Fat Mass Is Positively Associated with Estimated Hip Bone Strength among Chinese Men Aged 50 Years and above with Low Levels of Lean Mass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Body Composition, BMD and Hip Geometry Measurements
2.3. Covariates Assessment
2.4. Statistical Analysis
3. Results
3.1. General Characteristics of the Subjects
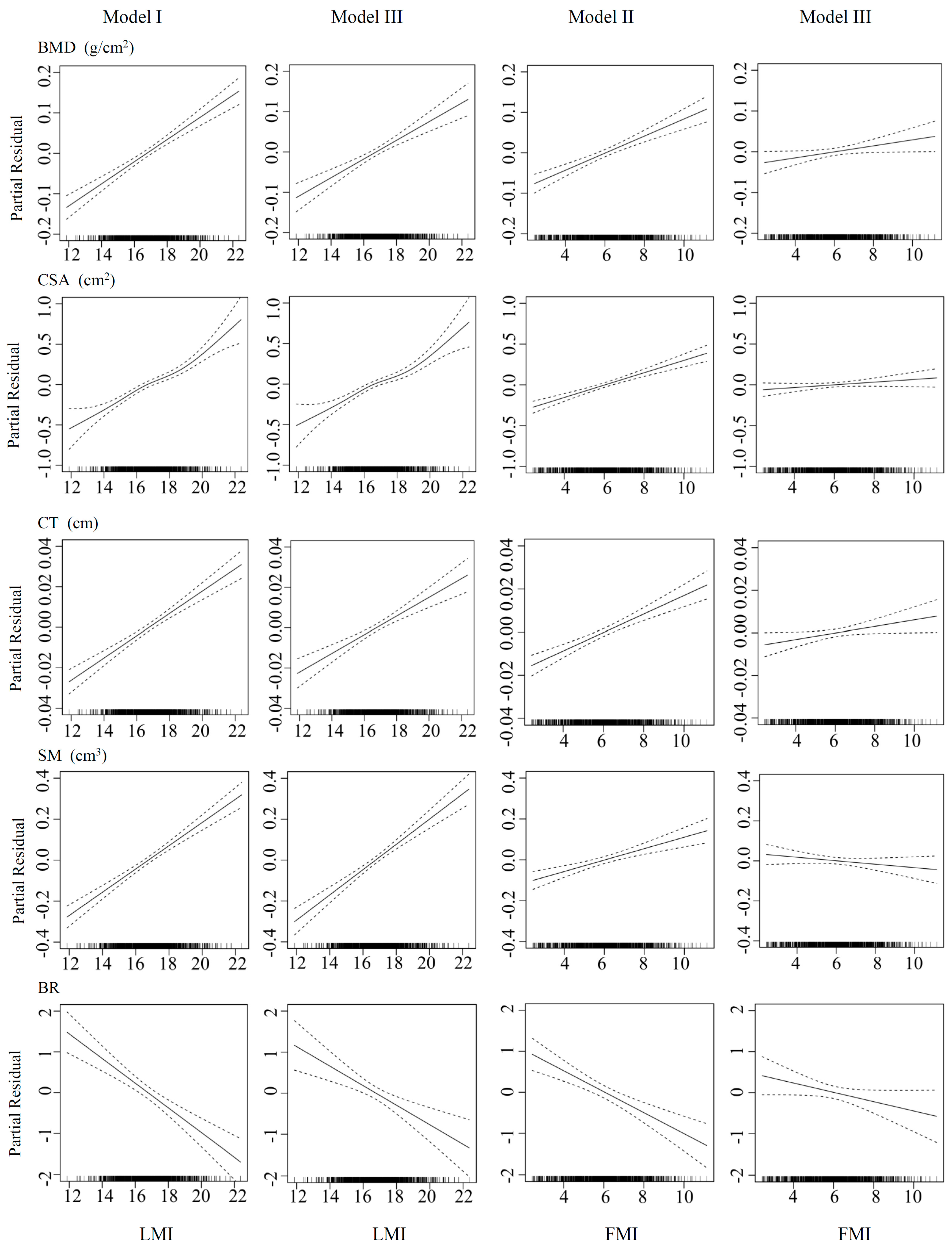
3.2. Associations of FMI and LMI with Bone Phenotypes
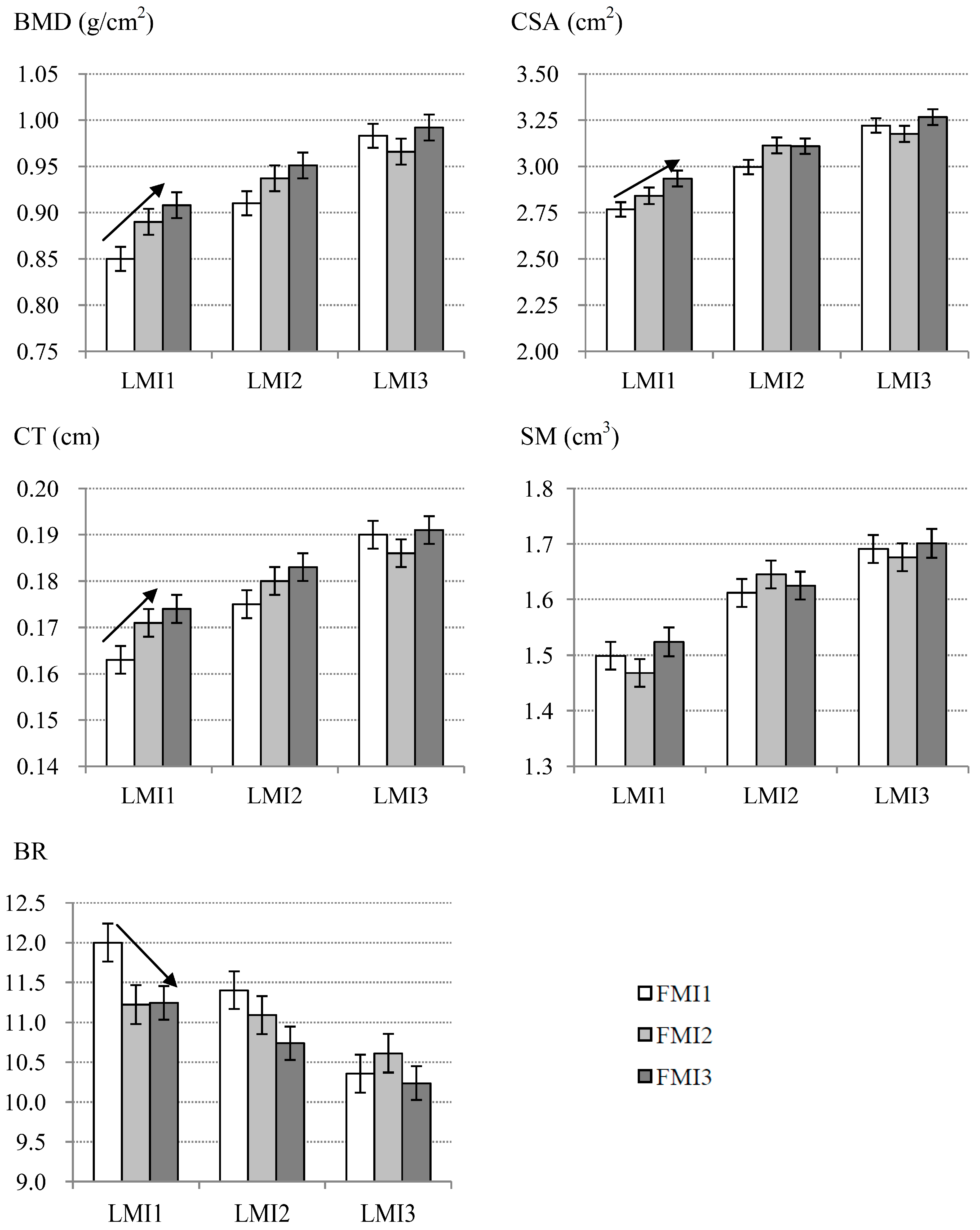
3.3. Specific Associations between FMI and Bone Phenotypes within Each LMI Subgroup
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kanis, J.A. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 2002, 359, 1929–1936. [Google Scholar] [CrossRef]
- Silva, M.J. Biomechanics of osteoporotic fractures. Injury 2007, 38 (Suppl. 3), S69–S76. [Google Scholar] [CrossRef] [PubMed]
- Kaptoge, S.; Beck, T.J.; Reeve, J.; Stone, K.L.; Hillier, T.A.; Cauley, J.A.; Cummings, S.R. Prediction of incident hip fracture risk by femur geometry variables measured by hip structural analysis in the study of osteoporotic fractures. J. Bone Miner. Res. 2008, 23, 1892–1904. [Google Scholar] [CrossRef] [PubMed]
- LaCroix, A.Z.; Beck, T.J.; Cauley, J.A.; Lewis, C.E.; Bassford, T.; Jackson, R.; Wu, G.; Chen, Z. Hip structural geometry and incidence of hip fracture in postmenopausal women: What does it add to conventional bone mineral density? Osteoporos. Int. 2010, 21, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Ito, M. Recent progress in bone imaging for osteoporosis research. J. Bone Miner. Metab. 2011, 29, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Palermo, L.; Black, D.M.; Eastell, R. Prediction of incident hip fracture with the estimated femoral strength by finite element analysis of DXA scans in the study of osteoporotic fractures. J. Bone Miner. Res. 2014, 29, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- Ahlborg, H.G.; Nguyen, N.D.; Nguyen, T.V.; Center, J.R.; Eisman, J.A. Contribution of hip strength indices to hip fracture risk in elderly men and women. J. Bone Miner. Res. 2005, 20, 1820–1827. [Google Scholar] [CrossRef] [PubMed]
- De Laet, C.; Kanis, J.A.; Oden, A.; Johanson, H.; Johnell, O.; Delmas, P.; Eisman, J.A.; Kroger, H.; Fujiwara, S.; Garnero, P.; et al. Body mass index as a predictor of fracture risk: A meta-analysis. Osteoporos. Int. 2005, 16, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Beck, T.J.; Petit, M.A.; Wu, G.; LeBoff, M.S.; Cauley, J.A.; Chen, Z. Does obesity really make the femur stronger? BMD, geometry, and fracture incidence in the women’s health initiative-observational study. J. Bone Miner. Res. 2009, 24, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.L.; Paggiosi, M.A.; Eastell, R.; Walsh, J.S. Bone density, microstructure and strength in obese and normal weight men and women in younger and older adulthood. J. Bone Miner. Res. 2015, 30, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Ho-Pham, L.T.; Nguyen, U.D.; Nguyen, T.V. Association between lean mass, fat mass, and bone mineral density: A meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, M.; Buettner, C.; Sun, L.; Iqbal, J. Minireview: The link between fat and bone: Does mass beget mass? Endocrinology 2012, 153, 2070–2075. [Google Scholar] [CrossRef] [PubMed]
- Travison, T.G.; Araujo, A.B.; Esche, G.R.; Beck, T.J.; McKinlay, J.B. Lean mass and not fat mass is associated with male proximal femur strength. J. Bone Miner. Res. 2008, 23, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Leslie, W.D.; Orwoll, E.S.; Nielson, C.M.; Morin, S.N.; Majumdar, S.R.; Johansson, H.; Oden, A.; McCloskey, E.V.; Kanis, J.A. Estimated lean mass and fat mass differentially affect femoral bone density and strength index but are not frax independent risk factors for fracture. J. Bone Miner. Res. 2014, 29, 2511–2519. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Ma, X.; Lu, H.; He, W.; Wang, Z.; Zhu, S. Associations of fat mass and fat distribution with bone mineral density in pre- and postmenopausal Chinese women. Osteoporos. Int. 2011, 22, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.S. Relationship of lean body mass with bone mass and bone mineral density in the general Korean population. Endocrine 2014, 47, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Arguelles, L.M.; Liu, X.; Tsai, H.J.; Hsu, Y.H.; Wang, B.; Zhang, S.; Li, Z.; Tang, G.; Liu, X.; et al. Percent fat mass is inversely associated with bone mass and hip geometry in rural chinese adolescents. J. Bone Miner. Res. 2010, 25, 1544–1554. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Kim, S.; Kim, K.H.; Park, S.M. Importance of fat mass and lean mass on bone health in men: The fourth Korean national health and nutrition examination survey (KNHANES IV). Osteoporos. Int. 2014, 25, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Nur, H.; Toraman, N.F.; Arica, Z.; Sarier, N.; Samur, A. The relationship between body composition and bone mineral density in postmenopausal Turkish women. Rheumatol. Int. 2013, 33, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Mizuma, N.; Mizuma, M.; Yoshinaga, M.; Iwamoto, I.; Matsuo, T.; Douchi, T.; Osame, M. Difference in the relative contribution of lean and fat mass components to bone mineral density with generation. J. Obstet. Gynaecol. Res. 2006, 32, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Kouda, K.; Fujita, Y.; Sato, Y.; Ohara, K.; Nakamura, H.; Uenishi, K.; Iki, M. Fat mass is positively associated with bone mass in relatively thin adolescents: Data from Kitakata Kids Health Study. Bone 2014, 64, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, C.; Guan, K.; Xu, Y.; Su, Y.X.; Chen, Y.M. Association of magnesium in serum and urine with carotid intima-media thickness and serum lipids in middle-aged and elderly chinese: A community-based cross-sectional study. Eur. J. Nutr. 2016, 55, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Beck, T.J. Extending dxa beyond bone mineral density: Understanding hip structure analysis. Curr. Osteoporos. Rep. 2007, 5, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, G.; Pan, X. China Food Composition; Peking University Medical Press: Beijing, China, 2002. [Google Scholar]
- Wang, P.; Chen, Y.M.; He, L.P.; Chen, C.G.; Zhang, B.; Xue, W.Q.; Su, Y.X. Association of natural intake of dietary plant sterols with carotid intima-media thickness and blood lipids in chinese adults: A cross-section study. PLoS ONE 2012, 7, e32736. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.F. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults—Study on optimal cut-off points of body mass index and waist circumference in chinese adults. Biomed. Environ. Sci. 2002, 15, 83–96. [Google Scholar] [PubMed]
- Reid, I.R. Fat and bone. Arch. Biochem. Biophys. 2010, 503, 20–27. [Google Scholar] [CrossRef] [PubMed]
- The Chinese Nutrition Society. Chinese Dietary Reference Intakes; China Light Industry Press: Beijing, China, 2000. [Google Scholar]
- El Hage, R. Geometric indices of hip bone strength in obese, overweight, and normal-weight adolescent boys. Osteoporos. Int. 2012, 23, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Sjoblom, S.; Suuronen, J.; Rikkonen, T.; Honkanen, R.; Kroger, H.; Sirola, J. Relationship between postmenopausal osteoporosis and the components of clinical sarcopenia. Maturitas 2013, 75, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Verschueren, S.; Gielen, E.; O’Neill, T.W.; Pye, S.R.; Adams, J.E.; Ward, K.A.; Wu, F.C.; Szulc, P.; Laurent, M.; Claessens, F.; et al. Sarcopenia and its relationship with bone mineral density in middle-aged and elderly european men. Osteoporos. Int. 2013, 24, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Leung, J.; Woo, J. Incremental predictive value of sarcopenia for incident fracture in an elderly Chinese cohort: Results from the osteoporotic fractures in men (MrOs) study. J. Am. Med. Dir. Assoc. 2014, 15, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, C.; Wittrant, Y.; Davicco, M.J.; Walrand, S.; Coxam, V. Muscle and bone, two interconnected tissues. Ageing Res. Rev. 2015, 21, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Matsumoto, E.; Higashimaki, Y.; Katagiri, T.; Sugimoto, T.; Seino, S.; Kaji, H. Role of osteoglycin in the linkage between muscle and bone. J. Biol. Chem. 2012, 287, 11616–11628. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, M.W.; McNeil, P.L.; Patterson, S.L. Role of muscle-derived growth factors in bone formation. J. Musculoskelet. Neuronal Interact. 2010, 10, 64–70. [Google Scholar] [PubMed]
- Kawao, N.; Kaji, H. Interactions between muscle tissues and bone metabolism. J. Cell. Biochem. 2015, 116, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Bialek, P.; Parkington, J.; Li, X.; Gavin, D.; Wallace, C.; Zhang, J.; Root, A.; Yan, G.; Warner, L.; Seeherman, H.J.; et al. A myostatin and activin decoy receptor enhances bone formation in mice. Bone 2014, 60, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Ilich, J.Z.; Kelly, O.J.; Inglis, J.E.; Panton, L.B.; Duque, G.; Ormsbee, M.J. Interrelationship among muscle, fat, and bone: Connecting the dots on cellular, hormonal, and whole body levels. Ageing Res. Rev. 2014, 15, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Di Comite, M.; Mori, G.; et al. The myokine irisin increases cortical bone mass. Proc. Natl. Acad. Sci. USA 2015, 112, 12157–12162. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostrom, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Dincer, F.; Mesfum, E.; Mantzoros, C.S. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int. J. Obes. (Lond.) 2014, 38, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Argiles, J.M.; Lopez-Soriano, J.; Almendro, V.; Busquets, S.; Lopez-Soriano, F.J. Cross-talk between skeletal muscle and adipose tissue: A link with obesity? Med. Res. Rev. 2005, 25, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Elefteriou, F.; Levasseur, R.; Liu, X.; Zhao, L.; Parker, K.L.; Armstrong, D.; Ducy, P.; Karsenty, G. Leptin regulates bone formation via the sympathetic nervous system. Cell 2002, 111, 305–317. [Google Scholar] [CrossRef]
- Travison, T.G.; Araujo, A.B.; Esche, G.R.; McKinlay, J.B. The relationship between body composition and bone mineral content: Threshold effects in a racially and ethnically diverse group of men. Osteoporos. Int. 2008, 19, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthi, K.; Ahmad, O.; Engelke, K.; Taylor, R.H.; Zhu, K.; Gustafsson, S.; Prince, R.L.; Wilson, K.E. An in vivo comparison of hip structure analysis (HSA) with measurements obtained by QCT. Osteoporos. Int. 2012, 23, 543–551. [Google Scholar] [CrossRef] [PubMed]
| Variable | Mean ± SD/Median (25th–75th)/n (%) |
|---|---|
| Age (years) | 62.0 (58.0–66.0) |
| Height (cm) | 165.9 ± 5.7 |
| Weight (kg) | 66.2 ± 9.3 |
| BMI (kg/m2) | 24.0 ± 2.9 |
| Obesity status | |
| underweight | 17 (1.9%) |
| normal | 439 (49.4%) |
| overweight | 362 (40.7%) |
| obese | 71 (8.0%) |
| Physical activity (MET·h/d) a | 13.9 (9.8–24.1) |
| Smoking status | |
| yes | 309 (34.8%) |
| no | 580 (65.2%) |
| Drinking status | |
| yes | 154 (17.3%) |
| no | 735 (82.7) |
| Calcium supplement intake | |
| yes | 173 (19.5%) |
| no | 716 (80.5%) |
| Dietary-calcium intake (mg/d) | 537.8 (401.7–689.1) |
| Dietary-protein intake (g/d) | 75.7 (59.4–87.8) |
| Energy intake (kcal/d) | 1810.4 (1477.7–2067.0) |
| Percent fat mass (%) | 25.5 (22.6–28.1) |
| FM (kg) | 16.7 ± 4.5 |
| FMI (kg/m2) | 6.0 ± 1.6 |
| LM (kg) | 46.2 ± 5.4 |
| LMI (kg/m2) | 16.8 ± 1.6 |
| BMD (g/cm2) | 0.932 ± 0.143 |
| CSA (cm2) | 3.047 ± 0.478 |
| CT (cm) | 0.179 ± 0.029 |
| SM (cm3) | 1.604 ± 0.298 |
| BR | 10.988 ± 2.332 |
| Variable | Model I | Model II | Model III | Partial R2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | β | SE | p | ||
| BMD (g/cm2) | ||||||||||
| LMI (kg/m2) | 0.027 | 0.003 | <0.001 | 0.023 | 0.003 | <0.001 | 0.088 | |||
| FMI (kg/m2) | 0.021 | 0.003 | <0.001 | 0.007 | 0.004 | 0.040 | 0.050 | |||
| CSA (cm2) | ||||||||||
| LMI (kg/m2) | 0.110 | 0.009 | <0.001 | 0.101 | 0.011 | <0.001 | 0.128 | |||
| FMI (kg/m2) | 0.075 | 0.009 | <0.001 | 0.016 | 0.011 | 0.147 | 0.057 | |||
| CT (cm) | ||||||||||
| LMI (kg/m2) | 0.006 | 0.001 | <0.001 | 0.005 | 0.001 | <0.001 | 0.084 | |||
| FMI (kg/m2) | 0.004 | 0.001 | <0.001 | 0.002 | 0.001 | 0.039 | 0.048 | |||
| SM (cm3) | ||||||||||
| LMI (kg/m2) | 0.057 | 0.005 | <0.001 | 0.062 | 0.006 | <0.001 | 0.087 | |||
| FMI (kg/m2) | 0.028 | 0.006 | <0.001 | −0.008 | 0.007 | 0.201 | 0.020 | |||
| BR | ||||||||||
| LMI (kg/m2) | −0.302 | 0.049 | <0.001 | −0.238 | 0.060 | <0.001 | 0.040 | |||
| FMI (kg/m2) | −0.254 | 0.050 | <0.001 | −0.114 | 0.061 | 0.061 | 0.027 | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, G.; Chen, Y.-M.; Huang, H.; Chen, Z.; Jing, L.; Xiao, S.-M. Fat Mass Is Positively Associated with Estimated Hip Bone Strength among Chinese Men Aged 50 Years and above with Low Levels of Lean Mass. Int. J. Environ. Res. Public Health 2017, 14, 453. https://doi.org/10.3390/ijerph14040453
Han G, Chen Y-M, Huang H, Chen Z, Jing L, Xiao S-M. Fat Mass Is Positively Associated with Estimated Hip Bone Strength among Chinese Men Aged 50 Years and above with Low Levels of Lean Mass. International Journal of Environmental Research and Public Health. 2017; 14(4):453. https://doi.org/10.3390/ijerph14040453
Chicago/Turabian StyleHan, Guiyuan, Yu-Ming Chen, Hua Huang, Zhanyong Chen, Lipeng Jing, and Su-Mei Xiao. 2017. "Fat Mass Is Positively Associated with Estimated Hip Bone Strength among Chinese Men Aged 50 Years and above with Low Levels of Lean Mass" International Journal of Environmental Research and Public Health 14, no. 4: 453. https://doi.org/10.3390/ijerph14040453






