Synthetic Receptors Induce Anti Angiogenic and Stress Signaling on Human First Trimester Cytotrophoblast Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information
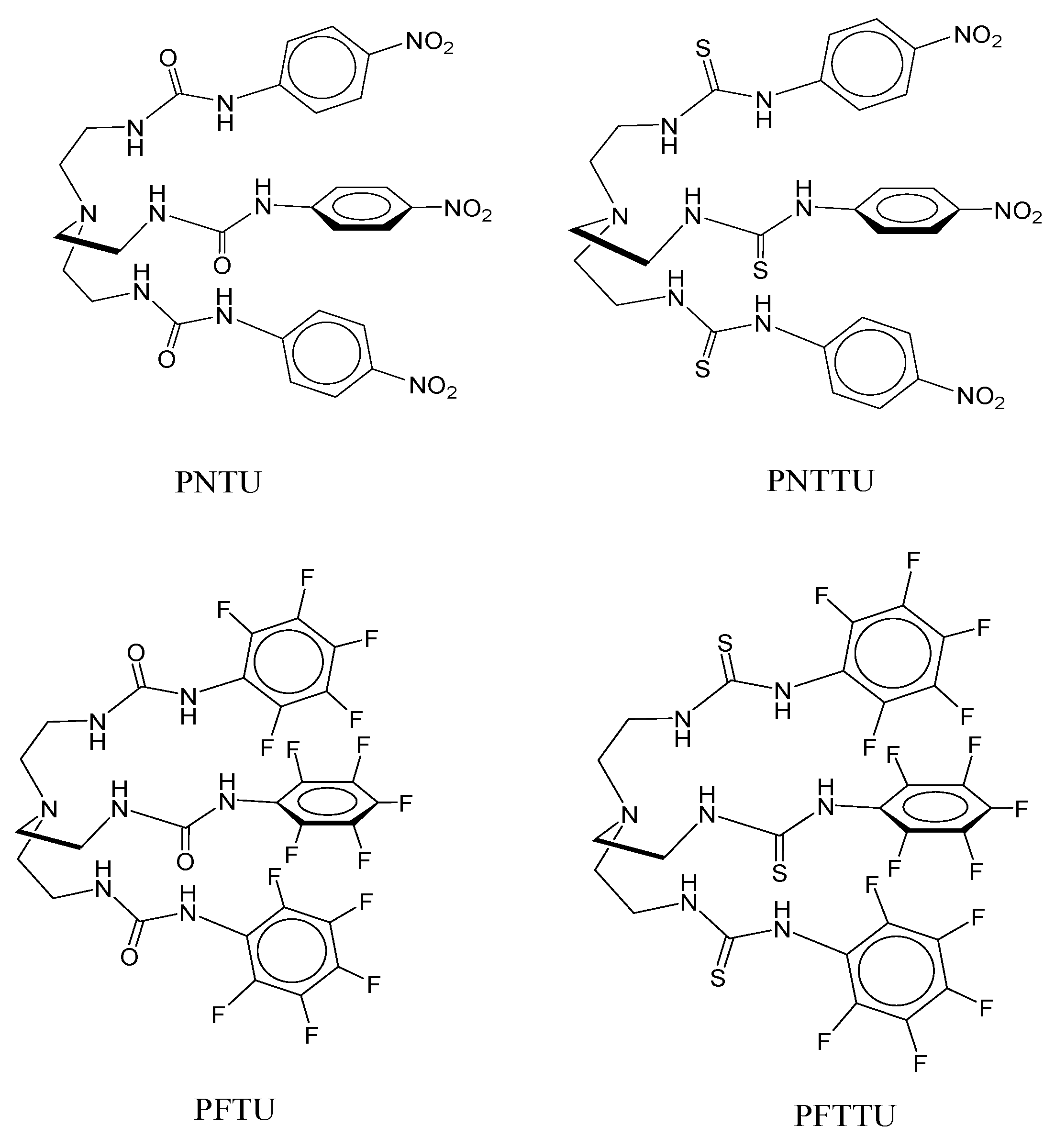
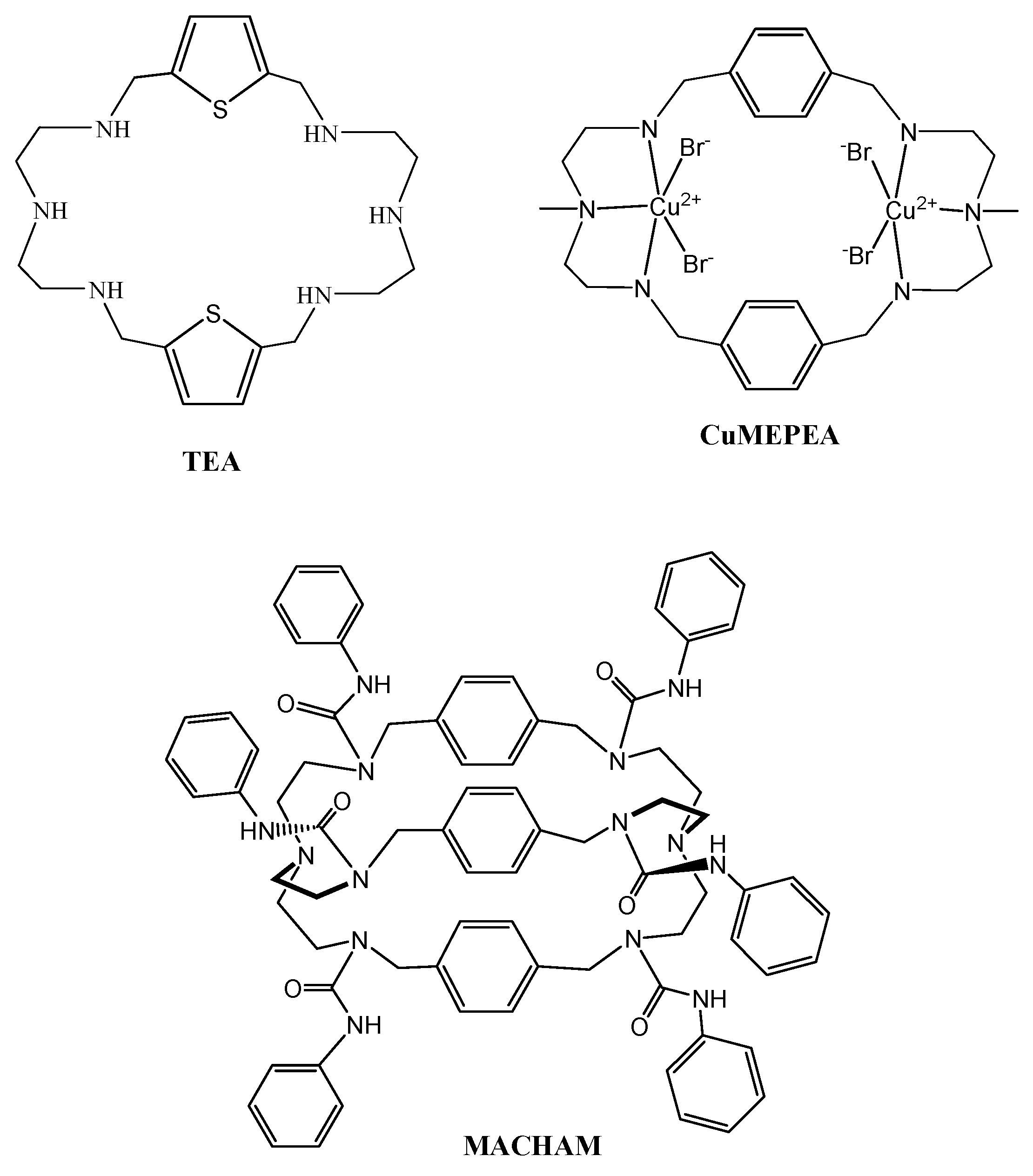
2.2. Synthesis
2.3. Cell Culture
2.4. Treatment of Cells
2.5. ELISA for Angiogenic and Anti-Angiogenic Factors
2.6. Western Blots for VEGFR-1, AT1, and AT2 Receptors
2.7. Statistical Method
3. Results
3.1. PNTU, PFTU, PNTTU, PFTTU, and CuMEPEA Downregulated Angiogenic Factors
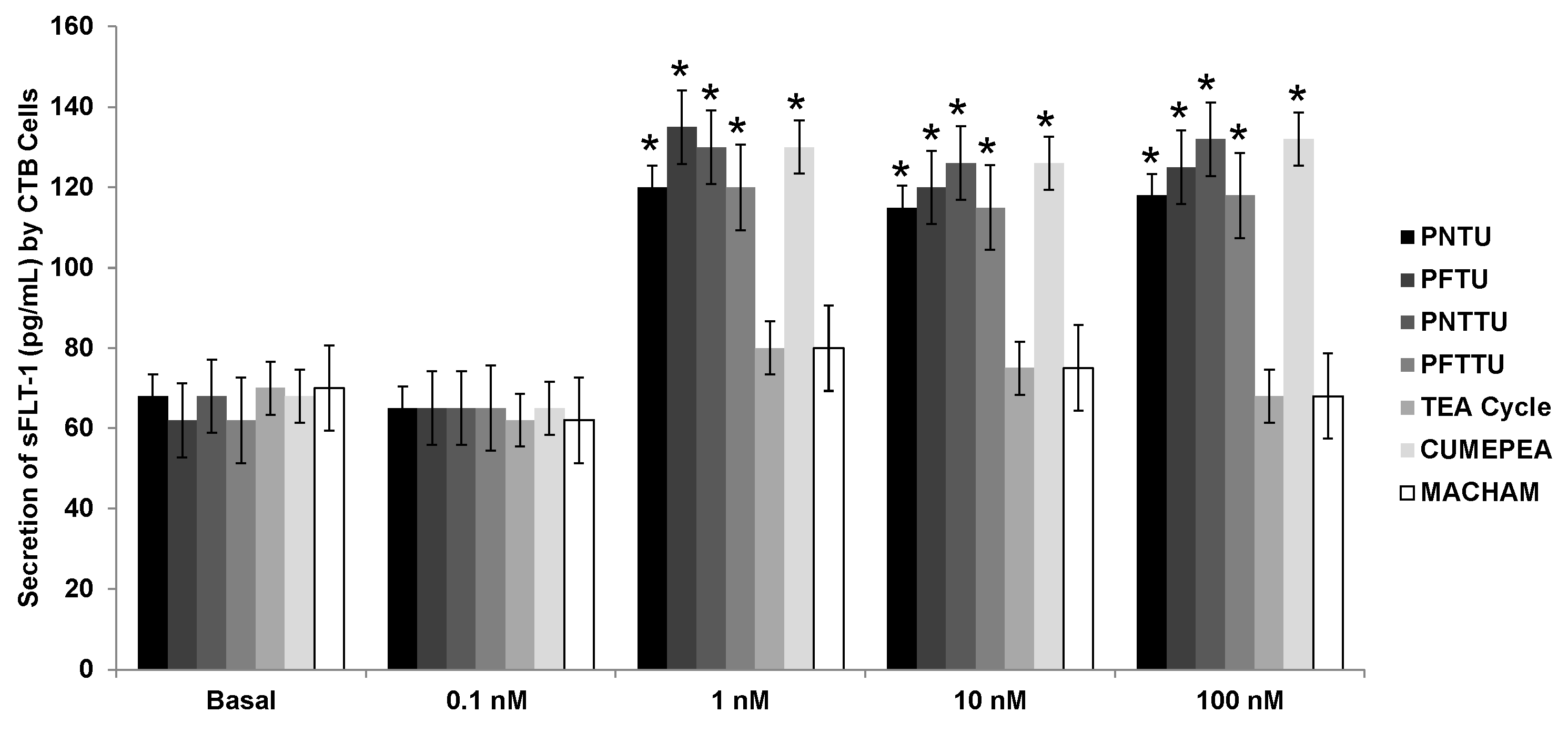
3.2. PNTU, PFTU, PNTTU, PFTTU Upregulated Anti-Angiogenic Factors
3.3. PNTU, PFTU, PNTTU, PFTTU, CuMEPEA, TEA Cycle and MACHAM Upregulated AT2 Receptor Expression
3.4. PNTU, PFTU, PNTTU, PFTTU, CuMEPEA, TEA and MACHAM Downregulated VEGFR-1 and AT1 Receptor Expression
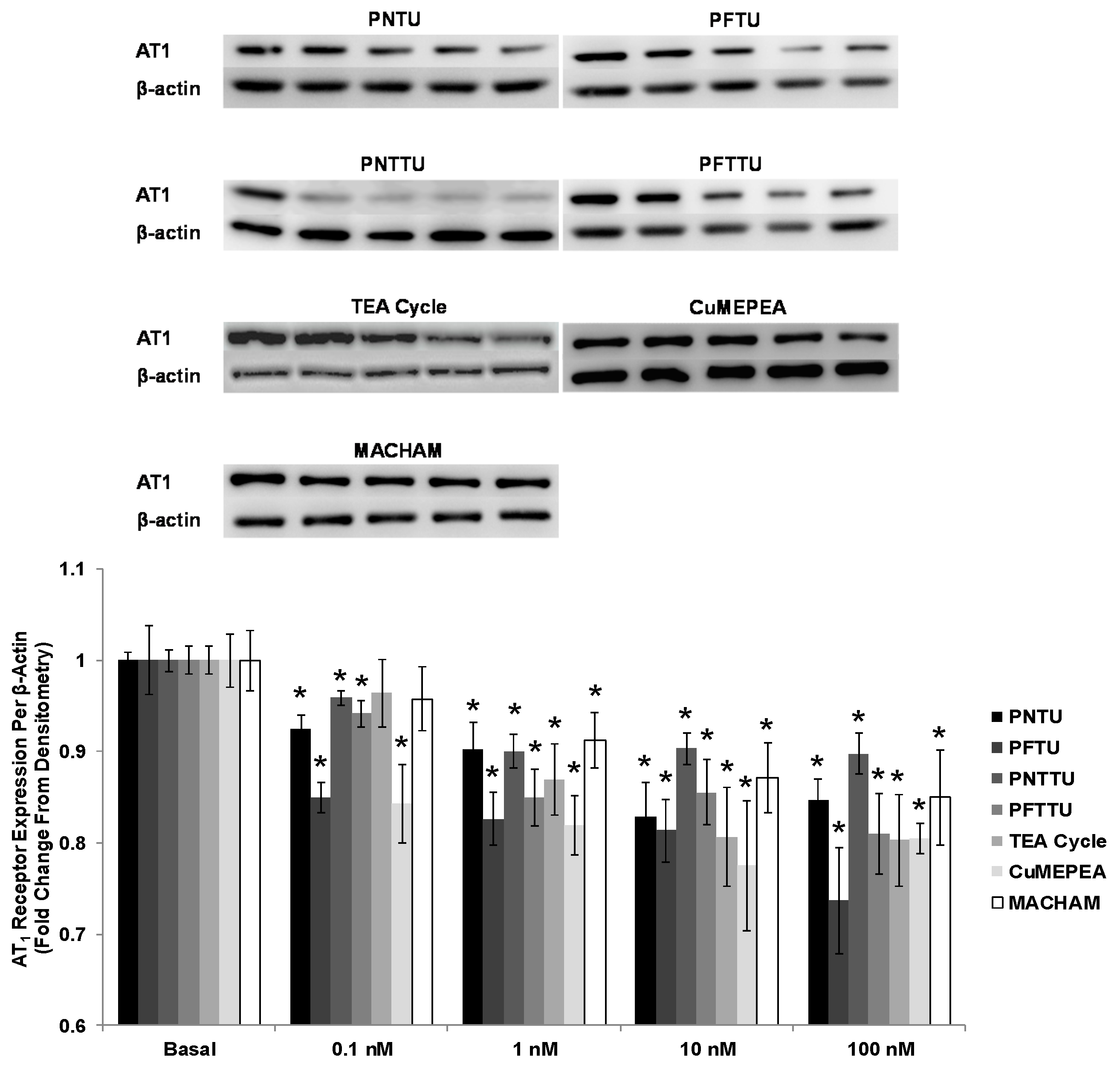
3.5. PNTU, PFTU, PNTTU, PFTTU, and CuMEPEA Downregulated AT1
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Von Dadelszen, P.; Magee, L.A. Pre-eclampsia: An update. Curr. Hypertens. Rep. 2014, 16, 454. [Google Scholar] [CrossRef] [PubMed]
- Steegers, E.A.; Von Dadelszen, P.; Duvekot, J.J.; Pijnenborg, R. Pre-eclampsia. Lancet 2010, 376, 631–644. [Google Scholar] [CrossRef]
- Puschett, J.B.; Agunanne, E.; Uddin, M.N. Emerging role of the bufadienolides in cardiovascular and kidney diseases. Am. J. Kidney Dis. 2010, 56, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Allen, S.R.; Jones, R.O.; Zawieja, D.C.; Kuehl, T.J. Pathogenesis of pre-eclampsia: Marinobufagenin and angiogenic imbalance as biomarkers of the syndrome. Transl. Res. 2012, 160, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.S.; Wojdyla, D.; Say, L.; Gulmezoglu, A.M.; Van Look, P.F. WHO analysis of causes of maternal death: A systematic review. Lancet 2006, 367, 1066–1074. [Google Scholar] [CrossRef]
- Duley, L. The global impact of pre-eclampsia and eclampsia. Semin. Perinatol. 2009, 33, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Berg, C.J.; Mackay, A.P.; Qin, C.; Callaghan, W.M. Overview of maternal morbidity during hospitalization for labor and delivery in the United States: 1993–1997 and 2001–2005. Obstet. Gynecol. 2009, 113, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Wallis, A.B.; Saftlas, A.F.; Hsia, J.; Atrash, H.K. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am. J. Hypertens. 2008, 21, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Leonard, D.; Co, M.A.; Mukhopadhyay, D.; Giri, B.; Perger, L.; Beeram, M.R.; Kuehl, T.J.; Uddin, M.N. Pre-eclampsia has an adverse impact on maternal and fetal health. Transl. Res. 2015, 165, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Bagrov, A.Y.; Shapiro, J.I.; Fedorova, O.V. Endogenous cardiotonic steroids: Physiology, pharmacology, and novel therapeutic targets. Pharmacol. Rev. 2009, 61, 9–38. [Google Scholar] [CrossRef] [PubMed]
- Buckalew, V.M. Endogenous digitalis-like factors: An overview of the history. Front. Endocrinol. 2015, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, O.V.; Agalakova, N.I.; Morrell, C.H.; Lakatta, E.G.; Bagrov, A.Y. ANP differentially modulates marinobufagenin-induced sodium pump inhibition in kidney and aorta. Hypertension 2006, 48, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Schoner, W.; Scheiner-Bobis, G. Endogenous and exogenous cardiac glycosides: Their roles in hypertension, salt metabolism, and cell growth. Am. J. Physiol. Cell. Physiol. 2007, 293, 509–536. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, O.V.; Tapilskaya, N.I.; Bzhelyansky, A.M.; Frolova, E.V.; Nikitina, E.R.; Reznik, V.A.; Kashkin, V.A.; Bagrov, A.Y. Interaction of Digibind with endogenous cardiotonic steroids from preeclamptic placentae. J. Hypertens. 2010, 28, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shapiro, J.I. Regulation of sodium pump endocytosis by cardiotonic steroids: Molecular mechanisms and physiological implications. Pathophysiology 2007, 14, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Agunanne, E.E.; Horvat, D.; Puschett, J.B. Resibufogenin administration prevents oxidative stress in a rat model of human preeclampsia. Hypertens. Pregnancy 2012, 31, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, O.V.; Simbirtsev, A.S.; Kolodkin, N.I.; Kotov, A.Y.; Agalakova, N.I.; Kashkin, V.A.; Tapilskaya, N.I.; Bzhelyansky, A.; Reznik, V.A.; Frolova, E.V.; et al. Monoclonal antibody to an endogenous bufadienolide, marinobufagenin, reverses preeclampsia-induced Na/K-ATPase inhibition and lowers blood pressure in NaCl-sensitive hypertension. Hypertension 2008, 24, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T. Vascular Na+/Ca2+ exchanger: Implications for the pathogenesis and therapy of salt-dependenthypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R536–R545. [Google Scholar] [CrossRef] [PubMed]
- Graves, S.W.; Williams, G.H. An endogenous ouabain-like factor associated with hypertensive pregnant women. J. Clin. Endocrinol. MeTable 1984, 59, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Goodlin, R.C. Antidigoxin antibodies in eclampsia. N. Engl. J. Med. 1988, 318, 518–519. [Google Scholar] [PubMed]
- Adair, C.D.; Buckalew, V.; Taylor, K.; Ernest, J.M.; Frye, A.H.; Evans, C.; Veille, J.C. Elevated endoxin-like factor complicating a multifetal second trimester pregnancy: Treatment with digoxin-binding immunoglobulin. Am. J. Nephrol. 1996, 16, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Lopatin, D.A.; Ailamazian, E.K.; Dmitrieva, R.I.; Shpen, V.M.; Fedorova, O.V.; Doris, P.A.; Bagrov, A.Y. Circulating bufadienolide and cardenolide sodium pump inhibitors in preeclampsia. J. Hypertens. 1999, 17, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Abi-Ghanem, D.; Lai, X.; Berghman, L.R.; Horvat, D.; Li, J.; Romo, D.; Uddin, M.N.; Kamano, Y.; Nogawa, T.; Xu, J.P.; et al. A chemifluorescent immunoassay for the determination of marinobufagenin in body fluids. J. Immunoassay Immunochem. 2011, 32, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Agunanne, E.; Horvat, D.; Harrison, R.; Uddin, M.N.; Jones, R.; Kuehl, T.J.; Ghanem, D.A.; Berghman, L.R.; Lai, X.; Li, J.; et al. Marinobufagenin Levels in Preeclamptic Patients: A Preliminary Report. Am. J. Perinatol. 2011, 28, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Averina, I.V.; Tapilskaya, N.I.; Reznik, V.A.; Frolova, E.V.; Fedorova, O.V.; Lakatta, E.G.; Bagrov, A.Y. Endogenous Na/K-ATPase inhibitors in patients with preeclampsia. Cell. Mol. Biol. 2006, 52, 19–23. [Google Scholar] [PubMed]
- LaMarca, H.L.; Morris, C.A.; Pettit, G.R.; Nagowa, T.; Puschett, J.B. Marinobufagenin impairs first trimester cytotrophoblast differentiation. Placenta 2006, 27, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Horvat, D.; Glaser, S.S.; Danchuk, S.; Mitchell, B.M.; Sullivan, D.E.; Morris, C.A.; Puschett, J.B. Marinobufagenin inhibits proliferation and migration of cytotrophoblast and CHO cells. Placenta 2008, 29, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Horvat, D.; Glaser, S.S.; Mitchell, B.M.; Puschett, J.B. Examination of the cellular mechanisms by which marinobufagenin inhibits cytotrophoblast function. J. Biol. Chem. 2008, 283, 17946–17953. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Horvat, D.; Childs, E.W.; Puschett, J.B. Marinobufagenin causes endothelial cell monolayer hyperpermeability by altering apoptotic signaling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1726–R1734. [Google Scholar] [CrossRef] [PubMed]
- Ing, N.H.; Berghman, L.; Abi-Ghanem, D.; Abbas, K.; Kaushik, A.; Riggs, P.K.; Puschett, J.B. Marinobufagenin regulates permeability and gene expression of brain endothelial cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R918–R924. [Google Scholar] [CrossRef] [PubMed]
- Afroze, S.H.; Sloan, J.; Grace-Ann, C.; Osuji, G.C.; Drever, N.; Pilkinton, K.; Zawieja, D.C.; Kuehl, T.; Uddin, M.N. Cinobufotalin impedes Sw.71 cytotrophoblast cell line function via cell cycle arrest and apoptotic signaling. Mol. Cell. Biochem. 2016, 422, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J. Pathogenesis and promising non-invasive markers for preeclampsia. Obstet. Gynecol. Sci. 2013, 56, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Ehrig, J.C.; Horvat, D.; Allen, S.R.; Jones, R.O.; Kuehl, T.J.; Uddin, M.N. Cardiotonic steroids induce anti-angiogenic and anti-proliferative profiles in first trimester extravillouscytotrophoblast cells. Placenta 2014, 35, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Gurusinghe, S.; Wallace, E.M.; Lim, R. The relationship between Activin A and anti-angiogenic factors in the development of pre-eclampsia. Pregnancy Hypertens. 2014, 4, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Maynard, S.; Karumanchi, S. Angiogenic factors and preeclampsia. MBG is elevated in both animal models of volume expansion and patients with preE. Semin. Nephrol. 2001, 31, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.; Maynard, S.; Qian, C.; Lim, K.H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, I.; Karumanchi, S.A. Preeclampsia and the anti-angiogenic state. Pregnancy Hypertens. 2011, 1, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Maynard, S.E.; Min, J.Y.; Merchan, J.; Lim, K.H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Cerdeira, A.S.; Karumanchi, S.A. Angiogenic factors in preeclampsia and related disorders. Cold Spring Harb. Perspect. Med. 2012, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Verlohren, S.; Herraiz, I.; Lapaire, O.; Schlembach, D.; Moertl, M.; Zeisler, H.; Calda, P.; Holzgreve, W.; Galindo, A.; Engels, T.; et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am. J. Obstet. Gynecol. 2012, 206, 58.e1–58.e8. [Google Scholar] [CrossRef] [PubMed]
- Stepan, H.; Herraiz, I.; Schlembach, D.; Verlohren, S.; Brennecke, S.; Chantraine, F.; Klein, E.; Lapaire, O.; Llurba, E.; Ramoni, A.; et al. Implementation of the sFlt-1/PlGF ratio for prediction and diagnosis of pre-eclampsia in singleton pregnancy: Implications for clinical practice. Ultrasound Obstet. Gynecol. 2015, 45, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Nikitina, E.R.; Mikhailov, A.V.; Nikandrova, E.S.; Frolova, E.V.; Fadeev, A.V.; Shman, V.V.; Shilova, V.Y.; Tapilskaya, N.I.; Shapiro, J.I.; Fedorova, O.V.; et al. In preeclampsia endogenous cardiotonic steroids induce vascular fibrosis and impair relaxation of umbilical arteries. J. Hypertens. 2011, 29, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Khansari, M.E.; Johnson, C.R.; Basaran, I.; Nafis, A.; Wang, J.; Leszczynski, J.; Hossain, M.A. Synthesis and anion binding studies of tris(3-aminopropyl)amine-based tripodal urea and thiourea receptors: Proton transfer-induced selectivity for hydrogen sulfate over sulfate. RSC Adv. 2015, 5, 17606–17614. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A. Inclusion complexes of halide anions with macrocyclic receptors. Curr. Org. Chem. 2008, 12, 1231–1256. [Google Scholar] [CrossRef]
- Busschaert, N.; Wenzel, M.; Light, M.E.; Iglesias-Hernandez, P.; Perez-Tomas, R.; Gale, P.A. Structure-activity relationships in tripodal transmembrane anion transporters: The effect of fluorination. J. Am. Chem. Soc. 2011, 133, 14136–14148. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.A.; Saeed, M.A.; Jahan, A.; Wang, J.; Leszczynski, J.; Hossain, M.A. Experimental and theoretical aspects of anion complexes with a thiophene-based cryptand. Comments Inorg. Chem. 2016, 36, 305–326. [Google Scholar] [CrossRef] [PubMed]
- Rhaman, M.M.; Alamgir, A.; Wong, B.M.; Powell, D.R.; Hossain, M.A. A highly efficient dinuclear Cu(II) chemosensor for colorimetric and fluorescent detection of cyanide in water. RSC Adv. 2014, 4, 54263–54267. [Google Scholar] [CrossRef] [PubMed]
- Rhaman, M.M.; Fronczek, F.R.; Powell, D.; Hossain, M.A. Colourimetric and fluorescent detection of oxalate in water by a new macrocycle-based dinuclear nickel complex: A remarkable red shift of fluorescence band. Dalton Trans. 2014, 43, 4618–4621. [Google Scholar] [CrossRef] [PubMed]
- Rhaman, M.M.; Ahmed, L.; Wang, J.; Powell, D.; Leszczynski, J.; Hossain, M.A. Encapsulation and selectivity of sulfate with a furan-based hexaaza macrocyclic receptor in water. Org. Biomol. Chem. 2014, 12, 2045–2048. [Google Scholar] [CrossRef] [PubMed]
- Khansari, M.E.; Wallace, K.D.; Hossain, M.A. Synthesis and anion recognition studies of a dipodalthiourea-based sensor for anions. Tetrahedron Lett. 2014, 55, 438–440. [Google Scholar] [CrossRef]
- Pramanik, A.; Powell, D.R.; Wong, B.M.; Hossain, M.A. Spectroscopic, structural, and theoretical studies of halide complexes with a urea-based tripodal receptor. Inorg. Chem. 2012, 51, 4274–4284. [Google Scholar] [CrossRef] [PubMed]
- Horvat, D.; Khansari, M.E.; Pramanik, A.; Beeram, M.R.; Kuehl, T.J.; Hossain, M.A.; Uddin, M.N. A synthetic thiourea-based tripodal receptor that impairs the function of human first trimester cytotrophoblast cells. Int. J. Environ. Res. Public Health 2014, 11, 7456–7469. [Google Scholar] [CrossRef] [PubMed]
- Penniyaynen, V.A.; Kipenko, A.V.; Lopatina, E.V.; Bagrov, A.Y.; Krylov, B.V. The effect of marinobufagenin on the growth and proliferation of cells in the organotypic culture. Dokl. Biol. Sci. 2015, 462, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Afroze, S.H.; Munshi, M.K.; Martínez, A.K.; Uddin, M.N.; Gergely, M.; Szynkarski, C.; Guerrier, M.; Nizamutdinov, D.; Dostal, D.; Glaser, S. Activation of the renin-angiotensin system stimulates biliary hyperplasia during cholestasis induced by extrahepatic bile duct ligation. Am. J. Physiol. Gastrointest. Liver Physiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Sadoshima, J. Cytokine actions of angiotensin II. Circ. Res. 2000, 86, 1187–1189. [Google Scholar] [CrossRef] [PubMed]
- Ehrig, J.C.; Afroze, S.H.; Reyes, M.; Allen, S.R.; Drever, N.S.; Pilkinton, K.A.; Kuehl, T.J.; Uddin, M.N. A p38 mitogen-activated protein kinase inhibitor attenuates cardiotonic steroids-induced apoptotic and stress signaling in a Sw-71 cytotrophoblast cell line. Placenta 2015, 36, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Berg, C.J.; Atrash, H.K.; Koonin, L.M.; Tucker, M. Pregnancy-related mortality in the United States, 1987–1990. Obstet. Gynecol. 1996, 88, 161–167. [Google Scholar] [CrossRef]
- Pridjian, G.; Puschett, J.B. Preeclampsia. Part 1: Clinical and pathophysiologic considerations. Obstet. Gynecol. Surv. 2002, 57, 598–618. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantho, A.F.; Price, M.; Ashraf, A.Z.; Wajid, U.; Khansari, M.E.; Jahan, A.; Afroze, S.H.; Rhaman, M.M.; Johnson, C.R.; Kuehl, T.J.; et al. Synthetic Receptors Induce Anti Angiogenic and Stress Signaling on Human First Trimester Cytotrophoblast Cells. Int. J. Environ. Res. Public Health 2017, 14, 517. https://doi.org/10.3390/ijerph14050517
Pantho AF, Price M, Ashraf AZ, Wajid U, Khansari ME, Jahan A, Afroze SH, Rhaman MM, Johnson CR, Kuehl TJ, et al. Synthetic Receptors Induce Anti Angiogenic and Stress Signaling on Human First Trimester Cytotrophoblast Cells. International Journal of Environmental Research and Public Health. 2017; 14(5):517. https://doi.org/10.3390/ijerph14050517
Chicago/Turabian StylePantho, Ahmed F., Mason Price, AHM Zuberi Ashraf, Umaima Wajid, Maryam Emami Khansari, Afsana Jahan, Syeda H. Afroze, Md Mhahabubur Rhaman, Corey R. Johnson, Thomas J. Kuehl, and et al. 2017. "Synthetic Receptors Induce Anti Angiogenic and Stress Signaling on Human First Trimester Cytotrophoblast Cells" International Journal of Environmental Research and Public Health 14, no. 5: 517. https://doi.org/10.3390/ijerph14050517




