1. Introduction
Both public debate and epidemiological research show evidence of an increase of stress symptoms and stress-associated diseases over the past decade. This has been identified on an international level [
1], but it is also found in Germany specifically [
2]. According to a recent statement issued by German health insurance companies [
3], 16.2% of all employee sickness-related absences are attributable to mental health disorders, with many of them associated to stress. This is an extreme increase, since only 2% of paid sick leave was attributed to mental health disorders about 40 years ago. The discussion about stress has also reached the school context, at least after a significant school reform in Germany, which reduced high-school duration by one year with almost the same curriculum [
4,
5].
From a developmental neurobiology perspective, childhood and adolescence can be described as very vulnerable phases in which biological systems develop. Stress experience during this age can influence an individual’s response to stressful events for their lifetime, mainly via the effects of an increased activation of the hypothalamic-pituitary-adrenal (HPA) axis, the main biological stress system, on the brain [
6]. Stress exposure during childhood might therefore lead to a biologically-based susceptibility to stress-related illnesses later in life [
7]. This is also related to lower academic achievement [
8]. Measures to reduce stress and to build up stress resilience in schools need to be found.
There is promising research that exposure to green environments has some positive effects on mental health. Green environments can be described as areas with a certain amount of non-built spaces, for instance, public parks, lakes, rivers or forests. ‘Green’ does therefore not necessarily mean ‘green’ as a color, but rather stands as a synonym for nature with all its different shapes. In their recent systematic literature review, James et al. found that neighborhood greenness, or vegetation, may affect health behaviors and outcomes, and increased physical activity and social contacts may result in decreasing stress [
9]. Their findings accord with a previous systematic literature review by Lee and Maheswaran, who concluded that most studies reported findings that generally supported the view of green environments having a beneficial health effect. However, they found that many studies were limited by poor study design, failure to exclude confounding, bias or reverse causality, and weak statistical associations [
10]. On a general level, Roe et al. could associate more green space in deprived urban neighborhoods in Scotland to lower levels of perceived stress and improved physiological stress. This was measured by diurnal secretion patterns of the stress hormone cortisol and a steeper (healthier) diurnal cortisol decline with 104 subjects [
11]. A similar correlation of green space and mental health factors seems to hold also for short-term visits of green space. Aspinal et al. found a relationship between green environment, behavior settings and emotions. They investigated the emotional experience of a group of walkers in three types of urban environments, including a green space setting, using mobile electroencephalography (EEG) as a method to record and analyze the
n = 12 subjects’ emotional experience. Their findings showed evidence when moving into the green space zone of lower frustration, engagement, arousal, and higher meditation; respondents showed higher engagement when moving out of it [
12]. Certainly in accordance with these findings, Brantman and colleagues investigated the effect of a 90 min walk in a natural environment and found a reduction of blood flow in the subgenual anterior cingulate cortex, a region associated with stress regulation, as well as a reduction of rumination, a cognitive style associated with depression [
13]. Interestingly, a lower activation in this brain region during acute social stress was found in individuals who grew up in a rural environment, compared to those who grew up in an urban environment [
14]. A systematic literature review [
15] compared effects of physical activity in outdoor natural environments with indoor environments on physical and psychological wellbeing. In contrast to being physically active indoors, physical activity in outdoor natural environments—so called green exercise—is associated with a decrease in tension, anger and depression. However, the authors conclude that the methodological quality of evaluated studies is poor and more high quality large-scale studies are needed in this field of research. On this basis, Rogerson, et al. [
16] tested 331 participants before and after a 5 km run in four different natural environments, applying appropriate measurements and statistical analyses. Participants’ stress and mood improved from pre- to post-run, independent of the specific green environment. The authors concluded that exercise in green environments offers possible benefits to psychological wellbeing.
In a series of studies, van den Berg and her team describe green space as a buffer between stressful life events and health for
n = 4529 Dutch respondents [
17]. They confirm the hypothesis that more time spent in green space is associated with higher scores on mental health and vitality scales, independent of cultural and climatic contexts, by comparing
n = 3748 observations from four European cities [
18]. They also describe gardening as an effective measure to promote neuroendocrine and affective restoration from stress in
n = 30 active private gardeners [
19].
A systematic literature review on the effects of school-based outdoor education programs on students’ health, physical activity, social and learning dimensions, however, revealed that only very limited research has been reported so far [
20]. Of more than 7800 articles analyzed, only 13 have met the inclusion criteria, of which six are so-called qualitative case studies, and seven apply (mostly poor) quantitative methodology. All studies are consistent in describing at least some positive effects of outdoor teaching to the various variables, health effects, physical activity, social and learning behavior. With respect to stress and mental health in the outdoor teaching context, we could identify only one slightly relevant study in the literature: Gustafsson and his colleagues describe that mental problems decreased in boys, but not in girls, in an outdoor teaching setting when compared to a control condition without outdoor teaching. These statistically significant effects were observed for a “difficulties total score”, as well as for “emotional symptoms”, “conduct problems”, and “hyperactivity”. Data were collected with a parent-report questionnaire [
21]. Additionally, a promising quasi-experimental study design of the impacts of education outside the classroom on students’ physical activity, well-being, and learning has been recently published. This study with
n = 834 observations of children aged 9–13 years had been performed in Denmark in the past years, and the results are due in late 2017 [
22]. Those results may be able to close some of the above-mentioned gaps in recent research and theory construction in “green exercise”.
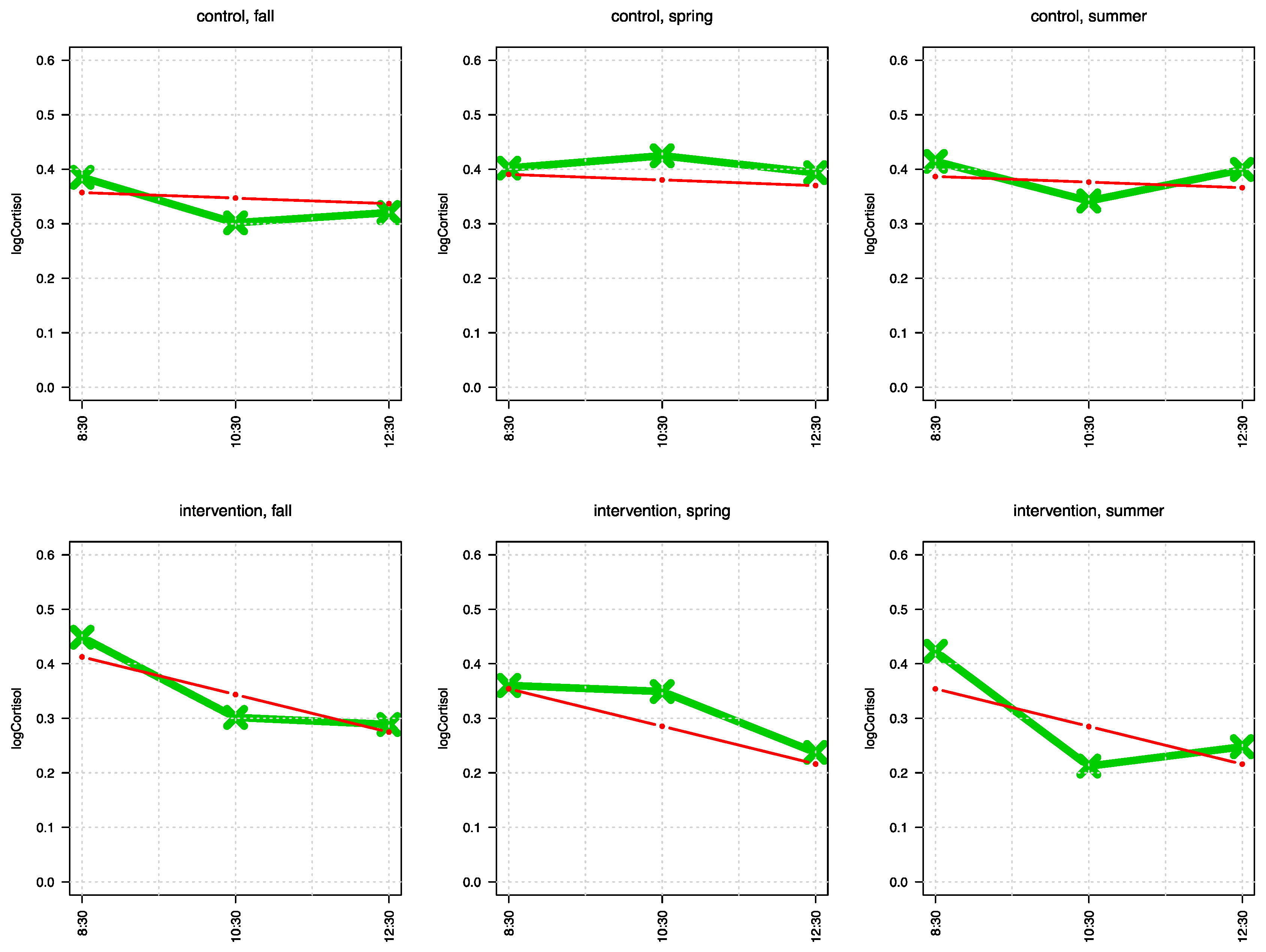
Taken together, the studies mentioned above give some evidence for a protective effect of a natural environment or outdoor setting on biological stress systems that might be related to mental health, also in the school context. However, to our knowledge, there is no prospective control group study investigating the effect of outdoor teaching on biological measures of stress. Therefore, we conducted the present pilot study, in which we hypothesized that regular intervals of outdoor teaching over the course of one school year will have a stress protective effect and, accordingly, will result in less activation of the HPA-axis, as reflected by a steeper decrease of cortisol secretion over the school day. The normal diurnal cortisol rhythm displays high cortisol values directly after getting up, with a steady decrease over the day [
23,
24,
25]. However, it has been reported that adolescents with a high score on the Children’s Depression Inventory show a reduced decline of morning cortisol, when compared to low scoring children [
25]. Thus, cortisol appears to be a fitting measure for stress, with respect to mental health.
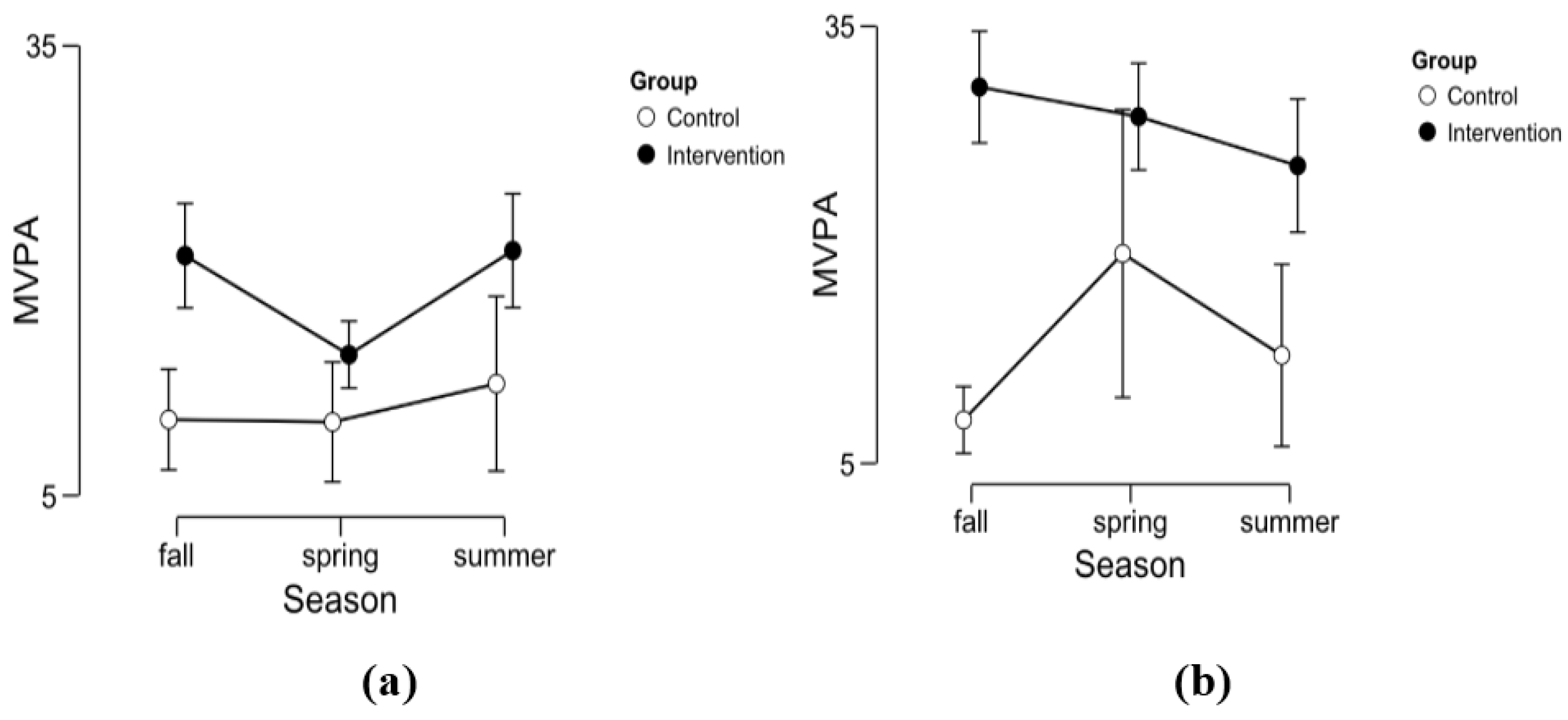
In addition, using an explorative approach, we investigated children’s physical activity (PA) levels. Previous research indicates that children’s PA-levels are consistently higher in outdoor education settings using natural environments, compared to normal indoor settings [
26,
27]. Since it is known that high PA can lead to higher cortisol levels [
28], we needed to control whether the expected PA differences between outdoor and indoor would modulate potential differences in the stress response.