Developing a Water Quality Index (WQI) for an Irrigation Dam
Abstract
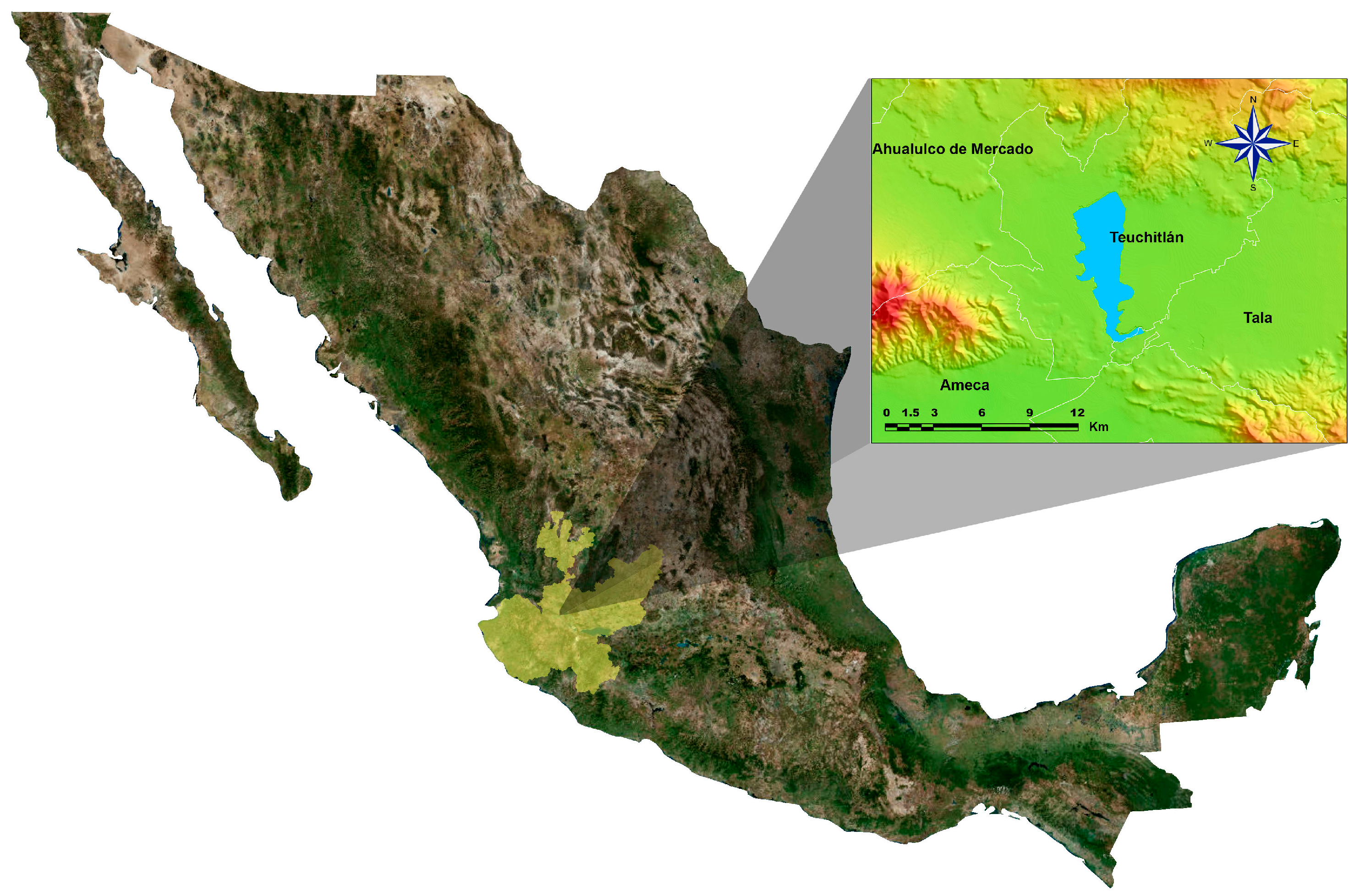
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characteristics Water in the La Vega Dam Reservoir
3.2. Water Quality Index
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sánchez, E.; Colmenarejo, M.F.; Vicente, J.; Rubio, A.; García, M.G.; Travieso, L.; Borja, R. Use of the water quality index and dissolved oxygen deficit as simple indicators of watersheds pollution. Ecol. Indic. 2007, 7, 315–328. [Google Scholar] [CrossRef]
- Simeonov, V.; Stratis, J.A.; Samara, C.; Zachariadis, G.; Voutsa, D.; Anthemidis, A.; Sofoniou, M.; Kouimtzis, T. Assessment of the surface water quality in Northern Greece. Water Res. 2003, 37, 4119–4124. [Google Scholar] [CrossRef]
- Brainwood, M.A.; Burgin, S.; Maheshwari, B. Temporal variations in water quality of farm dams: Impacts of land use and water sources. Agric. Water Manag. 2004, 70, 151–175. [Google Scholar] [CrossRef]
- Gruszowski, K.E.; Foster, I.D.; Lees, J.A.; Charlesworth, S.M. Sediment sources and transport pathways in a rural catchment, Herefordshire, UK. Hydrol. Process. 2003, 17, 2665–2681. [Google Scholar] [CrossRef]
- De la Mora-Orozco, C.; Rubio-Arias, H.; García-Velasco, J. Índice de calidad de agua en el lago de Chapala, Jalisco, México. In Contribución al Estudio de los Servicios Ambientales; Libro Técnico Num. 1; CIRPAC-INIFAP: Mexico City, Mexico, 2005; pp. 33–52. [Google Scholar]
- Zalidis, G.; Stamatiadis, S.; Takavakoglou, V.; Eskridge, K.; Misopolinos, N. Impacts of agricultural practices on soil and water quality in the Mediterranean region and proposed assessment methodology. Agric. Ecosyst. Environ. 2002, 88, 137–146. [Google Scholar] [CrossRef]
- Tong, T.Y.S.; Chen, W. Modeling the relationship between land use and surface water quality. J. Environ. Manag. 2002, 66, 377–393. [Google Scholar] [CrossRef]
- Beamonte, C.E.; Martínez, C.A.; Ferrer, V.E. Water quality indicators: Comparison of a probabilistic index and a general quality index. The case of the Confederación Hidrográfica del Júcar (Spain). Ecol. Indic. 2010, 10, 1049–1054. [Google Scholar] [CrossRef]
- Pesce, S.F.; Wunderlin, D.A. Use of water quality indices to verify the impact of Cordoba City (Argentina) on Suquía River. Water Res. 2000, 34, 2915–2926. [Google Scholar] [CrossRef]
- Jonnalagadda, S.B.; Mhere, G. Water quality of the Odzi River in the eastern highlands of Zimbabwe. Water Res. 2001, 35, 2371–2376. [Google Scholar] [CrossRef]
- Wei, M.; Nan, Z.; Yuan, Z.; Binghui, Z. Integrated assessment of river health based on water quality, aquatic life and physical habitat. J. Environ. Sci. 2009, 21, 1017–1027. [Google Scholar]
- Bordalo, A.A.; Nilsumranchi, W.; Chalermwat, K. Water quality and uses of the Bangpakong River (Eastern Thailand). Water Res. 2001, 35, 3635–3642. [Google Scholar] [CrossRef]
- Swamee, P.K.; Tyagi, A. Describing water quality with aggregate index. J. Environ. Eng. 2000, 126, 451–455. [Google Scholar] [CrossRef]
- Cude, C.G. Oregon water quality index: A tool for evaluating water quality management effectiveness. J. Am. Water Resour. Assoc. 2001, 37, 125–137. [Google Scholar] [CrossRef]
- Nagel, J.W.; Davies-Colley, R.J.; Smith, D.G. A water quality index for contact recreation in New Zealand. Water Sci. Technol. 2001, 43, 285–292. [Google Scholar]
- Liou, S.M.; Lo, S.L.; Hu, C.Y. Application of two-stage fuzzy set theory to river quality evaluation in Taiwan. Water Res. 2003, 37, 1406–1416. [Google Scholar] [CrossRef]
- Barrel, R.A.E.; Hunter, P.R.; Nichols, G. Microbiological standards for water and their relationship to health risk. Commun. Dis. Public Health 2000, 3, 8–13. [Google Scholar]
- Barrera-Escorcia, G.; Fernández-Rendón, C.L.; Wong-Chang, I.; Ramírez, P.R. La sensibilidad del grupo coliforme como indicador de la presencia de enterobacterias patógenas en cuatro cuerpos acuáticos de México. Hidrobiológica 2013, 23, 87–96. [Google Scholar]
- Tomasini-Ortiz, A.C.; Moeller-Chávez, G.; Sánchez-Chávez, J.J.; Bravo-Inclán, L.A. Cianobacterias y cianotoxinas en el Lago de Pátzcuaro, Michoacán, México. Revista AIDIS de Ingeniería y Ciencias Ambientales: Investigación Desarrollo y Práctica 2012, 5, 93–101. [Google Scholar]
- Rosales-Hoz, L.; Cundy, A.B.; Bahena-Manjarrez, J.L. Heavy metals in sediment cores from a tropical estuary affected by anthropogenic discharges: Coatzacoalcos estuary, Mexico. Estuar. Coast. Shelf Sci. 2003, 58, 117–126. [Google Scholar] [CrossRef]
- De La Mora-Orozco, C.; Flores-López, H.E.; Chávez-Durán, A.A. Calidad del Agua de la Presa La Vega y su Impacto en Tierras Agrícolas Bajo Riego; Libro Técnico Num. 6; CIRPAC-INIFAP: Guadalajara, Mexico, 2013; pp. 65–101. [Google Scholar]
- Comisión Estatal del Agua de Jalisco (CEA). Reportes de Monitoreo y Análisis de Calidad del Agua; Laboratorio de Calidad del Agua de la Comisión Estatal del Agua de Jalisco: Guadalajara, Mexico, 2008; p. 55. [Google Scholar]
- Norma Mexicana NMX-AA-014-1980. Cuerpos Receptores—Muestreo/Receiver Bodies—Sampling; Secretaría de Comercio y Fomento Industrial: Mexico City, Mexico, 1980. [Google Scholar]
- Norma Mexicana NMX-AA-008-SCFI-2000. Análisis de Agua—Determinación del pH—Método de Prueba/Water Analysis—Determination of pH—Test Method; Secretaría de Comercio y Fomento Industrial: Mexico City, Mexico, 2000. [Google Scholar]
- Norma Mexicana NMX-AA-093-SCFI-2000. Análisis de Agua—Determinación de la Conductividad Electrolítica—Método de Prueba/Water Analysis—Determination of Electrolitical Conductivity—Test Method; Secretaría de Comercio y Fomento Industrial: Mexico City, Mexico, 2000. [Google Scholar]
- Norma Mexicana NMX-AA-012-SCFI-2001. Análisis de Agua—Determinación de Oxígeno Disuelto en Aguas Naturales, Residuales y Residuales Tratadas—Método de Prueba/Water Analysis—Determination of Disolved Oxygen in Natural, Wastewaters and Wastewaters Treated—Test Method; Secretaría de Comercio y Fomento Industrial: Mexico City, Mexico, 2001. [Google Scholar]
- American Public Health Association; American Water Workers Association; Water Pollution Control Federation. Standard Methods for the Examination of Water and Waste Water; American Public Health Association: Washington, DC, USA, 1992; 516p. [Google Scholar]
- Norma Mexicana NMX-AA-072-SCFI-2001. Análisis de Agua—Determinación de Dureza Total en Aguas Naturales, Residuales y Residuales Tratadas—Método de Prueba/Water Analysis—Determination of Total Hardness in Natural, Wastewaters and Wastewaters Treated—Test Method; Secretaría de Comercio y Fomento Industrial: Mexico City, Mexico, 2001. [Google Scholar]
- Norma Mexicana NMX-AA-073-SCFI-2001. Análisis de Agua—Determinación de Cloruros Totales en Aguas Naturales, Residuales y Residuales Tratadas—Método de Prueba/Water Analysis—Determination of Total Chlorine in Natural Water, Wastewaters and Wastewaters Treated—Test Method; Secretaría de Comercio y Fomento Industrial: Mexico City, Mexico, 2001. [Google Scholar]
- Norma Mexicana NMX-AA-082-1986. Contaminación del Agua-Determinación de Nitrógeno de Nitrato-Método Espectrofotométrico Ultravioleta/Water Contamination—Determination of Nitrogen Nitrate—Ultraviolet Spectrophotometric Method; Secretaría de Comercio y Fomento Industrial: Mexico City, Mexico, 1986. [Google Scholar]
- Norma Mexicana NMX-AA-074-1981. Análisis de Agua—Determinación del Ion Sulfato/Analysis of Water—Determination of Sulfate Ion; Secretaría de Comercio y Fomento Industrial: Mexico City, Mexico, 1981. [Google Scholar]
- Norma Mexicana NMX-AA-029-SCFI-2001. Análisis de Aguas—Determinación de Fósforo Total en Aguas Naturales, Residuales y Residuales Tratadas—Método de Prueba/Water Analysis—Determination of Total Phosphorus in Natural, Wastewaters and Wastewaters Treated—Test Method; Secretaría de Comercio y Fomento Industrial: Mexico City, Mexico, 2001. [Google Scholar]
- Norma Mexicana NMX-AA-051-SCFI-2001. Análisis de Agua—Determinación de Metales por Absorción Atómica en Aguas Naturales, Potables, Residuales y Residuales Tratadas—Método de Prueba/Water Analysis—Determination of Metals by Atomic Absorption in Natural, Drinking, Wastewaters and Wastewaters Treated—Test Method; Secretaría de Comercio y Fomento Industrial: Mexico City, Mexico, 2001. [Google Scholar]
- Conesa, F.V.V. Methodological Guide for Environmental Impact Evaluation (Guía Metodológica para la Evaluación del Impacto Ambiental); Mundi-Prensa: Madrid, Spain, 1995; p. 390. [Google Scholar]
- León-Vizcaíno, R. Índices de Calidad del Agua, Formas de Estimarlos y Aplicación en la Cuenca Lerma-Chapala, México; Instituto Tecnológico del Agua: Morelos, Mexico, 1991; pp. 1–7. [Google Scholar]
- Gebremariam, Y.S.; Beutel, W.M. Nitrate removal and DO levels in batch wetland mesocosms: Cattail (Typha spp.) versus bulrush (Scirpus spp.). Ecol. Eng. 2008, 34, 1–6. [Google Scholar] [CrossRef]
- Algoazany, A.S.; Kalita, P.K.; Mitchell, J.K.; Cooke, R.A.C.; Hirschi, M.C. A long-Term Monitoring of Agricultural Chemical Transport from a Flat Tile Drained Watershed. In Proceedings of the ASAE Annual Meeting, Tampa, FL, USA, 17–20 July 2005; Paper Number 052255; American Society of Agricultural and Biological Engineers: St Joseph, MI, USA, 2005. [Google Scholar]
- Schueneman, T.J. Characterization of sulfur sources in the EAA. Soil Crop Sci. Soc. Fla. Proc. 2001, 60, 49–52. [Google Scholar]
- Linnik, P.M.; Zubenko, I.B. Role of bottom sediments in the secondary pollution of aquatic environments by heavy metal compounds. Lakes Res. Res. Manag. 2000, 5, 11–21. [Google Scholar] [CrossRef]
- Ogoyi, D.O.; Mwita, C.J.; Nguu, E.K.; Shiundu, M. Determination of Heavy Metal Content in Water, Sediment and Microalgae from Lake Victoria, East Africa. Open Environ. Eng. J. 2011, 4, 156–161. [Google Scholar]
- Secretaría de Desarrollo Urbano y Ecología (Mexico). Criterios Ecológicos de Calidad del Agua; CE-CCA-001/89; SEGOB: Mexico City, Mexico, 1989. [Google Scholar]
- Kiss, A.S.; Oncsik, M.; Dombovári, J.; Veres, S.; Ács, G. Dangers of arsenic drinking and irrigation water to plants and humans. Antagonism of arsenic and magnesium. Acta Agron. Hung. 1992, 41, 3–9. [Google Scholar]
- Hood, R.D. Cacodylic Acid: Agricultural Uses, Biologic Effects, and Environmental Fate; VA Monograph; Veterans Administration: Washington, DC, USA, 1985; pp. 150–171. [Google Scholar]
- Roselli, L.; Fabbrocini, A.; Manzo, C.; D’Adamo, R. Hydrological heterogeneity, nutrient dynamics and water quality of a non-tidal lentic ecosystem (Lesina Lagoon, Italy). Estuar. Coast. Shelf Sci. 2009, 84, 539–552. [Google Scholar] [CrossRef]
- Razmkhah, H.; Abrishamchi, A.; Torkian, A. Evaluation of spatial and temporal variation in water quality by pattern recognition techniques: A case study on Jajrood River (Tehran, Iran). J. Environ. Manag. 2010, 91, 852–860. [Google Scholar] [CrossRef] [PubMed]
- González-Ortegón, E.; Subida, M.D.; Cuesta, J.A.; Arias, A.M.; Fernández-Delgado, C.; Drake, P. The impact of extreme turbidity events on the nursery function of a temperate European estuary with regulated freshwater inflow. Estuar. Coast. Shelf Sci. 2010, 87, 311–324. [Google Scholar] [CrossRef]
- Chilundo, M.; Kelderman, P.; Ókeeffe, J.H. Design of a water quality monitoring network for the Limpopo River Basin in Mozambique. Phys. Chem. Earth Parts A/B/C 2008, 33, 655–665. [Google Scholar] [CrossRef]
- Rubio-Arias, H.; Contreras-Caraveo, M.; Quintana, R.M.; Saucedo-Teran, R.A.; Pinales-Munguia, A. An Overall Water Quality Index (WQI) for a Man-Made Aquatic Reservoir in Mexico. Int. J. Environ. Res. Public Health 2012, 9, 1687–1698. [Google Scholar] [CrossRef] [PubMed]
- Alobaidy, A.; Maulood, B.; Kadhem, A. Evaluating raw and treated water quality of Tigris River within Baghdad by index analysis. J. Water Resour. Prot. 2010, 2, 629–635. [Google Scholar] [CrossRef]
- Ravikumar, P.; Mehmood, M.A.; Somashekar, R.K. Water quality index to determine the surface water quality of Sankey tank and Mallathahalli lake, Bangalore urban district, Karnataka, India. Appl. Water Sci. 2013, 3, 247–261. [Google Scholar] [CrossRef]
- Moriguti, T.; Shibata, T.; Nakamura, E. Lithium, boron and lead isotope and trace element systematics of Quaternary basaltic volcanic rocks in northeastern Japan: Mineralogical controls on slab-derived fluid composition. Chem. Geol. 2004, 212, 81–100. [Google Scholar] [CrossRef]
- Dotsika, E.; Poutoukis, D.; Michelot, J.L.; Kloppmann, W. Stable isotope and chloride, boron study for tracing sources of boron contamination in groundwater: Boron contents in fresh and thermal water in different areas in Greece. Water Air Soil Pollut. 2006, 174, 19–32. [Google Scholar] [CrossRef]
- Gemici, Ü.; Tarcan, G. Distribution of boron in thermal waters of western Anatolia, Turkey, and examples of their environmental impacts. Environ. Geol. 2002, 43, 87–98. [Google Scholar] [CrossRef]
| Parameter | Mexican Standards | Analytical Method |
|---|---|---|
| pH | NMX-AA-008-SCFI-2000 [24] | Potentiometer |
| EC (dS·m−1) | NMX-AA-093-SCFI-2000 [25] | Potentiometer |
| DO (mg·L−1) | NMX-AA-012-SCFI-2001 [26] | Potentiometer |
| TDS (mg·L−1) | Standard Methods 2540 c [27] | Potentiometer |
| TH (mg·L−1) | NMX-AA-072-SCFI-2001 [28] | Titration |
| Alk (mg·L−1) | NMX-AA-072-SCFI-2001 [28] | Titration |
| Cl− (mg·L−1) | NMX-AA-073-SCFI-2001 [29] | Potentiometer with silver nitrate |
| NO3 (mg·L−1) | NMX-AA-082-1996 [30] | Cadmium column (Griess-Ilosvay Met.) |
| SO4 (mg·L−1) | NMX-AA-074-1981 [31] | Spectrophotometry |
| TP (mg·L−1) | NMX-AA-029-SCFI-2001 [32] | Spectrophotometry |
| Ca (mg·L−1) | NMX-AA-51-SCFI-2001 [33] | Atomic Absorption |
| Mg (mg·L−1) | NMX-AA-51-SCFI-2001 [33] | Atomic Absorption |
| Na (mg·L−1) | NMX-AA-51-SCFI-2001 [33] | Atomic Absorption |
| K (mg·L−1) | NMX-AA-51-SCFI-2001 [33] | Atomic Absorption |
| B (mg·L−1) | NMX-AA-51-SCFI-2001 [33] | Spectrophotometry |
| As (mg·L−1) | NMX-AA-51-SCFI-2001 [33] | Atomic Absorption |
| Cu (mg·L−1) | NMX-AA-51-SCFI-2001 [33] | Atomic Absorption |
| Zn (mg·L−1) | NMX-AA-51-SCFI-2001 [33] | Atomic Absorption |
| Parameter | pH | EC | DO | TDS | TH | Alk | Cl− | NO3 | SO4 | Value |
|---|---|---|---|---|---|---|---|---|---|---|
| dS·m−1 | mg·L−1 | mg·L−1 | mg·L−1 | mg·L−1 | mg·L−1 | mg·L−1 | mg·L−1 | % | ||
| Analytical value | 1/14 | >16.00 | 0 | >1.500 | >1.500 | >1.500 | >400 | >55 | >250 | 0 |
| 2/13 | 12 | 1 | 1.5 | 1 | 1 | 350 | 50 | 225 | 10 | |
| 3/12 | 8 | 2 | 1 | 800 | 800 | 300 | 45 | 200 | 20 | |
| 4/11 | 5 | 3 | 800 | 600 | 600 | 250 | 40 | 175 | 30 | |
| 5/10 | 3 | 3.5 | 600 | 500 | 500 | 200 | 35 | 150 | 40 | |
| 6/9.5 | 2.5 | 4 | 500 | 400 | 400 | 150 | 30 | 130 | 50 | |
| 6.5 | 2 | 5 | 400 | 300 | 300 | 100 | 25 | 100 | 60 | |
| 9 | 1.5 | 6 | 300 | 200 | 200 | 50 | 20 | 75 | 70 | |
| 8.5 | 1.25 | 6.5 | 200 | 100 | 100 | 25 | 10 | 50 | 80 | |
| 8 | 1 | 7 | 100 | 50 | 50 | 10 | 5 | 25 | 90 | |
| 7 | <750 | 7.5 | <100 | <25 | <25 | <10 | <5 | <10 | 100 |
| Parameter | TP | Ca | Mg | Na | K | B | As | Cu | Zn | Value |
|---|---|---|---|---|---|---|---|---|---|---|
| mg·L−1 | mg·L−1 | mg·L−1 | mg·L−1 | mg·L−1 | mg·L−1 | mg·L−1 | mg·L−1 | mg·L−1 | % | |
| Analytical value | >0.65 | >250 | >50 | >90 | >35 | >3.0 | >0.30 | >0.30 | >4.0 | 0 |
| 0.6 | 225 | 45 | 80 | 30 | 2.5 | 0.2 | 0.28 | 3.6 | 10 | |
| 0.55 | 200 | 40 | 70 | 25 | 2 | 0.18 | 0.26 | 3.2 | 20 | |
| 0.5 | 175 | 35 | 60 | 20 | 1.5 | 0.14 | 0.24 | 2.8 | 30 | |
| 0.45 | 150 | 30 | 50 | 15 | 1 | 0.12 | 0.22 | 2.4 | 40 | |
| 0.4 | 120 | 24 | 40 | 10 | 0.7 | 0.1 | 0.2 | 2 | 50 | |
| 0.3 | 100 | 20 | 30 | 8 | 0.5 | 0.09 | 0.15 | 1.6 | 60 | |
| 0.25 | 75 | 15 | 20 | 6 | 0.4 | 0.08 | 0.1 | 1.2 | 70 | |
| 0.2 | 50 | 10 | 10 | 4 | 0.3 | 0.07 | 0.05 | 0.8 | 80 | |
| 0.15 | 25 | 5 | 5 | 2 | 0.2 | 0.06 | 0.03 | 0.4 | 90 | |
| <0.10 | <20 | <5 | <5 | <1 | <0.1 | <0.05 | <0.01 | <0.02 | 100 |
| Parameter | Weight (Wi) | Parameter | Weight (Wi) |
|---|---|---|---|
| pH | 2 | TP | 1 |
| EC | 3 | Ca | 2 |
| DO | 1 | Mg | 3 |
| TDS | 2 | Na | 5 |
| TH | 2 | K | 5 |
| Alk | 3 | B | 5 |
| Cl− | 3 | As | 4 |
| NO3 | 1 | Cu | 2 |
| SO4 | 5 | Zn | 2 |
| Sampling Site 1 | Sampling Site 2 | Sampling Site 3 | Sampling Site 4 | Sampling Site 5 | Sampling Site 6 | Sampling Site 7 | |
|---|---|---|---|---|---|---|---|
| pH | 8.23 ± 0.56 | 8.27 ± 0.50 | 8.17 ± 0.56 | 8.42 ± 0.43 | 8.61 ± 0.66 | 8.43 ± 0.42 | 8.41 ± 0.56 |
| EC (dS·m−1) | 449 ± 165 | 447 ± 144 | 483 ± 149 | 457 ± 138 | 480 ± 184 | 445 ± 136 | 447 ± 123 |
| DO (mg·L−1) | 5.34 ± 0.83 | 5.39 ± 1.41 | 5.42 ± 0.75 | 5.47 ± 0.61 | 6.01 ± 0.55 | 6.18 ± 0.47 | 6.14 ± 0.50 |
| TDS (mg·L−1) | 219 ± 80.1 | 219 ± 71.3 | 237 ± 73.7 | 224 ± 67.8 | 236 ± 72.9 | 218 ± 67.2 | 219 ± 60.7 |
| TH (mg·L−1) | 45.2 ± 38.7 | 61.2 ± 44.3 | 59.2 ± 43.8 | 42.5 ± 39.5 | 57.7 ± 46.8 | 31.7 ± 40.9 | 42.9 ± 40.6 |
| Alk (mg·L−1) | 14.2 ± 12.1 | 13.3 ± 12.5 | 14.8 ± 11.3 | 13.1 ± 12.4 | 18.1 ± 14.7 | 9.96 ± 12.8 | 11.3 ± 13.6 |
| Cl− (mg·L−1) | 31.9 ± 11.5 | 36.4 ± 8.14 | 37.9 ± 10.2 | 36.2 ± 7.03 | 37.3 ± 6.96 | 30.1 ± 10.2 | 32.3 ± 9.97 |
| NO3 (mg·L−1) | 0.76 ± 1.01 | 0.54 ± 0.57 | 1.37 ± 2.24 | 0.50 ± 0.62 | 1.08 ± 2.01 | 1.37 ± 1.84 | 0.96 ± 1.27 |
| TP (mg·L−1) | 0.59 ± 0.32 | 0.45 ± 0.15 | 0.45 ± 0.24 | 0.30 ± 0.19 | 0.15 ± 0.12 | 0.31 ± 0.18 | 0.29 ± 0.13 |
| SO4 (mg·L−1) | 10.3 ± 7.52 | 11.1 ± 9.39 | 11.1 ± 6.12 | 14.1 ± 9.22 | 9.80 ± 2.22 | 11.7 ± 14.1 | 13.8 ± 7.24 |
| Ca (mg·L−1) | 7.25 ± 6.20 | 6.99 ± 6.29 | 6.80 ± 6.15 | 6.81 ± 6.34 | 9.26 ± 7.51 | 5.09 ± 6.57 | 6.14 ± 6.72 |
| Mg (mg·L−1) | 5.47 ± 2.07 | 5.39 ± 2.05 | 5.40 ± 1.80 | 5.86 ± 1.58 | 5.93 ± 2.48 | 6.20 ± 1.66 | 5.57 ± 1.87 |
| Na (mg·L−1) | 76.5 ± 28.5 | 77.2 ± 26.7 | 85.3 ± 25.3 | 69.6 ± 37.8 | 79.3 ± 25.3 | 74.6 ± 23.9 | 76.0 ± 19.9 |
| K (mg·L−1) | 11.6 ± 2.83 | 11.9 ± 1.81 | 12.2 ± 1.86 | 11.9 ± 1.53 | 11.9 ± 2.10 | 12.4 ± 1.60 | 11.3 ± 1.38 |
| B (mg·L−1) | 3.80 ± 0.84 | 3.85 ± 0.86 | 4.24 ± 0.73 | 3.84 ± 0.61 | 3.89 ± 0.62 | 3.80 ± 0.59 | 3.75 ± 0.59 |
| As (mg·L−1) | 0.17 ± 0.02 | 0.16 ± 0.03 | 0.18 ± 0.04 | 0.15 ± 0.01 | 0.16 ± 0.01 | 0.13 ± 0.02 | 0.13 ± 0.02 |
| Cu (mg·L−1) | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Zn (mg·L−1) | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Date | Sampling Site 1 | Sampling Site 2 | Sampling Site 3 | Sampling Site 4 | Sampling Site 5 | Sampling Site 6 | Sampling Site 7 |
|---|---|---|---|---|---|---|---|
| March | 57.34 | 56.60 | 54.15 | 56.81 | 56.17 | 59.04 | 58.51 |
| June | 55.32 | 54.79 | 54.89 | 55.85 | 55.53 | 60.85 | 58.72 |
| July | 63.72 | 62.98 | 62.77 | 64.89 | 64.68 | 66.38 | 67.66 |
| September | 70.96 | 69.26 | 65.85 | 73.30 | 69.26 | 69.79 | 69.57 |
| December | 61.17 | 63.11 | 60.85 | 61.70 | 56.06 | 61.17 | 66.70 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De La Mora-Orozco, C.; Flores-Lopez, H.; Rubio-Arias, H.; Chavez-Duran, A.; Ochoa-Rivero, J. Developing a Water Quality Index (WQI) for an Irrigation Dam. Int. J. Environ. Res. Public Health 2017, 14, 439. https://doi.org/10.3390/ijerph14050439
De La Mora-Orozco C, Flores-Lopez H, Rubio-Arias H, Chavez-Duran A, Ochoa-Rivero J. Developing a Water Quality Index (WQI) for an Irrigation Dam. International Journal of Environmental Research and Public Health. 2017; 14(5):439. https://doi.org/10.3390/ijerph14050439
Chicago/Turabian StyleDe La Mora-Orozco, Celia, Hugo Flores-Lopez, Hector Rubio-Arias, Alvaro Chavez-Duran, and Jesus Ochoa-Rivero. 2017. "Developing a Water Quality Index (WQI) for an Irrigation Dam" International Journal of Environmental Research and Public Health 14, no. 5: 439. https://doi.org/10.3390/ijerph14050439







