Accessing Disadvantaged Pregnant Women in Houston, Texas, and Characterizing Biomarkers of Metal Exposure: A Feasibility Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Calderon, J.; Ortiz-Perez, D.; Yanez, L.; Diaz-Barriga, F. Human exposure to metals. Pathways of exposure, biomarkers of effect, and host factors. Ecotoxicol. Environ. Saf. 2003, 56, 93–103. [Google Scholar] [CrossRef]
- Hu, H. Exposure to metals. Prim. Care 2000, 27, 983–996. [Google Scholar] [CrossRef]
- Iyengar, G.V.; Rapp, A. Human placenta as a ‘dual’ biomarker for monitoring fetal and maternal environment with special reference to potentially toxic trace elements. Part 3: Toxic trace elements in placenta and placenta as a biomarker for these elements. Sci. Total Environ. 2001, 280, 221–238. [Google Scholar] [CrossRef]
- Bellinger, D.C. Teratogen update: Lead and pregnancy. Birth Defects Res. A Clin. Mol. Teratol. 2005, 73, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Kumarathasan, P.; Gomes, J. Infant and mother related outcomes from exposure to metals with endocrine disrupting properties during pregnancy. Sci. Total Environ. 2016, 569–570, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.L.; Ebisu, K. Environmental inequality in exposures to airborne particulate matter components in the United States. Environ. Health Perspect. 2012, 120, 1699–1704. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.T.; Aelion, C.M.; Liu, J.; Burch, J.B.; Cai, B.; Lawson, A.B.; McDermott, S. Potential sources and racial disparities in the residential distribution of soil arsenic and lead among pregnant women. Sci. Total Environ. 2016, 551–552, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.B. Trends and variability in blood lead concentrations among US children and adolescents. Environ. Sci. Pollut. Res. Int. 2016, 23, 7880–7889. [Google Scholar] [CrossRef] [PubMed]
- Pirkle, J.L.; Kaufmann, R.B.; Brody, D.J.; Hickman, T.; Gunter, E.W.; Paschal, D.C. Exposure of the U.S. population to lead, 1991–1994. Environ. Health Perspect. 1998, 106, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Morello-Frosch, R.; Shenassa, E.D. The environmental “riskscape” and social inequality: Implications for explaining maternal and child health disparities. Environ. Health Perspect. 2006, 114, 1150–1153. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Duran, N.; Norris, K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am. J. Public Health 2014, 104, e16–e31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Lu, L.; Pan, Y.J.; Ding, C.G.; Xu, D.Y.; Huang, C.F.; Pan, X.F.; Zheng, W. Baseline blood levels of manganese, lead, cadmium, copper, and zinc in residents of Beijing suburb. Environ. Res. 2015, 140, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, A.L.; Leroy, Z.C.; Logue, K.M.; Glanz, K.; Dunlop, B.P. Preconsent education about research processes improved African Americans' willingness to participate in clinical research. J. Clin. Epidemiol. 2011, 64, 872–877. [Google Scholar] [CrossRef] [PubMed]
- McDermott, S.; Salzberg, D.C.; Anderson, A.P.; Shaw, T.; Lead, J. Systematic Review of Chromium and Nickel Exposure During Pregnancy and Impact on Child Outcomes. J. Toxicol. Environ. Health A 2015, 78, 1348–1368. [Google Scholar] [CrossRef] [PubMed]
- Quansah, R.; Arman, F.A.; Essumang, D.K.; Luginaah, I.; Clarke, E.; Marfoh, K.; Cobbina, S.J.; Nketiah-Amponsah, E.; Namujju, P.B.; Obiri, S.; et al. Association of arsenic with adverse pregnancy outcomes/infant mortality: A systematic review and meta-analysis. Environ. Health Perspect. 2015, 123, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.E.; Maxson, P.; Miranda, M.L.; Fry, R.C. Cadmium levels in a North Carolina cohort: Identifying risk factors for elevated levels during pregnancy. J. Expo Sci. Environ. Epidemiol. 2015, 25, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Sanders, A.P.; Flood, K.; Chiang, S.; Herring, A.H.; Wolf, L.; Fry, R.C. Towards prenatal biomonitoring in North Carolina: Assessing arsenic, cadmium, mercury, and lead levels in pregnant women. PLoS ONE 2012, 7, e31354. [Google Scholar] [CrossRef] [PubMed]
- Foster, W.G.; Cheung, A.P.; Davis, K.; Graves, G.; Jarrell, J.; Leblanc, A.; Liang, C.L.; Leech, T.; Walker, M.; Weber, J.P.; et al. Circulating metals and persistent organic pollutant concentrations in Canadian and non-Canadian born primiparous women from five Canadian centres: Results of a pilot biomonitoring study. Sci. Total Environ. 2012, 435–436, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Arbuckle, T.E.; Liang, C.L.; Morisset, A.S.; Fisher, M.; Weiler, H.; Cirtiu, C.M.; Legrand, M.; Davis, K.; Ettinger, A.S.; Fraser, W.D. Maternal and fetal exposure to cadmium, lead, manganese and mercury: The MIREC study. Chemosphere 2016, 163, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, T.J.; Zota, A.R.; Schwartz, J.M. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ. Health Perspect. 2011, 119, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.E.; Valentiner, E.; Maxson, P.; Miranda, M.L.; Fry, R.C. Maternal cadmium levels during pregnancy associated with lower birth weight in infants in a North Carolina cohort. PLoS ONE 2014, 9, e109661. [Google Scholar] [CrossRef] [PubMed]
- King, K.E.; Darrah, T.H.; Money, E.; Meentemeyer, R.; Maguire, R.L.; Nye, M.D.; Michener, L.; Murtha, A.P.; Jirtle, R.; Murphy, S.K.; et al. Geographic clustering of elevated blood heavy metal levels in pregnant women. BMC Public Health 2015, 15, 1035. [Google Scholar] [CrossRef] [PubMed]

| All Women (n = 31) | Women with a Blood Draw (n = 22) | |
|---|---|---|
| n (%) | n (%) | |
| Maternal age [mean (SD)] | 29.0 (4.9) | 30.4 (4.5) |
| Gestational age at screening [mean (SD)] a | 25.3 (4.1) | 24.6 (3.8) |
| Race b | ||
| Black, non-Hispanic | 13 (41.9) | 9 (40.9) |
| Hispanic | 9 (29.0) | 6 (27.3) |
| White, non-Hispanic | 3 (9.7) | 3 (13.6) |
| Other | 3 (9.7) | 2 (9.1) |
| Missing | 3 (9.7) | 2 (9.1) |
| Marital Status | ||
| Divorced | 2 (6.5) | 1 (4.5) |
| In a relationship/married | 16 (41.9) | 12 (54.5) |
| Never married | 11 (35.5) | 8 (36.4) |
| Missing | 2 (6.5) | 1 (4.5) |
| College Degree | ||
| No | 23 (74.2) | 16 (72.7) |
| Yes | 6 (19.4) | 5 (22.7) |
| Missing | 2 (6.5) | 1 (4.5) |
| Annual Household Income | ||
| <$15,000 | 14 (45.2) | 13 (59.1) |
| $15,000–20,000 | 5 (16.1) | 3 (13.6) |
| >$20,000 | 4 (12.9) | 4 (18.2) |
| Don’t know | 6 (19.4) | 4 (18.2) |
| Missing | 2 (6.5) | 1 (4.5) |
| Smoked during pregnancy (yes) | 3 (9.7) | 3 (13.6) |
| Other smokers in the home (yes) | 3 (9.7) | 2 (9.1) |
| Works outside home (yes) | 11 (35.5) | 8 (36.4) |
| Moved during pregnancy (yes) | 6 (19.4) | 5 (22.7) |
| In a Future, Hypothetical Research Study of Pregnant Women and Their Babies, Would You Be Willing to…. | n | % |
|---|---|---|
| allow researchers to obtain a sample of your infant’s cord blood? | ||
| Yes | 16 | 51.6 |
| No | 4 | 12.9 |
| Don’t Know | 11 | 35.5 |
| allow researchers to access to your child’s medical records? | ||
| Yes | 14 | 45.2 |
| No | 5 | 16.1 |
| Don’t Know | 12 | 38.7 |
| allow researchers to access your child’s birth certificate? | ||
| Yes | 13 | 41.9 |
| No | 8 | 25.8 |
| Don’t Know | 10 | 32.3 |
| allow researchers to obtain air samples from inside your home? | ||
| Yes | 17 | 54.8 |
| No | 5 | 16.1 |
| Don’t Know | 8 | 25.8 |
| Refused | 1 | 3.2 |
| allow researchers to obtain air samples from outside your home? | ||
| Yes | 27 | 87.1 |
| No | 0 | 0.0 |
| Don’t Know | 3 | 9.7 |
| Refused | 1 | 3.2 |
| wear a personal badge for 2–3 days? | ||
| Yes | 27 | 87.1 |
| No | 2 | 6.5 |
| Don’t Know | 2 | 6.5 |
| get additional ultrasounds not part of your routine prenatal care? | ||
| Yes | 26 | 83.9 |
| No | 2 | 6.5 |
| Don’t Know | 3 | 9.7 |
| have additional blood draws that are not part of your routine prenatal care? | ||
| Yes | 20 | 64.5 |
| No | 7 | 22.6 |
| Don’t Know | 4 | 12.9 |
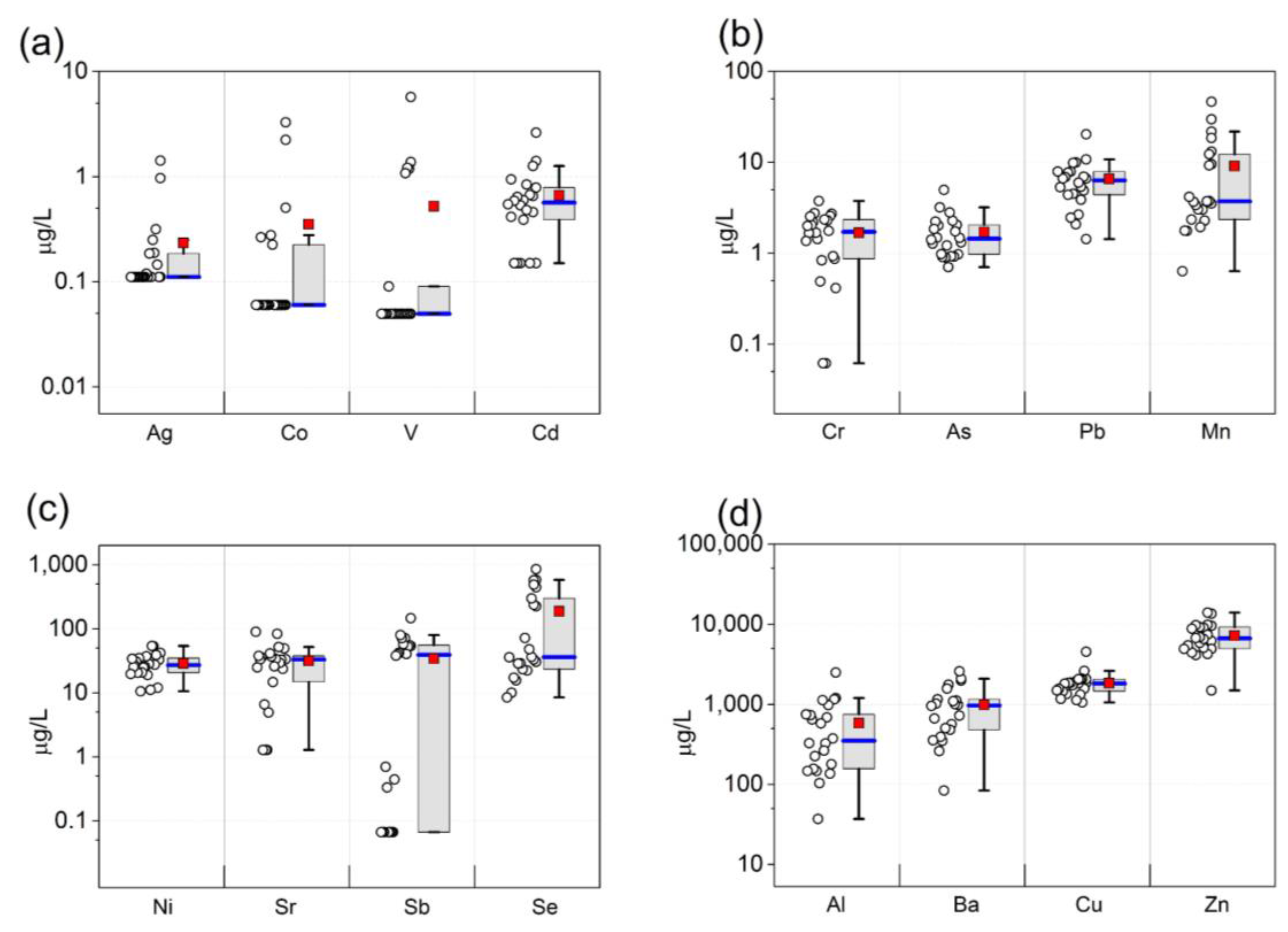
| Metal | LOD | n > LOD (%) | GM | Median | Mean ± SD (Range) |
|---|---|---|---|---|---|
| Aluminum | 0.88 | 22 (100%) | 374 | 351 | 581 ± 566 (37.1–2490) |
| Vanadium | 0.07 | 6 (27%) | 0.11 | 0.05 | 0.52 ± 1.3 (0.05–5.7) |
| Chromium | 0.09 | 20 (91%) | 1.2 | 1.7 | 1.7 ± 0.97 (0.06–3.8) |
| Manganese | 0.09 | 22 (100%) | 5.1 | 3.7 | 9.1 ± 11 (0.63–47) |
| Cobalt | 0.09 | 6 (27%) | 0.09 | 0.06 | 0.35 ± 0.81 (0.06–3.3) |
| Nickel | 0.10 | 22 (100%) | 26 | 27 | 29 ± 12 (11–54) |
| Copper | 0.13 | 22 (100%) | 1744 | 1821 | 1838 ± 713 (1059–4546) |
| Zinc | 0.26 | 22 (100%) | 6531 | 6661 | 7202 ± 3058 (1488–13,991) |
| Arsenic | 0.14 | 22 (100%) | 1.5 | 1.4 | 1.7 ± 0.98 (0.71–5.0) |
| Selenium | 0.48 | 22 (100%) | 77 | 36 | 202 ± 276 (17–1011) |
| Strontium | 1.81 | 20 (91%) | 19 | 33 | 32 ± 24 (1.3–90) |
| Silver | 0.16 | 8 (36%) | 0.13 | 0.11 | 0.22 ± 0.33 (0.11–1.4) |
| Cadmium | 0.21 | 17 (77%) | 0.44 | 0.57 | 0.65 ± 0.56 (0.15–2.6) |
| Antimony | 0.10 | 15 (68%) | 3.3 | 39 | 34 ± 38 (0.07–146) |
| Barium | 0.20 | 22 (100%) | 767 | 971 | 987 ± 658 (84–2582) |
| Lead | 0.21 | 22 (100%) | 5.6 | 6.3 | 6.6 ± 4.0 (1.4–20) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whitworth, K.W.; Han, I.; Afshar, M.; Mei, Y.; Berens, P.D.; Sharma, S.V.; Symanski, E. Accessing Disadvantaged Pregnant Women in Houston, Texas, and Characterizing Biomarkers of Metal Exposure: A Feasibility Study. Int. J. Environ. Res. Public Health 2017, 14, 474. https://doi.org/10.3390/ijerph14050474
Whitworth KW, Han I, Afshar M, Mei Y, Berens PD, Sharma SV, Symanski E. Accessing Disadvantaged Pregnant Women in Houston, Texas, and Characterizing Biomarkers of Metal Exposure: A Feasibility Study. International Journal of Environmental Research and Public Health. 2017; 14(5):474. https://doi.org/10.3390/ijerph14050474
Chicago/Turabian StyleWhitworth, Kristina W., Inkyu Han, Masoud Afshar, Yuan Mei, Pamela D. Berens, Shreela V. Sharma, and Elaine Symanski. 2017. "Accessing Disadvantaged Pregnant Women in Houston, Texas, and Characterizing Biomarkers of Metal Exposure: A Feasibility Study" International Journal of Environmental Research and Public Health 14, no. 5: 474. https://doi.org/10.3390/ijerph14050474





