What Type of Transitional Care Effectively Reduced Mortality and Improved ADL of Stroke Patients? A Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Data Sources and Literature Searches
2.2. Study Selection
2.3. Data Extraction and Study Quality Assessment
2.4. Data Synthesis and Analysis
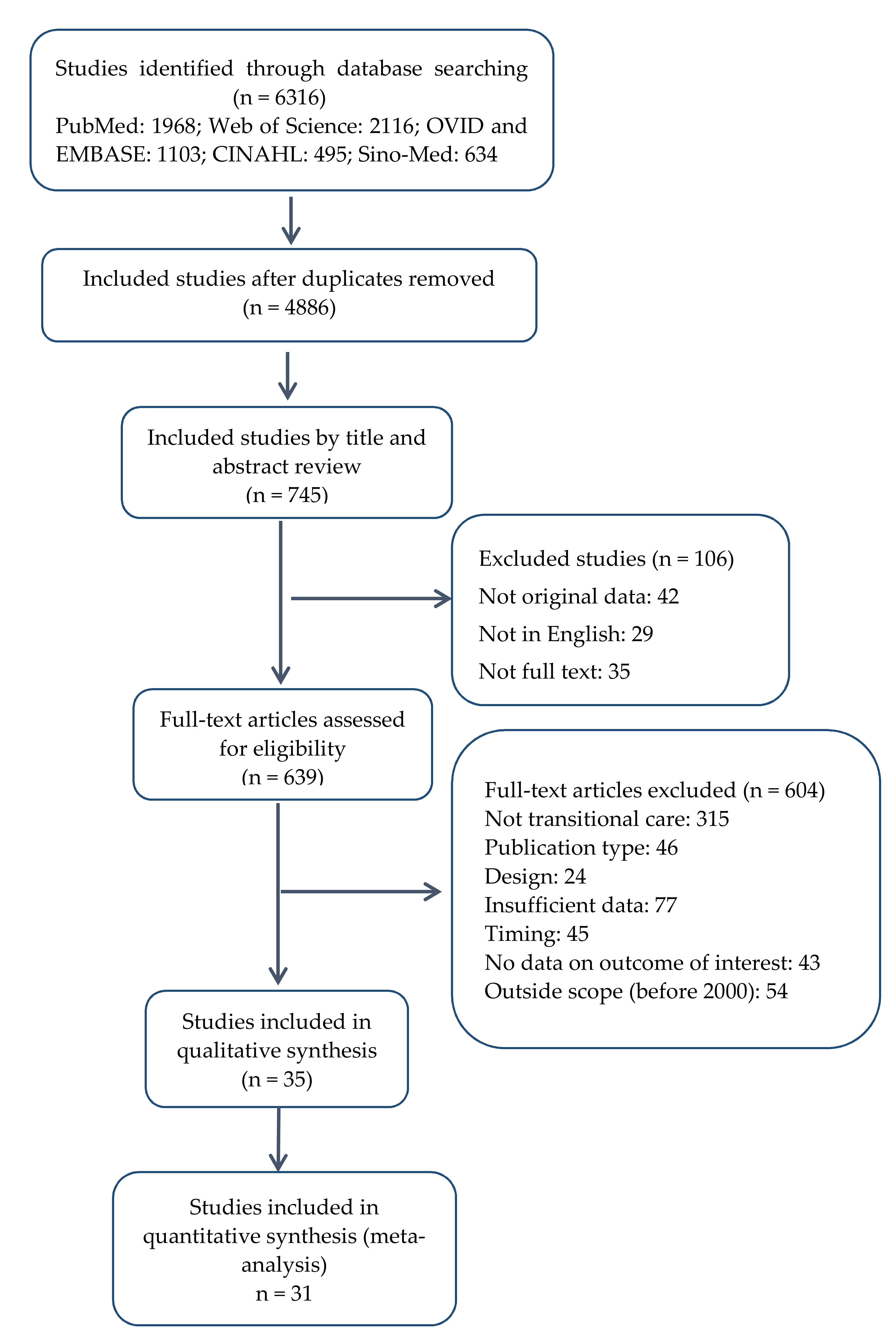
3. Results
3.1. Basic Characteristics of Included Studies
3.2. Methodological Quality
3.3. Interventions Characteristics of Included Studies
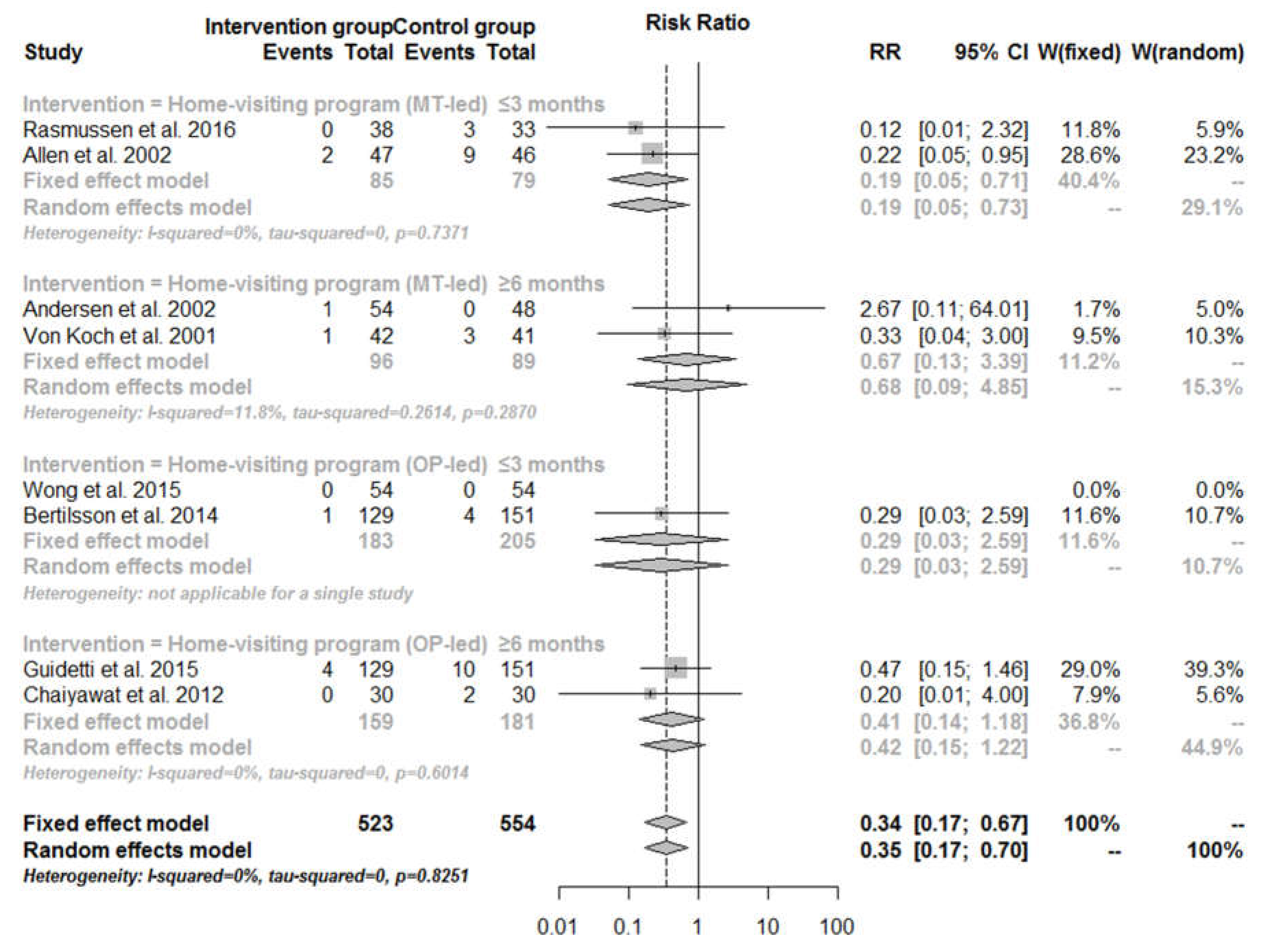
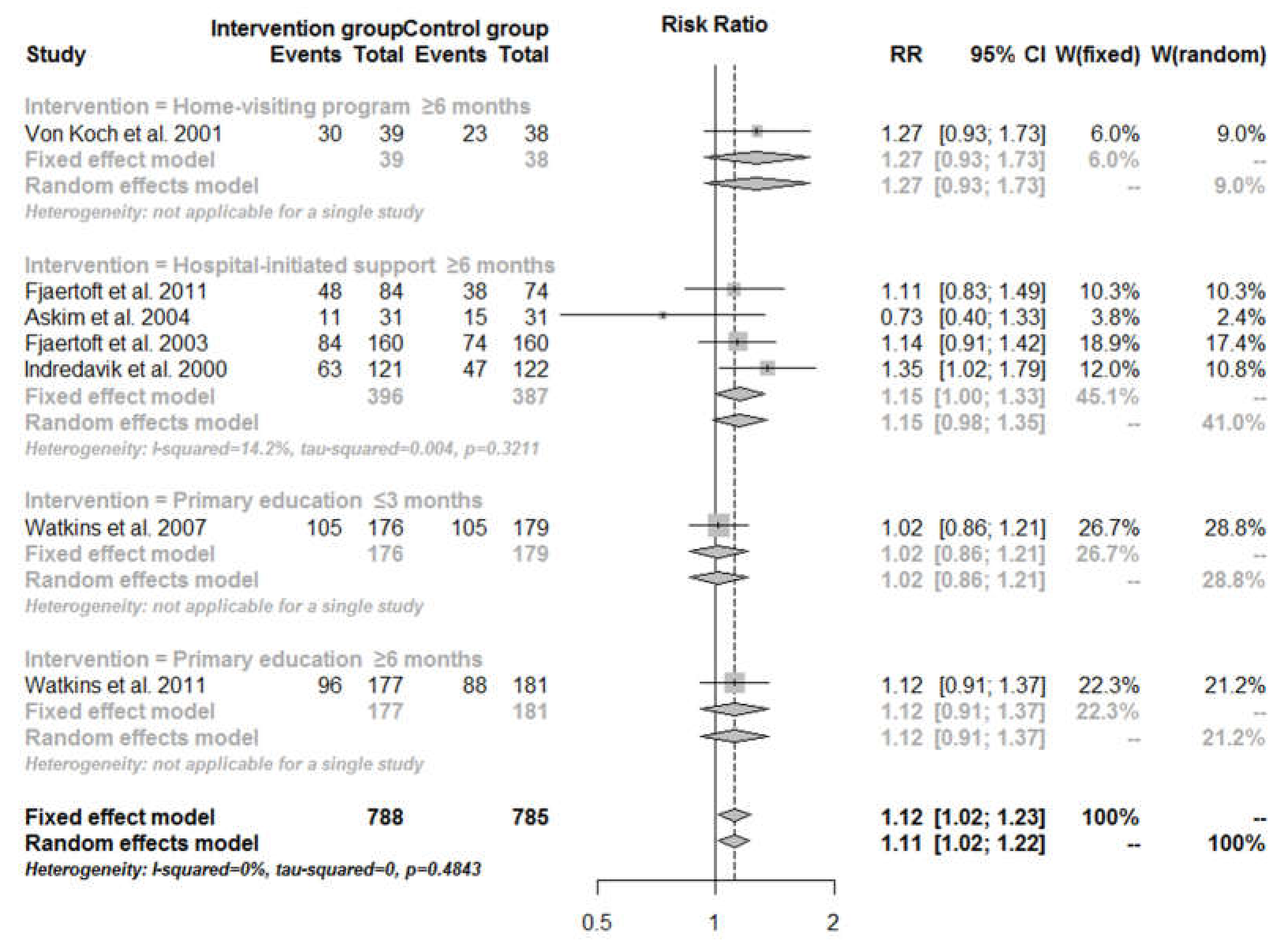
3.4. Mortality
3.5. Barthel ADL Index
3.6. Sensitivity Analysis
3.7. Publication Bias
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Feigin, V.L.; Forouzanfar, M.H.; Krishnamurthi, R.; Mensah, G.A.; Connor, M.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.; Truelsen, T.; et al. Global and regional burden of stroke during 1990–2010: Findings from the global burden of disease study 2010. Lancet 2014, 383, 245–254. [Google Scholar] [CrossRef]
- Gillespie, D.; Campbell, F. Effect of stroke on family carers and family relationships. Nurs. Stand. 2011, 26, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Hankey, G.J. Informal care giving for disabled stroke survivors—Training the care giver benefits the patient, the care giver, and the community. Br. Med. J. 2004, 328, 1085–1086. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the global burden of disease study 2013. Lancet 2015, 386, 743–800. [Google Scholar]
- Intercollegiate Stroke Working Party. National Clinical Guideline for Stroke, 4th ed.; Royal College of Physicians: London, UK, 2012. [Google Scholar]
- Coleman, E.A.; Boult, C. Improving the quality of transitional care for persons with complex care needs. J. Am. Geriatr. Soc. 2003, 51, 556–557. [Google Scholar] [CrossRef]
- Naylor, M.D.; Aiken, L.H.; Kurtzman, E.T.; Olds, D.M.; Hirschman, K.B. The care span: The importance of transitional care in achieving health reform. Health Aff. 2011, 30, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Bradway, C.; Trotta, R.; Bixby, M.B.; McPartland, E.; Wollman, M.C.; Kapustka, H.; McCauley, K.; Naylor, M.D. A qualitative analysis of an advanced practice nurse-directed transitional care model intervention. Gerontologist 2012, 52, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Bettger, J.P.; Alexander, K.P.; Dolor, R.J.; Olson, D.M.; Kendrick, A.S.; Wing, L.; Coeytaux, R.R.; Graffagnino, C.; Duncan, P.W. Transitional care after hospitalization for acute stroke or myocardial infarction: A systematic review. Ann. Intern. Med. 2012, 157, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Askim, T.; Mørkved, S.; Engen, A.; Roos, K.; Aas, T.; Indredavik, B.; Askim, T.; Mørkved, S.; Engen, A.; Roos, K.; et al. Effects of a community-based intensive motor training program combined with early supported discharge after treatment in a comprehensive stroke unit: A randomized, controlled trial. Stroke 2010, 41, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Hofstad, H.; Gjelsvik, B.E.; Naess, H.; Eide, G.E.; Skouen, J.S. Early supported discharge after stroke in bergen (ESD Stroke Bergen): Three and six months results of a randomised controlled trial comparing two early supported discharge schemes with treatment as usual. BMC Neurol. 2014, 14, 239. [Google Scholar] [CrossRef] [PubMed]
- Askim, T.; Rohweder, G.; Lydersen, S.; Indredavik, B. Evaluation of an extended stroke unit service with early supported discharge for patients living in a rural community: A randomized controlled trial. Clin. Rehabil. 2004, 18, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Fagerberg, B.; Claesson, L.; Gosman-Hedstrom, G.; Blomstrand, C. Effect of acute stroke unit care integrated with care continuum versus conventional treatment: A randomized 1-year study of elderly patients: The goteborg 70+ stroke study. Stroke 2000, 31, 2578–2584. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Rubenach, S.; Mhurchu, C.N.; Clark, M.; Spencer, C.; Winsor, A. Home or hospital for stroke rehabilitation? Results of a randomized controlled trial I: Health outcomes at 6 months. Stroke 2000, 31, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Chichester, UK, 2011. [Google Scholar]
- Fjaertoft, H.; Rohweder, G.; Indredavik, B. Stroke unit care combined with early supported discharge improves 5-year outcome: A randomized controlled trial. Stroke 2011, 42, 1707–1711. [Google Scholar] [CrossRef] [PubMed]
- Watkins, C.L.; Wathan, J.V.; Leathley, M.J.; Auton, M.F.; Deans, C.F.; Dickinson, H.A.; Jack, C.I.; Sutton, C.J.; van den Broek, M.D.; Lightbody, C.E.; et al. The 12-month effects of early motivational interviewing after acute stroke: A randomized controlled trial. Stroke 2011, 42, 1956–1961. [Google Scholar] [CrossRef] [PubMed]
- Watkins, C.L.; Auton, M.F.; Deans, C.F.; Dickinson, H.A.; Jack, C.I.; Lightbody, C.E.; Sutton, C.J.; van den Broek, M.D.; Leathley, M.J.; Watkins, C.L.; et al. Motivational interviewing early after acute stroke: A randomized, controlled trial. Stroke 2007, 38, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, A.M.; Holmqvist, L.W.; de Pedro-Cuesta, J.; von Koch, L. A randomized controlled trial of early supported discharge and continued rehabilitation at home after stroke—Five-year follow-up of patient outcome. Stroke 2005, 36, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Fjaertoft, H.; Indredavik, B.; Lydersen, S. Stroke unit care combined with early supported discharge: Long-term follow-up of a randomized controlled trial. Stroke 2003, 34, 2687–2691. [Google Scholar] [CrossRef] [PubMed]
- Von Koch, L.; de Pedro-Cuesta, J.; Kostulas, V.; Almazan, J.; Widen Holmqvist, L. Randomized controlled trial of rehabilitation at home after stroke: One-year follow-up of patient outcome, resource use and cost. Cerebrovasc. Dis. 2001, 12, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Indredavik, B.; Fjaertoft, H.; Ekeberg, G.; Loge, A.D.; Morch, B. Benefit of an extended stroke unit service with early supported discharge—A randomized, controlled trial. Stroke 2000, 31, 2989–2994. [Google Scholar] [CrossRef] [PubMed]
- Sit, J.W.H.; Chair, S.Y.; Choi, K.C.; Chan, C.W.H.; Lee, D.T.F.; Chan, A.W.K.; Cheung, J.L.K.; Tang, S.W.; Chan, P.S.; Taylor-Piliae, R.E. Do empowered stroke patients perform better at self-management and functional recovery after a stroke? A randomized controlled trial. Clin. Interv. Aging 2016, 11, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.K.Y.; Yeung, S.M. Effects of a 4-week transitional care programme for discharged stroke survivors in Hongkong: A randomised controlled trial. Health Soc. Care Community 2015, 23, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Chaiyawat, P.; Kulkantrakorn, K. Effectiveness of home rehabilitation program for ischemic stroke upon disability and quality of life: A randomized controlled trial. Clin. Neurol. Neurosurg. 2012, 114, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Langhammer, B.; Lindmark, B.; Stanghelle, J.K. Stroke patients and long-term training: Is it worthwhile? A randomized comparison of two different training strategies after rehabilitation. Clin. Rehabil. 2007, 21, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.E.; Eriksen, K.; Brown, A.; Schultz-Larsen, K.; Forchhammer, B.H. Follow-up services for stroke survivors after hospital discharge—A randomized control study. Clin. Rehabil. 2002, 16, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Forster, A.; Young, J.; Chapman, K.; Nixon, J.; Patel, A.; Holloway, I.; Mellish, K.; Anwar, S.; Breen, R.; Knapp, M.; et al. Cluster randomized controlled trial: Clinical and cost-effectiveness of a system of longer-term stroke care. Stroke 2015, 46, 2212–2219. [Google Scholar] [CrossRef] [PubMed]
- Sackley, C.; Wade, D.T.; Mant, D.; Atkinson, J.C.; Yudkin, P.; Cardoso, K.; Levin, S.; Lee, V.B.; Reel, K. Cluster randomized pilot controlled trial of an occupational therapy intervention for residents with stroke in UK care homes. Stroke 2006, 37, 2336–2341. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, M.; Power, M.; Russell, M.; Fullerton, K. Randomized controlled trial of an early discharge rehabilitation service: The Belfast community stroke trial. Stroke 2004, 35, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Jiang, W.H.; Chen, B.W.; Zheng, J.G.; Wan-Qing, L.I.; Jie, L.I. Application of meta package of R in the meta-analysis. J. Evid.-Based Med. 2011, 11, 305–309. [Google Scholar]
- Rasmussen, R.S.; Østergaard, A.; Kjær, P.; Skerris, A.; Skou, C.; Christoffersen, J.; Seest, L.S.; Poulsen, M.B.; Rønholt, F.; Overgaard, K. Stroke rehabilitation at home before and after discharge reduced disability and improved quality of life: A randomised controlled trial. Clin. Rehabil. 2016, 30, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, S.; Ranner, M.; Tham, K.; Andersson, M.; Ytterberg, C.; von Koch, L. A “client-centred activities of daily living” intervention for persons with stroke: One-year follow-up of a randomized controlled trial. J. Rehabil. Med. 2015, 47, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Bertilsson, A.S.; Ranner, M.; von Koch, L.; Eriksson, G.; Johansson, U.; Ytterberg, C.; Guidetti, S.; Tham, K. A client-centred adl intervention: Three-month follow-up of a randomized controlled trial. Scand. J. Occup. Ther. 2014, 21, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.R.; Hazelett, S.; Jarjoura, D.; Wickstrom, G.C.; Hua, K.; Weinhardt, J.; Wright, K. Effectiveness of a postdischarge care management model for stroke and transient ischemic attack: A randomized trial. J. Stroke Cerebrovasc. Dis. 2002, 11, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Tilling, K.; Wilson-Barnett, J.; Newham, D.J.; Wolfe, C.D.A. Effect of recommended positioning on stroke outcome at six months: A randomized controlled trial. Clin. Rehabil. 2005, 19, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Sulch, D.; Perez, I.; Melbourn, A.; Kalra, L.; Sulch, D.; Perez, I.; Melbourn, A.; Kalra, L. Randomized controlled trial of integrated (managed) care pathway for stroke rehabilitation. Stroke 2000, 31, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Irewall, A.L.; Ogren, J.; Bergstrom, L.; Laurell, K.; Soderstrom, L.; Mooe, T. Nurse-led, telephone-based, secondary preventive follow-up after stroke or transient ischemic attack improves blood pressure and LDL cholesterol: Results from the first 12 months of the randomized, controlled nailed stroke risk factor trial. PLoS ONE 2015, 10, e0139997. [Google Scholar] [CrossRef] [PubMed]
- Boter, H. Multicenter randomized controlled trial of an outreach nursing support program for recently discharged stroke patients. Stroke 2004, 35, 2867–2872. [Google Scholar] [CrossRef] [PubMed]
- Barker-Collo, S.; Krishnamurthi, R.; Witt, E.; Feigin, V.; Jones, A.; McPherson, K.; Starkey, N.; Parag, V.; Jiang, Y.; Barber, P.A.; et al. Improving adherence to secondary stroke prevention strategies through motivational interviewing: Randomized controlled trial. Stroke 2015, 46, 3451–3458. [Google Scholar] [CrossRef] [PubMed]
- Forster, A.; Dickerson, J.; Young, J.; Patel, A.; Kalra, L.; Nixon, J.; Smithard, D.; Knapp, M.; Holloway, I.; Anwar, S.; et al. A structured training programme for caregivers of inpatients after stroke (tracs): A cluster randomised controlled trial and cost-effectiveness analysis. Lancet 2013, 382, 2069–2076. [Google Scholar] [CrossRef]
- Higgins, J.; Salbach, N.M.; Wood-Dauphinee, S.; Richards, C.L.; Cote, R.; Mayo, N.E. The effect of a task-oriented intervention on arm function in people with stroke: A randomized controlled trial. Clin. Rehabil. 2006, 20, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Forster, A.; Bogle, S.; Young, J. Physiotherapy for patients with mobility problems more than 1 year after stroke: A randomised controlled trial. Lancet 2002, 359, 199–203. [Google Scholar] [CrossRef]
- Meyer, M.J.; Teasell, R.; Thind, A.; Koval, J.; Speechley, M. A synthesis of peer-reviewed literature on team-coordinated and delivered early supported discharge after stroke. Can. J. Neurol. Sci. 2016, 43, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Feltner, C.; Jones, C.D.; Cene, C.W.; Zheng, Z.J.; Sueta, C.A.; Coker-Schwimmer, E.J.; Arvanitis, M.; Lohr, K.N.; Middleton, J.C.; Jonas, D.E. Transitional care interventions to prevent readmissions for persons with heart failure: A systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Fens, M.; Vluggen, T.P.; van Haastregt, J.C.; Verbunt, J.A.; Beusmans, G.H.; van Heugten, C.M. Multidisciplinary care for stroke patients living in the community: A systematic review. J. Rehabil. Med. 2013, 45, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.K.; Brown, R.M.; Erikssen, L.; Tieman, J.J. Multidisciplinary care planning in the primary care management of completed stroke: A systematic review. BMC Fam. Pract. 2008, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Prvu Bettger, J.A.; Stineman, M.G. Effectiveness of multidisciplinary rehabilitation services in postacute care: State-of-the-science. A review. Arch. Phys. Med. Rehabil. 2007, 88, 1526–1534. [Google Scholar] [CrossRef] [PubMed]
- Warner, G.; Packer, T.; Villeneuve, M.; Audulv, A.; Versnel, J. A systematic review of the effectiveness of stroke self-management programs for improving function and participation outcomes: Self-management programs for stroke survivors. Disabil. Rehabil. 2015, 37, 2141–2163. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.; Shelling, L.; Dennett, R.; Ayers, T.; Evans, P.H.; Campbell, J.L. The effectiveness of various models of primary care-based follow-up after stroke: A systematic review. Prim. Health Care Res. Dev. 2011, 12, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Puhr, M.I.; Thompson, H.J. The use of transitional care models in patients with stroke. J. Neurosci. Nurs. 2015, 47, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Brady, B.K.; McGahan, L.; Skidmore, B. Systematic review of economic evidence on stroke rehabilitation services. Int. J. Technol. Assess. Health Care 2005, 21, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.Y.; Chair, S.Y.; Chau, J.P. The effectiveness of caregiver psychosocial interventions on the psychosocial wellbeing, physical health and quality of life of stroke family caregivers and their stroke survivors: A systematic review. JBI Lib. Syst. Rev. 2012, 10, 679–797. [Google Scholar] [CrossRef] [PubMed]

| Category | Definition |
|---|---|
| Hospital-initiated support | Stroke unit care was combined with early supported discharge (e.g., health education before discharge, discharge action plans, appropriate positioning training, or integrated care pathway service) for patients’ further rehabilitation, and follow-up in close cooperation with the primary healthcare system. |
| Home-visiting program | Home visits by healthcare providers, such as a physician, physiotherapist, occupational therapist, nurse, or pharmacist, who educated, reinforced self-care instructions, performed physical examination, or provided other care (e.g., individual counselling, which focused on education, applying information learned in practical situations, and solving problems occurring at home, was offered to the caregiver if needed, and physical therapy, occupational therapy, or medication reconciliation). These interventions were provided by various providers separately or by a multidisciplinary team. |
| Structured telephone support | Monitoring, education, or self-care management (e.g., lifestyle counselling and assessment of pharmacological treatment) using simple telephone technology after discharge in a structured format (e.g., series of scheduled calls with a specific goal, structured questioning). |
| Outpatient setting- based support | Services provided in a community (e.g., community physiotherapy service, stroke care coordinator service/care, rehabilitation setting, nursing home), except patients’ home. |
| Primary education | Patient education (care management) delivered before or at discharge with motivational interviewing or empowerment intervention for self-management, or structured training program for caregivers. |
| References | Country | Design | Control Group | Intervention Group | Only First-Ever Stroke | Stroke Subtype Described | Duration of Follow-Up (Month) | BI Score Described | Intervention | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N, Male (%) | Age (Mean, y) | N, Male (%) | Age (Mean, y) | ||||||||
| Rasmussen et al. 2016 [34] | Denmark | Single-center | 33 (42.0) | 79 | 38 (42.0) | 78 | NR | No | 3 | Yes | Home-visiting program (MT-led) |
| Guidetti et al. 2015 [35] | Sweden | Multicenter | 151 (63.0) | 71 | 129 (57.0) | 74 | No | No | 12 | Yes | Home-visiting program (OP-led) |
| Wong et al. 2015 [24] | China | Multicenter | 54 (37.0) | 71.5 | 54 (37.0) | 67.5 | No | Yes | 2 | No | Home-visiting program (OP-led) |
| Bertilsson et al. 2014 [36] | Sweden | Multicenter | 151 (63.0) | 71 | 129 (57.0) | 74 | No | No | 3 | Yes | Home-visiting program (OP-led) |
| Chaiyawat et al. 2012 [25] | Thailand | Single-center | 30 (43.0) | 66 | 30 (47.0) | 67 | NR | No | 24 | Yes | Home-visiting program (OP-led) |
| Thorsen et al. 2005 [19] | Spain | Single-center | 41 (58.3) | 71 | 42 (50.0) | 71 | No | Yes | 60 | No | Home-visiting program (MT-led) |
| Donnelly et al. 2004 [30] | UK | Multicenter | 54 (43.0) | 68 | 59 (43.0) | 71 | NR | No | 12 | Yes | Home-visiting program (MT-led) |
| Andersen et al. 2002 [27] | Denmark | Multicenter | 48 (56.3) | 68.3 | 54 (44.4) | 69.8 | No | Yes | 6 | Yes | Home-visiting program (MT-led) |
| Allen et al. 2002 [37] | USA | Single-center | 46 (46.0) | 72 | 47 (43.0) | 69 | NR | Yes | 3 | Yes | Home-visiting program (MT-led) |
| Von Koch et al. 2001 [21] | Sweden | Single-center | 41 (55.0) | 72 | 42 (55.0) | 72 | No | Yes | 12 | No | Home-visiting program (MT-led) |
| Anderson et al. 2000 [14] | Australia | Multicenter | 44 (50.0) | 71 | 42 (62.0) | 72 | No | Yes | 6 | Yes | Home-visiting program (MT-led) |
| Fjaertoft et al. 2011 [16] | Norway | Single-center | 160 (44.0) | 73.8 | 160 (54.0) | 74 | No | No | 60 | Yes | Hospital-initiated support |
| Jones et al. 2005 [38] | UK | Multicenter | 68 (50.0) | 71 | 52 (37.0) | 75 | Yes | No | 6 | Yes | Hospital-initiated support |
| Askim et al. 2004 [12] | Norway | Single-center | 31 (54.8) | 76.3 | 31 (51.6.0) | 76.9 | No | Yes | 12 | Yes | Hospital-initiated support |
| Fjaertoft et al. 2003 [20] | Norway | Single-center | 160 (44.0) | 73.8 | 160 (54.0) | 74 | No | No | 12 | Yes | Hospital-initiated support |
| Fagerberg et al. 2000 [13] | Sweden | Single-center | 83 (46.0) | 79.7 | 167 (34.0) | 80.1 | No | Yes | 12 | No | Hospital-initiated support |
| Indredavik et al. 2000 [22] | Norway | Single-center | 160 (44.0) | 73.8 | 16 (54.0) | 74 | No | No | 6 | Yes | Hospital-initiated support |
| Sulch et al. 2000 [39] | UK | Single-center | 76 (56.0) | 74 | 76 (46.0) | 75 | NR | Yes | 6 | Yes | Hospital-initiated support |
| Irewall et al. 2015 [40] | Sweden | Single-center | 271 (57.2) | 70.1 | 266 (56.8) | 71.5 | No | Yes | 12 | No | Structured telephone support |
| Boter et al. 2004 [41] | Netherlands | Multicenter | 273 (48.0) | 63 | 263 (49.0) | 66 | Yes | Yes | 6 | Yes | Structured telephone support |
| Sit et al. 2016 [23] | China | Single-center | 105 (52.4) | 70.7 | 105 (52.4) | 67.8 | Yes | Yes | 6 | No | Primary education |
| Barker-Collo et al. 2015 [42] | New Zealand | NR | 193 (NR) | NR | 193 (NR) | NR | No | No | 12 | No | Primary education |
| Forster et al. 2013 [43] | UK | Multicenter | 478 (32.0) | 60.8 | 450 (31.0) | 61.1 | No | Yes | 12 | No | Primary education |
| Watkins et al. 2011 [17] | UK | Single-center | 207 (58.9) | 70 | 204 (57.8) | 70 | No | Yes | 12 | Yes | Primary education |
| Watkins et al. 2007 [18] | UK | Single-center | 207 (58.9) | 70 | 204 (57.8) | 70 | No | Yes | 3 | Yes | Primary education |
| Forster et al. 2015 [28] | UK | Multicenter | 399 (54.6) | 72.5 | 401 (53.6) | 70.9 | NR | Yes | 12 | No | Outpatient setting-based |
| Askim et al. 2010 [10] | Norway | Single-center | 32 (55.2) | 77.6 | 30 (40.4) | 75.4 | No | No | 6 | Yes | Outpatient setting-based |
| Langhammer et al. 2007 [26] | Norway | Multicenter | 40 (NR) | 72 | 35 (NR) | 76 | Yes | Yes | 12 | Yes | Outpatient setting-based |
| Higgins et al. 2006 [44] | Canada | Multicenter | 44 (59.0) | 71 | 47 (64.0) | 73 | No | Yes | 1.5 | No | Outpatient setting-based |
| Sackley et al. 2006 [29] | UK | Multicenter | 55 (18.0) | 86.3 | 63 (17.0) | 88.6 | NR | No | 6 | Yes | Outpatient setting-based |
| Green et al. 2002 [45] | UK | Multicenter | 85 (54.0) | 73.5 | 85 (58.0) | 71.5 | NR | No | 9 | Yes | Outpatient setting-based |
| References | Randomization Methods Reported | Researcher/Participant Blinded | Allocation Concealment | Blinding of Assessors | Inclusion/Exclusion Criteria Described | Attrition Rate Reported | Participants Lost to Follow Up Described | Intention to Treat Analysis | Similarity at Baseline | Power Analysis | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rasmussen et al. 2016 [34] | Yes | No | Yes | Yes | Yes | No | Yes | NR | Yes | Yes | Low |
| Guidetti et al. 2015 [35] | No | NR | NR | Yes | Yes | No | Yes | Yes | NR | Yes | Unclear |
| Wong et al. 2015 [24] | Yes | NR | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Low |
| Bertilsson et al. 2014 [36] | NR | NR | NR | Yes | Yes | No | Yes | Yes | NR | Yes | High |
| Chaiyawat et al. 2012 [25] | Yes | No | Yes | No | Yes | No | Yes | Yes | Yes | Yes | High |
| Thorsen et al. 2005 [19] | Yes | NR | Yes | Yes | Yes | No | Yes | No | Yes | NR | Low |
| Donnelly et al. 2004 [30] | Yes | NR | Yes | Yes | Yes | No | Yes | NR | Yes | Yes | Low |
| Andersen et al. 2002 [27] | Yes | NR | Yes | Yes | Yes | No | Yes | Yes | Yes | NR | Low |
| Allen et al. 2002 [37] | Yes | NR | Yes | No | Yes | No | Yes | NR | Yes | Yes | High |
| Von Koch et al. 2001 [21] | Yes | NR | Yes | Yes | Yes | No | Yes | No | Yes | Yes | Low |
| Anderson et al. 2000 [14] | Yes | NR | Yes | Yes | Yes | No | Yes | Yes | No | NR | Low |
| Fjaertoft et al. 2011 [16] | NR | NR | NR | Yes | Yes | No | Yes | Yes | Yes | No | High |
| Jones et al. 2005 [38] | NR | No | NR | NR | Yes | Yes | Yes | Yes | No | Yes | High |
| Askim et al. 2004 [12] | Yes | NR | Yes | Yes | Yes | No | Yes | Yes | Yes | NR | Low |
| Fjaertoft et al. 2003 [20] | Yes | NR | Yes | Yes | Yes | No | No | Yes | Yes | NR | High |
| Fagerberg et al. 2000 [13] | NR | NR | NR | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear |
| Indredavik et al. 2000 [22] | NR | NR | NR | Yes | Yes | No | No | Yes | Yes | NR | Unclear |
| Sulch et al. 2000 [39] | Yes | NR | Yes | Yes | Yes | No | No | Yes | Yes | Yes | High |
| Irewall et al. 2015 [40] | Yes | No | NR | No | Yes | No | Yes | Yes | Yes | Yes | High |
| Boter et al. 2004 [41] | Yes | NR | NR | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear |
| Sit et al. 2016 [23] | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Barker-Collo et al. 2015 [42] | Yes | No | NR | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear |
| Forster et al. 2013 [43] | Yes | NR | Yes | NR | Yes | Yes | Yes | Yes | Yes | Yes | Unclear |
| Watkins et al. 2011 [17] | Yes | NR | Yes | Yes | Yes | No | Yes | Yes | Yes | NR | Low |
| Watkins et al. 2007 [18] | Yes | NR | Yes | Yes | Yes | No | Yes | Yes | Yes | NR | Low |
| Forster et al. 2015 [28] | Yes | No | NR | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear |
| Askim et al. 2010 [10] | Yes | No | NR | Yes | Yes | No | Yes | Yes | No | Yes | Unclear |
| Langhammer et al. 2007 [26] | Yes | Yes | NR | NR | Yes | No | Yes | Yes | Yes | Yes | Unclear |
| Higgins et al. 2006 [44] | Yes | NR | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Low |
| Sackley et al. 2006 [29] | Yes | NR | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Low |
| Green et al. 2002 [45] | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Low |
| Subcategory | Intervention Group | Control Group | Fixed Effect Model | Random Effect Model | Heterogeneity | ||||
|---|---|---|---|---|---|---|---|---|---|
| Events | Total | Events | Total | RR (95% CI) | RR (95% CI) | I2 (%) | τ2 | p | |
| Total effect of TCI | 331 | 3817 | 380 | 3820 | 0.86 (0.75–0.98) | 0.85 (0.72–1.01) | 17.50 | 0.03 | 0.20 |
| ≤3 months | 7 | 519 | 28 | 535 | 0.27 (0.12–0.58) | 0.27 (0.12–0.60) | 0.00 | 0.00 | 0.92 |
| ≥6 months | 324 | 3298 | 352 | 3820 | 0.91 (0.79–1.04) | 0.92 (0.80–1.05) | 1.8 | 0.00 | 0.20 |
| Home-visiting program | |||||||||
| Total effect | 20 | 666 | 47 | 693 | 0.46 (0.28–0.74) | 0.47 (0.29–0.79) | 0.00 | 0.00 | 0.62 |
| ≤3 months | 3 | 268 | 16 | 284 | 0.21 (0.07–0.65) | 0.22 (0.07–0.67) | 0.00 | 0.00 | 0.90 |
| ≥6 months | 17 | 398 | 31 | 409 | 0.58 (0.34–1.00) | 0.58 (0.33–1.01) | 0.00 | 0.00 | 0.59 |
| Hospital-initiated support | |||||||||
| Total effect | 178 | 805 | 161 | 738 | 0.99 (0.83–1.09) | 0.98 (0.82–1.17) | 0.00 | 0.00 | 0.73 |
| ≤3 months | - | - | - | - | - | - | - | - | - |
| ≥6 months | 178 | 805 | 161 | 738 | 0.99 (0.83–1.09) | 0.98 (0.82–1.17) | 0.00 | 0.00 | 0.73 |
| Structured telephone support | |||||||||
| Total effect | 16 | 529 | 15 | 544 | 1.17 (0.58, 2.38) | 1.17 (0.58, 2.38) | 0.00 | 0.00 | 0.63 |
| ≤3 months | - | - | - | - | - | - | - | - | - |
| ≥6 months | 16 | 529 | 15 | 544 | 1.17 (0.58, 2.38) | 1.17 (0.58, 2.38) | 0.00 | 0.00 | 0.63 |
| Primary education | |||||||||
| Total effect | 76 | 1156 | 94 | 1190 | 0.84 (0.63–1.12) | 0.74 (0.44–1.23) | 44.30 | 0.14 | 0.13 |
| ≤3 months | 4 | 204 | 12 | 207 | 0.34 (0.11–1.03) | 0.34 (0.11–1.03) | Not applicable for a single study | ||
| ≥6 month | 72 | 952 | 82 | 983 | 0.92 (0.68–1.24) | 0.86 (0.55–1.36) | 29.00 | 0.07 | 0.24 |
| Outpatient setting-based | |||||||||
| Total effect | 51 | 661 | 63 | 655 | 0.79 (0.56–1.11) | 0.70 (0.37–1.31) | 46.50 | 0.21 | 0.11 |
| ≤3 months | 0 | 47 | 0 | 44 | - | - | Not applicable for a single study | ||
| ≥6 months | 51 | 614 | 63 | 611 | 0.79 (0.56–1.11) | 0.70 (0.37–1.31) | 46.50 | 0.21 | 0.11 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yang, F.; Shi, H.; Yang, C.; Hu, H. What Type of Transitional Care Effectively Reduced Mortality and Improved ADL of Stroke Patients? A Meta-Analysis. Int. J. Environ. Res. Public Health 2017, 14, 510. https://doi.org/10.3390/ijerph14050510
Wang Y, Yang F, Shi H, Yang C, Hu H. What Type of Transitional Care Effectively Reduced Mortality and Improved ADL of Stroke Patients? A Meta-Analysis. International Journal of Environmental Research and Public Health. 2017; 14(5):510. https://doi.org/10.3390/ijerph14050510
Chicago/Turabian StyleWang, Yuncui, Fen Yang, Hao Shi, Chongming Yang, and Hui Hu. 2017. "What Type of Transitional Care Effectively Reduced Mortality and Improved ADL of Stroke Patients? A Meta-Analysis" International Journal of Environmental Research and Public Health 14, no. 5: 510. https://doi.org/10.3390/ijerph14050510





