of smoking status on the pharmacokinetics and pharmacodynamics of clopidogrel and prasugrel: The PARADOX study. J. Am. Coll. Cardiol. 2013, 62, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Park, K.W.; Kang, S.H.; Kang, J.; Jeon, K.H.; Park, J.J.; Han, J.K.; Koh, J.S.; Lee, S.E.; Yang, H.M.; Lee, H.Y.; et al. Enhanced clopidogrel response in smokers is reversed after discontinuation as assessed by VerifyNow assay: Additional evidence for the concept of “smokers’ paradox”. Heart 2012, 98, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Park, K.W.; Park, J.J.; Jeon, K.H.; Kang, S.H.; Oh, I.Y.; Yang, H.M.; Cho, H.J.; Lee, H.Y.; Kang, H.J.; Koo, B.K.; et al. Enhanced clopidogrel responsiveness in smokers: Smokers’ paradox is dependent on cytochrome P450 CYP1A2 status. Arterioscler. Thromb. Vasc. Biol. 2010, 31, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Suh, J.W.; Kang, S.H.; Park, J.J.; Yoon, C.H.; Cho, Y.S.; Youn, T.J.; Chae, I.H.; Choi, D.J.; Kim, H.S. Cigarette Smoking Does Not Enhance Clopidogrel Responsiveness After Adjusting VerifyNow P2Y12 Reaction Unit for the Influence of Hemoglobin Level. JACC Cardiovasc. Interv. 2016, 9, 1680–1690. [Google Scholar] [CrossRef] [PubMed]
- Ferreiro, J.L.; Bhatt, D.L.; Ueno, M.; Bauer, D.; Angiolillo, D.J. Impact of smoking on long-term outcomes in patients with atherosclerotic vascular disease treated with aspirin or clopidogrel: Insights from the CAPRIE trial (Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events). J. Am. Coll. Cardiol. 2014, 63, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Y.; Song, H.; Hou, C.; Cao, Q.; Dong, K.; Huang, X.; Feng, W.; Ovbiagele, B.; Wang, M.; et al. Clopidogrel and ischemic stroke outcomes by smoking status: Smoker’s paradox? J. Neurol. Sci. 2017, 373, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, T.; Tanguay, J.-F.; Bourassa, M.G. Management of coronary artery disease: Therapeutic options in patients with diabetes. J. Am. Coll. Cardiol. 2000, 36, 355–365. [Google Scholar] [CrossRef]
- Angiolillo, D.J.; Fernandez-Ortiz, A.; Bernardo, E.; Ramirez, C.; Sabate, M.; Jimenez-Quevedo, P.; Hernandez, R.; Moreno, R.; Escaned, J.; Alfonso, F.; et al. Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes 2005, 54, 2430–2435. [Google Scholar] [CrossRef] [PubMed]
- Serebruany, V.; Pokov, I.; Kuliczkowski, W.; Chesebro, J.; Badimon, J. Baseline platelet activity and response after clopidogrel in 257 diabetics among 822 patients with coronary artery disease. Thromb. Haemost. 2008, 100, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Angiolillo, D.J.; Bernardo, E.; Zanoni, M.; Vivas, D.; Capranzano, P.; Malerba, G.; Capodanno, D.; Prandini, P.; Pasquali, A.; Trabetti, E.; et al. Impact of insulin receptor substrate-1 genotypes on platelet reactivity and cardiovascular outcomes in patients with type 2 diabetes mellitus and coronary artery disease. J. Am. Coll. Cardiol. 2011, 58, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, X.; Liu, D.; Liu, T.; Cai, W.; Yan, C.; Han, Y. Association between insulin receptor substrate-1 polymorphisms and high platelet reactivity with clopidogrel therapy in coronary artery disease patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2016, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Angiolillo, D.J.; Jakubowski, J.A.; Ferreiro, J.L.; Tello-Montoliu, A.; Rollini, F.; Franchi, F.; Ueno, M.; Darlington, A.; Desai, B.; Moser, B.; et al. Impaired responsiveness to the platelet P2Y12 receptor antagonist clopidogrel in patients with type 2 diabetes and coronary artery disease. J. Am. Coll. Cardiol. 2014, 64, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Braunwald, E.; Angiolillo, D.J.; Meisel, S.; Dalby, A.J.; Verheugt, F.W.; Goodman, S.G.; Corbalan, R.; Purdy, D.A.; Murphy, S.A.; et al. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction 38. Circulation 2008, 118, 1626–1636. [Google Scholar] [PubMed]
- James, S.; Angiolillo, D.J.; Cornel, J.H.; Erlinge, D.; Husted, S.; Kontny, F.; Maya, J.; Nicolau, J.C.; Spinar, J.; Storey, R.F.; et al. Ticagrelor vs. clopidogrel in patients with acute coronary syndromes and diabetes: A substudy from the PLATelet inhibition and patient outcomes (PLATO) trial. Eur. Heart J. 2010, 31, 3006–3016. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.H.; Tantry, U.S.; Park, Y.; Kwon, T.J.; Park, J.R.; Hwang, S.J.; Bliden, K.P.; Koh, E.H.; Kwak, C.H.; Hwang, J.Y.; et al. Pharmacodynamic effect of cilostazol plus standard clopidogrel versus double-dose clopidogrel in patients with type 2 diabetes undergoing percutaneous coronary intervention. Diabetes Care 2012, 35, 2194–2197. [Google Scholar] [CrossRef] [PubMed]
- Best, P.J.M.; Lennon, R.; Ting, H.H.; Bell, M.R.; Rihal, C.S.; Holmes, D.R.; Berger, P.B. The impact of renal insufficiency on clinical outcomes in patients undergoing percutaneous coronary interventions. J. Am. Coll. Cardiol. 2002, 39, 1113–1119. [Google Scholar] [CrossRef]
- Angiolillo, D.J.; Bernardo, E.; Capodanno, D.; Vivas, D.; Sabate, M.; Ferreiro, J.L.; Ueno, M.; Jimenez-Quevedo, P.; Alfonso, F.; Bass, T.A.; et al. Impact of chronic kidney disease on platelet function profiles in diabetes mellitus patients with coronary artery disease taking dual antiplatelet therapy. J. Am. Coll. Cardiol. 2010, 55, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, O.K.; Smoot, K.J.; Dufour, A.B.; Cho, K.; Young, M.; Gagnon, D.R.; Ly, S.; Temiyasathit, S.; Faxon, D.P.; Gaziano, J.M.; et al. Outcomes with prolonged clopidogrel therapy after coronary stenting in patients with chronic kidney disease. Heart 2015, 101, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Mangiacapra, F.; Cavallari, I.; Barbato, E.; Ricottini, E.; Patti, G.; Vizzi, V.; D’Ambrosio, A.; De Bruyne, B.; Wijns, W.; Di Sciascio, G. Impact of chronic kidney disease on platelet reactivity and outcomes of patients receiving clopidogrel and undergoing percutaneous coronary intervention. Am. J. Cardiol. 2014, 113, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Baber, U.; Mehran, R.; Kirtane, A.J.; Gurbel, P.A.; Christodoulidis, G.; Maehara, A.; Witzenbichler, B.; Weisz, G.; Rinaldi, M.J.; Metzger, D.C.; et al. Prevalence and impact of high platelet reactivity in chronic kidney disease: results from the assessment of dual antiplatelet therapy with drug-eluting stents registry. Circ. Cardiovasc. Interv. 2015, 8, e001683. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.C.; Di Micco, L.; Razavian, M.; Craig, J.C.; Perkovic, V.; Pellegrini, F.; Copetti, M.; Graziano, G.; Tognoni, G.; Jardine, M.; et al. Effects of antiplatelet therapy on mortality and cardiovascular and bleeding outcomes in persons with chronic kidney disease: A systematic review and meta-analysis. Ann. Intern. Med. 2012, 156, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Weisz, G.; Smilowitz, N.R.; Kirtane, A.J.; Rinaldi, M.J.; Parvataneni, R.; Xu, K.; Stuckey, T.D.; Maehara, A.; Witzenbichler, B.; Neumann, F.J.; et al. Proton pump inhibitors, platelet reactivity, and cardiovascular outcomes after drug-eluting stents in clopidogrel-treated patients: The ADAPT-DES study. Circ. Cardiovasc. Interv. 2015, 8, e001952. [Google Scholar] [PubMed]
- Harvey, A.; Modak, A.; Dery, U.; Roy, M.; Rinfret, S.; Bertrand, O.F.; Larose, E.; Rodes-Cabau, J.; Barbeau, G.; Gleeton, O.; et al. Changes in CYP2C19 enzyme activity evaluated by the [(13)C]-pantoprazole breath test after co-administration of clopidogrel and proton pump inhibitors following percutaneous coronary intervention and correlation to platelet reactivity. J. Breath Res. 2016, 10, 017104. [Google Scholar] [CrossRef] [PubMed]
- Melloni, C.; Washam, J.B.; Jones, W.S.; Halim, S.A.; Hasselblad, V.; Mayer, S.B.; Heidenfelder, B.L.; Dolor, R.J. Conflicting results between randomized trials and observational studies on the impact of proton pump inhibitors on cardiovascular events when coadministered with dual antiplatelet therapy: Systematic review. Circ. Cardiovasc. Qual. Outcomes 2015, 8, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Cannon, C.P.; Cryer, B.L.; Liu, Y.; Hsieh, W.H.; Doros, G.; Cohen, M.; Lanas, A.; Schnitzer, T.J.; Shook, T.L.; et al. Efficacy and safety of proton-pump inhibitors in high-risk cardiovascular subsets of the COGENT trial. Am. J. Med. 2016, 129, 1002–1005. [Google Scholar] [CrossRef] [PubMed]
- Siller-Matula, J.M.; Lang, I.; Christ, G.; Jilma, B. Calcium-channel blockers reduce the antiplatelet effect of clopidogrel. J. Am. Coll. Cardiol. 2008, 52, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Park, K.W.; Kang, J.; Jeon, K.-H.; Kang, S.-H.; Ahn, H.S.; Han, J.-K.; Koh, J.-S.; Lee, S.E.; Yang, H.-M.; et al. CYP3A4 genetic status may be associated with increased vulnerability to the inhibitory effect of calcium-channel blockers on clopidogrel. Circ. J. 2013, 77, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Harmsze, A.M.; Robijns, K.; van Werkum, J.W.; Breet, N.J.; Hackeng, C.M.; Ten Berg, J.M.; Ruven, H.J.; Klungel, O.H.; de Boer, A.; Deneer, V.H. The use of amlodipine, but not of P-glycoprotein inhibiting calcium channel blockers is associated with clopidogrel poor-response. Thromb. Haemost. 2010, 103, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.C. Atorvastatin reduces the ability of clopidogrel to inhibit platelet aggregation: A new drug-drug interaction. Circulation 2002, 107, 32–37. [Google Scholar] [CrossRef]
- Neubauer, H. Lipophilic statins interfere with the inhibitory effects of clopidogrel on platelet function—A flow cytometry study. Eur. Heart J. 2003, 24, 1744–1749. [Google Scholar] [CrossRef]
- Park, Y.; Jeong, Y.H.; Tantry, U.S.; Ahn, J.H.; Kwon, T.J.; Park, J.R.; Hwang, S.J.; Gho, E.H.; Bliden, K.P.; Kwak, C.H.; et al. Accelerated platelet inhibition by switching from atorvastatin to a non-CYP3A4-metabolized statin in patients with high platelet reactivity (ACCEL-STATIN) study. Eur. Heart J. 2012, 33, 2151–2162. [Google Scholar] [CrossRef] [PubMed]
- Wienbergen, H.; Gitt, A.K.; Schiele, R.; Juenger, C.; Heer, T.; Meisenzahl, C.; Limbourg, P.; Bossaller, C.; Senges, J. Comparison of clinical benefits of clopidogrel therapy in patients with acute coronary syndromes taking atorvastatin versus other statin therapies. Am. J. Cardiol. 2003, 92, 285–288. [Google Scholar] [CrossRef]
- Ojeifo, O.; Wiviott, S.D.; Antman, E.M.; Murphy, S.A.; Udell, J.A.; Bates, E.R.; Mega, J.L.; Sabatine, M.S.; O’Donoghue, M.L. Concomitant administration of clopidogrel with statins or calcium-channel blockers: Insights from the TRITON-TIMI 38 (trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction 38). JACC Cardiovasc. Interv. 2013, 6, 1275–1281. [Google Scholar] [PubMed]
- Saw, J.; Brennan, D.M.; Steinhubl, S.R.; Bhatt, D.L.; Mak, K.H.; Fox, K.; Topol, E.J.; Investigators, C. Lack of evidence of a clopidogrel-statin interaction in the CHARISMA trial. J. Am. Coll. Cardiol. 2007, 50, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Hobl, E.L.; Stimpfl, T.; Ebner, J.; Schoergenhofer, C.; Derhaschnig, U.; Sunder-Plassmann, R.; Jilma-Stohlawetz, P.; Mannhalter, C.; Posch, M.; Jilma, B. Morphine decreases clopidogrel concentrations and effects: A randomized, double-blind, placebo-controlled trial. J. Am. Coll. Cardiol. 2014, 63, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Nimmo, W.S.; Heading, R.C.; Wilson, J.; Tothill, P.; Prescott, L.F. Inhibition of gastric emptying and drug absorption by narcotic analgesics. Br. J. Clin. Pharmacol. 1975, 2, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Parodi, G.; Bellandi, B.; Xanthopoulou, I.; Capranzano, P.; Capodanno, D.; Valenti, R.; Stavrou, K.; Migliorini, A.; Antoniucci, D.; Tamburino, C.; et al. Morphine is associated with a delayed activity of oral antiplatelet agents in patients with ST-elevation acute myocardial infarction undergoing primary percutaneous coronary intervention. Circ. Cardiovasc. Interv. 2015, 8, e001593. [Google Scholar] [CrossRef] [PubMed]
- Kubica, J.; Adamski, P.; Ostrowska, M.; Sikora, J.; Kubica, J.M.; Sroka, W.D.; Stankowska, K.; Buszko, K.; Navarese, E.P.; Jilma, B.; et al. Morphine delays and attenuates ticagrelor exposure and action in patients with myocardial infarction: The randomized, double-blind, placebo-controlled IMPRESSION trial. Eur. Heart J. 2016, 37, 245–252. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.H.; Bhatti, S.K.; Patil, H.R.; DiNicolantonio, J.J.; Lucan, S.C.; Lavie, C.J. Effects of habitual coffee consumption on cardiometabolic disease, cardiovascular health, and all-cause mortality. J. Am. Coll. Cardiol. 2013, 62, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Varani, K.; Portaluppi, F.; Gessi, S.; Merighi, S.; Ongini, E.; Belardinelli, L.; Borea, P.A. Dose and time effects of caffeine intake on human platelet adenosine A2A receptors functional and biochemical aspects. Circulation 2000, 102, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Lev, E.I.; Arikan, M.E.; Vaduganathan, M.; Alviar, C.L.; Tellez, A.; Mathuria, N.; Builes, A.; Granada, J.F.; del Conde, I.; Kleiman, N.S. Effect of caffeine on platelet inhibition by clopidogrel in healthy subjects and patients with coronary artery disease. Am. Heart J. 2007, 154, 694e1–694e7. [Google Scholar] [CrossRef] [PubMed]
| Polymorphisms | Samples | Influence on Pharmacokinetics and Pharmacodynamics | Influence on Clinical Outcome | References |
|---|---|---|---|---|
| ABCB1 C3435T | 60 CAD | Lower exposure to clop-AM | NA | [4] |
| 2208 AMI | NA | Increase in cardiovascular risk | [5] | |
| 2188 PCI-treated | Higher on-treatment platelet reactivity | Increase in cardiovascular risk | [6] | |
| NA | No negative effect on platelet reactivity | Increase in cardiovascular risk | [7] | |
| 401 ACS | Lower exposure to clop-AM and CLP | NA | [8] | |
| Higher on-treatment platelet reactivity | ||||
| 123 AMI | Lower exposure to CLP and 2-oxo- CLP | NA | [9] | |
| 42 PCI-treated | Lower exposure to CLP | NA | [10] | |
| 10153 subjects | NA | Inconclusive | [11] | |
| 1524 PCI-treated | inconclusive | NA | [12] | |
| CES1 rs8192950 | 377 ischemic stroke | NA | Decrease in cardiovascular risk | [13] |
| G143E | 566 healthy volunteers | Higher exposure to clop-AM | NA | [14] |
| 350 CAD | Lower on-treatment platelet reactivity | |||
| 1109 healthy volunteers | Higher exposure to clop-AM | NA | [15] | |
| Lower on-treatment platelet reactivity | ||||
| CES1P1 rs3785161 | 162 CAD | Higher on-treatment platelet reactivity | NA | [16] |
| PON1 Q192R | Many groups | Higher exposure to clop-AM | Decrease in cardiovascular risk | [17] |
| Lower on-treatment platelet reactivity | ||||
| 482 CAD | Inconclusive | NA | [18] | |
| 275 healthy volunteers | Inconclusive | Inconclusive | [19] | |
| 2922 ACS | ||||
| CYP2C19*2*3 | 28 healthy volunteers | Higher on-treatment platelet reactivity | NA | [20] |
| 110 ACS | Higher on-treatment platelet reactivity | Increase in cardiovascular risk | [21] | |
| 4819 atherothrombosis | NA | Decrease in bleeding risk but not increase in cardiovascular | [22] | |
| 429 healthy volunteers | Higher on-treatment platelet reactivity | Increase in cardiovascular risk | [23] | |
| 227 PCI-treated | ||||
| 162 healthy volunteers | Lower exposure to clop-AM | [24] | ||
| Higher on-treatment platelet reactivity | ||||
| 1477 ACS | Increase in cardiovascular risk | |||
| 259 MI | NA | Increase in cardiovascular risk | [25] | |
| 366 CAD | Lower exposure to CLP | NA | [26] | |
| *17 | 1524 PCI-treated | Lower on-treatment platelet reactivity | Increase in bleeding risk but not in cardiovascular | [27] |
| 820 CVD | Lower on-treatment platelet reactivity | Increase in bleeding risk | [28] | |
| CYP3A4*1G | 82 PCI-treated | Inconclusive | NA | [29] |
| CYP3A5*3 | 101 angina | Higher on-treatment platelet reactivity | NA | [30] |
| 35 healthy volunteers | Higher on-treatment platelet reactivity | NA | [31] | |
| 1258 PCI-treated | Higher on-treatment platelet reactivity | Increase in cardiovascular risk | [32] | |
| P2RY12 H2 | 98 healthy volunteers | Higher on-treatment platelet reactivity | NA | [33] |
| A-F | 1031 CAD | Higher on-treatment platelet reactivity | NA | [34] |
| T774C | 597 ACS | Inconclusive | NA | [35] |
| PEAR1 rs12041331 | 104 healthy volunteers | Platelet aggregation | NA | [36] |
| rs56260937 | ||||
| rs41273215 | 204 CHD | Higher on-treatment platelet reactivity | NA | [37] |
| rs57731889 | Lower on-treatment platelet reactivity | |||
| rs2768759 | 1486 healthy volunteers | Higher on-treatment platelet reactivity | NA | [38] |
| rs11264579 | 500 healthy volunteers | Higher on-treatment platelet reactivity | NA | [39] |
| rs12041331 | 565 healthy volunteers | Higher on-treatment platelet reactivity | Increase in cardiovascular risk | [40] |
| 227 PCI-treated | ||||
| 1000 CAD |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-J.; Li, M.-P.; Tang, J.; Chen, X.-P. Pharmacokinetic and Pharmacodynamic Responses to Clopidogrel: Evidences and Perspectives. Int. J. Environ. Res. Public Health 2017, 14, 301. https://doi.org/10.3390/ijerph14030301
Zhang Y-J, Li M-P, Tang J, Chen X-P. Pharmacokinetic and Pharmacodynamic Responses to Clopidogrel: Evidences and Perspectives. International Journal of Environmental Research and Public Health. 2017; 14(3):301. https://doi.org/10.3390/ijerph14030301
Chicago/Turabian StyleZhang, Yan-Jiao, Mu-Peng Li, Jie Tang, and Xiao-Ping Chen. 2017. "Pharmacokinetic and Pharmacodynamic Responses to Clopidogrel: Evidences and Perspectives" International Journal of Environmental Research and Public Health 14, no. 3: 301. https://doi.org/10.3390/ijerph14030301





