Replication of High Fetal Alcohol Spectrum Disorders Prevalence Rates, Child Characteristics, and Maternal Risk Factors in a Second Sample of Rural Communities in South Africa
Abstract
:1. Introduction
1.1. Diagnosing a Continuum
1.2. Epidemiology Studies of FASD in South Africa: Creating a Sampling Distribution
1.3. The Current Study
2. Materials and Methods
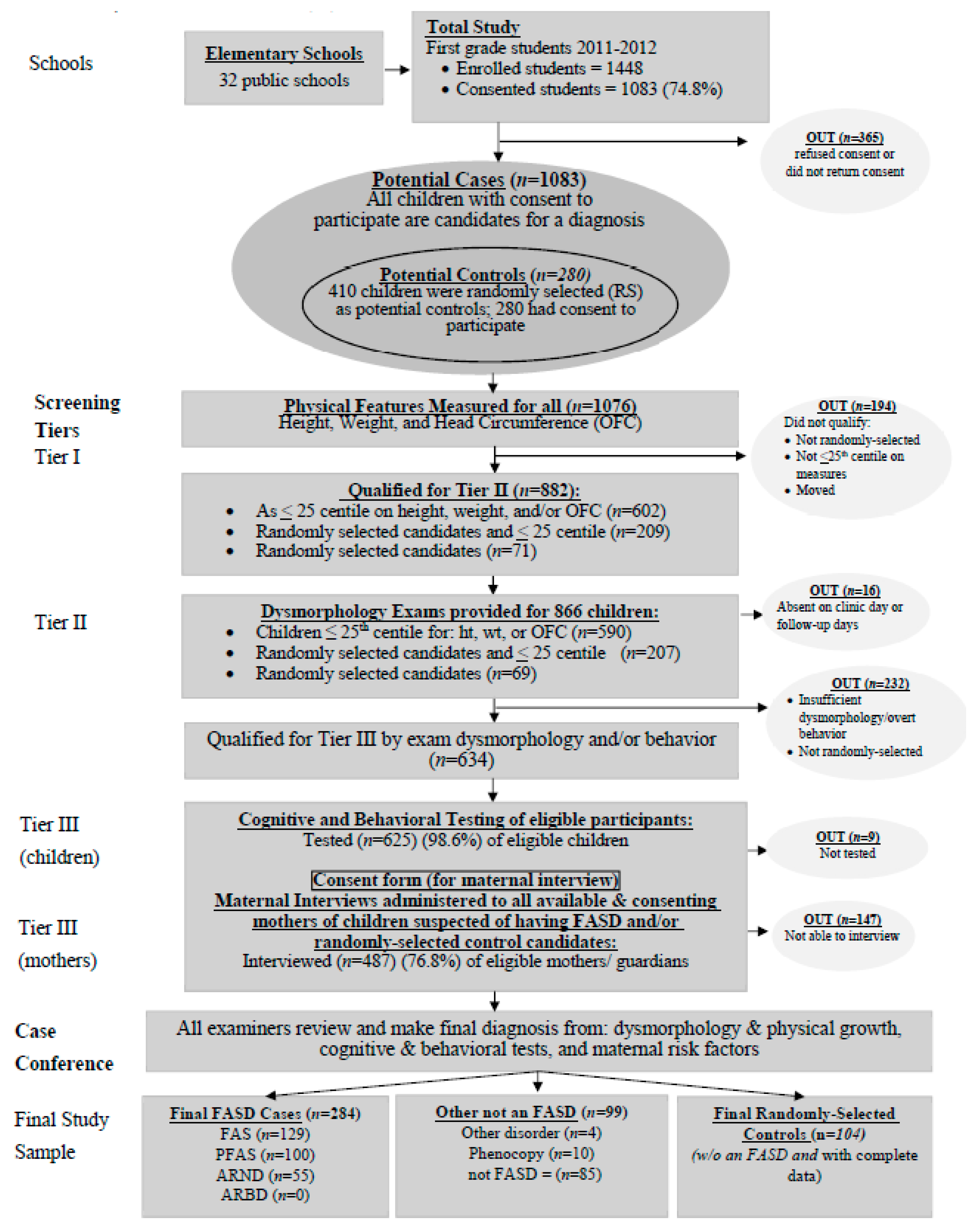
2.1. Sampling and Recruitment
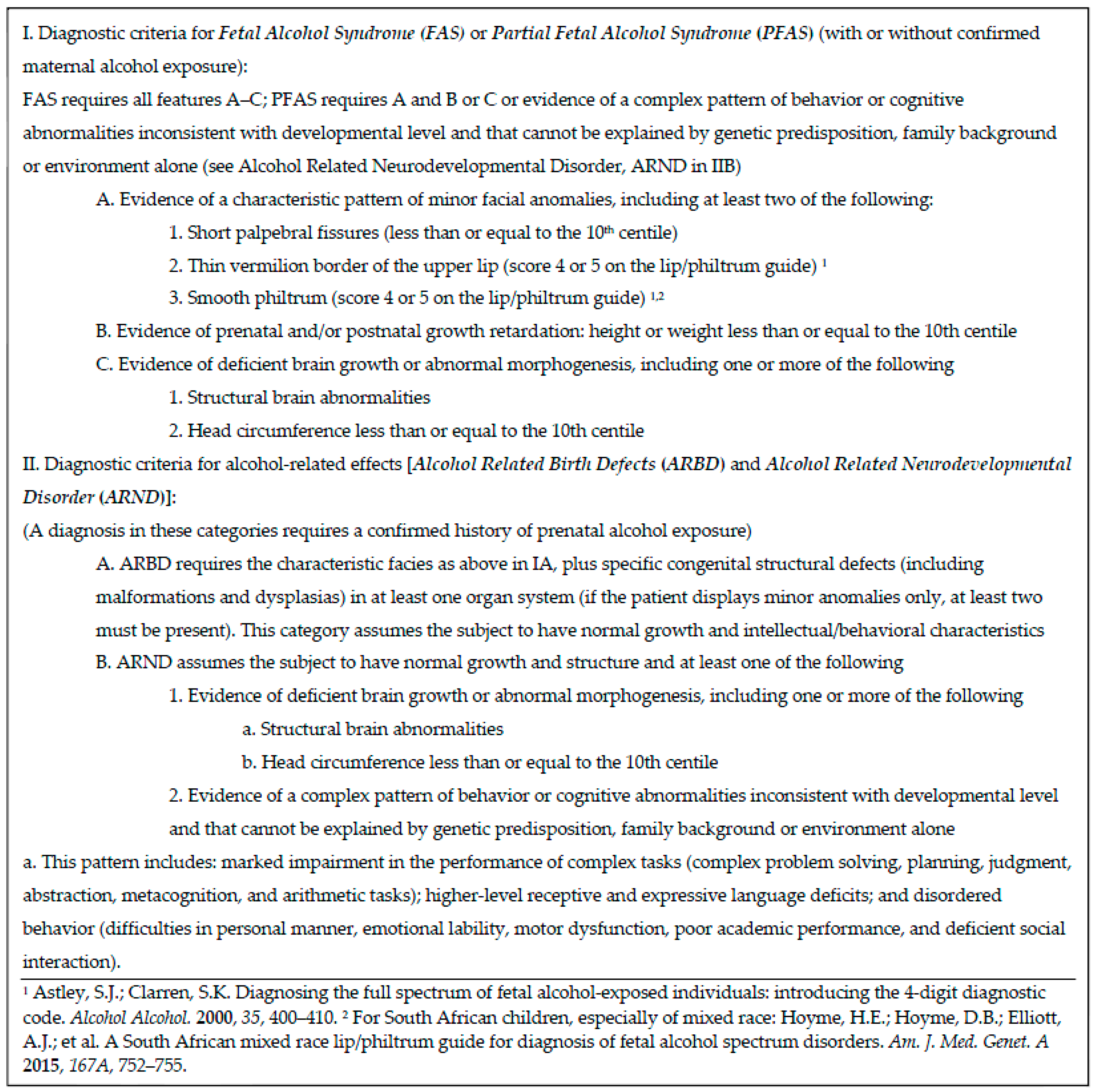
2.2. IOM Diagnostic Categories
2.3. Assessment of Cognitive and Behavioral Traits
2.4. Maternal Risk Factor Assessment: Proximal and Distal Variables
2.5. Multidisciplinary Case Conference for Final Diagnosis
2.6. Statistical Analysis
3. Results
3.1. Child Physical Growth and Development and Dysmorphology
3.2. Cognitive and Behavioral Traits
3.3. Proximal Maternal Risk Variables: Alcohol Use during Pregnancy
3.4. Correlating FASD Traits with Alcohol Use
3.5. Tobacco Use
3.6. Distal Maternal Risk Traits—Physical, Childbearing, and Demographic
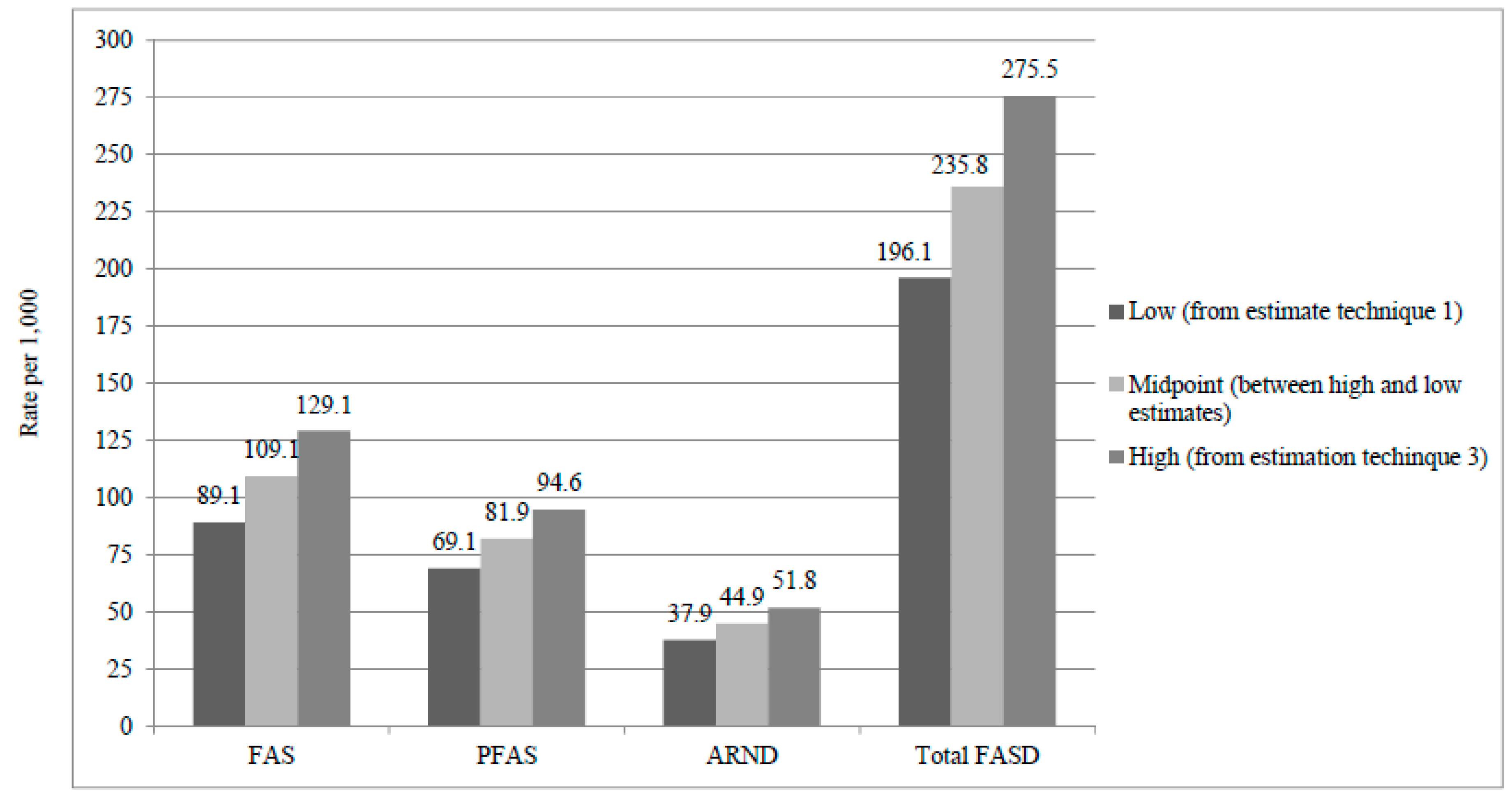
3.7. Prevalence Estimates by Three Methods, Their Calculation, and the Final Estimates
3.8. Comparing Sample 1 to Sample 2 in This Region
4. Discussion
4.1. Summary of Findings
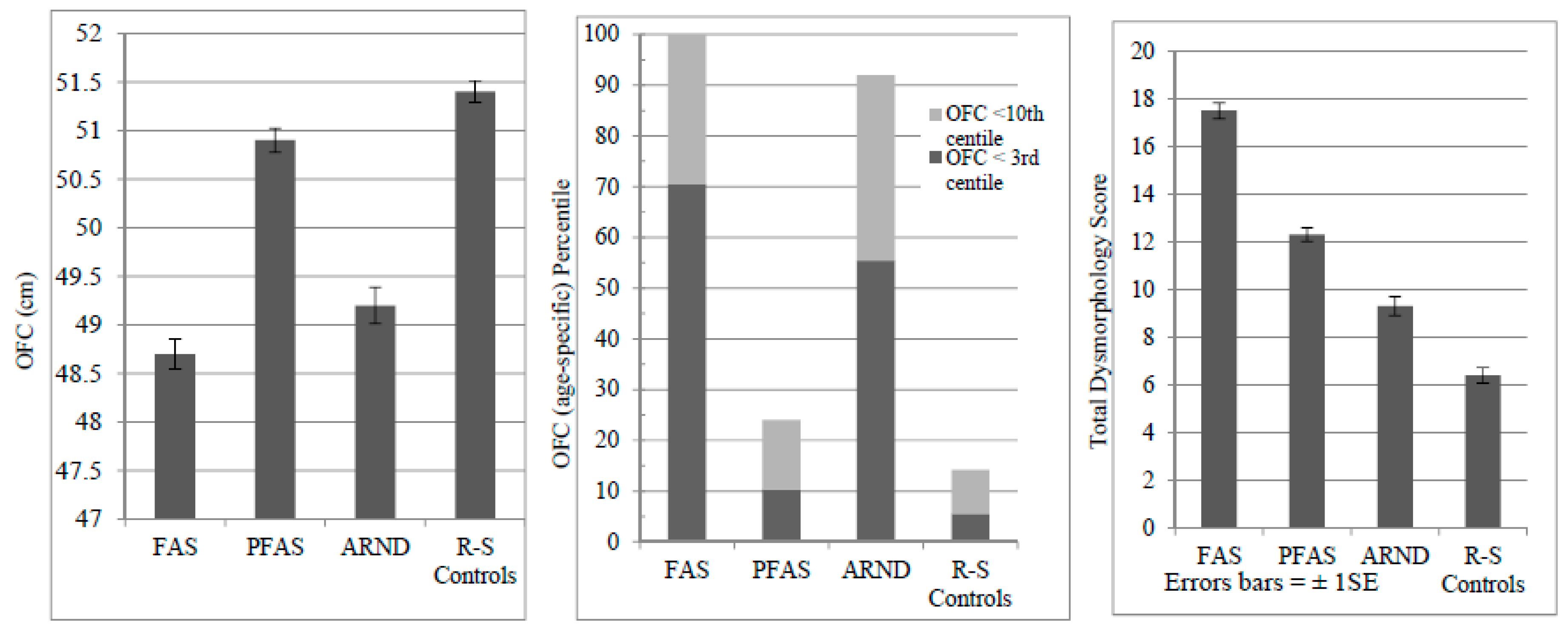
4.2. Head Circumference Is Suppressed in Children with FASD
4.3. Severity: Almost Half of All FASD Diagnosed in This Sample Meet Criteria for FAS
4.4. Convergence of Prevalence Rates from Estimation Techniques and Stability of Rates
4.5. Linking FASD to Prenatal Alcohol Use
4.6. Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jones, K.J.; Smith, D.W. Recognition of the fetal alcohol syndrome in early infancy. Lancet 1973, 302, 999–1001. [Google Scholar] [CrossRef]
- Stratton, K.R.; Howe, C.J.; Battaglia, F.C. Fetal Alcohol Syndrome Diagnosis, Epidemiology, Prevention, and Treatment; Institute of Medicine, National Academy Press: Washington, DC, USA, 1996. [Google Scholar]
- Hoyme, H.E.; May, P.A.; Kalberg, W.O.; Kodituwakku, P.; Gossage, J.P.; Trujillo, P.M.; Buckley, D.G.; Miller, J.; Khaole, N.; Viljoen, D.L.; et al. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: Clarification of the 1996 Institute of Medicine criteria. Pediatrics 2005, 115, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Hoyme, H.E.; Kalberg, W.O.; Elliott, A.J.; Blankenship, J.; Buckley, D.; Marais, A.S.; Manning, M.A.; Robinson, L.K.; Adam, M.P.; Abdul-Rahman, O.; et al. Updated clinical guidelines for diagnosing fetal alcohol spectrum disorders. Pediatrics 2016, 138, e20154256. [Google Scholar] [CrossRef] [PubMed]
- Streissguth, A.P.; O’Malley, K. Neuropsychiatric implications and long-term consequences of fetal alcohol spectrum disorders. Sem. Clin. Neuropsychol. 2000, 47, 177–190. [Google Scholar] [CrossRef]
- Warren, K.; Floyd, L.; Calhoun, F.; Stone, D.; Bertrand, J.; Streissguth, A.; Lierman, T.; Riley, E.; O’Malley, K.; Cook, J.; et al. Consensus Statement on FASD; National Organization on Fetal Alcohol Syndrome: Washington, DC, USA, 2004. [Google Scholar]
- Chasnoff, I.J.; Wells, A.M.; King, L. Misdiagnosis and missed diagnoses in fosters and adopted children with prenatal alcohol exposure. Pediatrics 2015, 135, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Fox, D.J.; Pettygrove, S.; Cunniff, C.; O’Leary, L.A.; Gilboa, S.M.; Bertrand, J.; Druschel, C.M.; Breen, A.; Robinson, L.; Ortiz, L.; et al. Centers for Disease Control and Prevention. Fetal alcohol syndrome among children aged 7–9 years—Arizona, Colorado and New York, 2015. MMWR 2015, 64, 54–57. [Google Scholar] [PubMed]
- Sampson, P.D.; Streissguth, A.P.; Bookstein, F.L.; Little, R.E.; Clarren, S.K.; Dehaene, P.; Hanson, J.W.; Graham, J.M., Jr. Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology 1997, 56, 317–326. [Google Scholar] [CrossRef]
- May, P.A.; Gossage, J.P.; Kalberg, W.O.; Robinson, L.K.; Buckley, D.; Manning, M.A.; Hoyme, H.E. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev. Disabil. Res. Rev. 2009, 15, 176–192. [Google Scholar] [CrossRef] [PubMed]
- May, P.A.; Fiorentino, D.; Coriale, G.; Kalberg, W.O.; Hoyme, H.E.; Aragon, A.S.; Buckley, D.; Stellavato, C.; Gossage, J.P.; Robinson, L.K.; et al. Prevalence of children with severe fetal alcohol spectrum disorders in communities near Rome, Italy: New estimated rates are higher than previous estimates. Int. J. Environ. Res. Public Health 2011, 8, 2331–2351. [Google Scholar] [CrossRef] [PubMed]
- May, P.A.; Baete, A.; Russo, J.; Elliott, A.J.; Blankenship, J.; Kalberg, W.O.; Buckley, D.; Brooks, M.; Hasken, J.; Abdul-Rahman, O.; et al. Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics 2014, 134, 855–866. [Google Scholar] [CrossRef] [PubMed]
- May, P.A.; Keaster, C.; Bozeman, R.; Goodover, J.; Blankenship, J.; Kalberg, W.O.; Buckley, D.; Brooks, M.; Hasken, J.; Gossage, J.P.; et al. Prevalence and characteristics of fetal alcohol syndrome and partial fetal alcohol syndrome in a Rocky Mountain Region City. Drug Alcohol Depend. 2015, 155, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Okulicz-Kozaryn, K.; Borkowska, M.; Brzozka, K. FASD prevalence among schoolchildren in Poland. J. Appl. Res. Intellect. Disabil. 2017, 30, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Petković, G.; Barišić, I. FAS prevalence in a sample of urban schoolchildren in Croatia. Reprod. Toxicol. 2010, 29, 237–272. [Google Scholar] [CrossRef] [PubMed]
- Petković, G.; Barišić, I. Prevalence of fetal alcohol syndrome and maternal characteristics in a sample of schoolchildren from a rural province of Croatia. Int. J. Environ. Res. Public Health 2013, 10, 1547–1561. [Google Scholar] [CrossRef] [PubMed]
- Roozen, S.; Peters, G.J.; Kok, G.; Townsend, D.; Nijhuis, J.; Curfs, L. Worldwide prevalence of fetal alcohol spectrum disorders: A systematic literature review including meta-analysis. Alcohol. Clin. Exp. Res. 2016, 40, 18–32. [Google Scholar] [CrossRef] [PubMed]
- May, P.A.; Brooke, L.E.; Gossage, J.P.; Croxford, J.; Adnams, C.M.; Jones, K.L.; Robinson, L.K.; Viljoen, D.L. The epidemiology of Fetal Alcohol Syndrome in a South African community in the Western Cape Province. Am. J. Public Health 2000, 90, 1905–1912. [Google Scholar] [PubMed]
- May, P.A.; Gossage, J.P.; Marais, A.S.; Adnams, C.M.; Hoyme, H.E.; Jones, K.L.; Robinson, L.K.; Khaole, N.C.; Snell, C.; Kalberg, W.O.; et al. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug Alcohol Depend. 2007, 88, 259–271. [Google Scholar] [CrossRef] [PubMed]
- May, P.A.; Blankenship, J.; Marais, A.S.; Gossage, J.P.; Kalberg, W.O.; Barnard, R.; de Vries, M.; Robinson, L.K.; Adnams, C.M.; Buckley, D.; et al. Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a South African population-based study. Alcohol. Clin. Exp. Res. 2013, 37, 818–830. [Google Scholar] [CrossRef] [PubMed]
- May, P.A.; Marais, A.S.; de Vries, M.M.; Kalberg, W.O.; Buckley, D.; Hasken, J.M.; Adnams, C.M.; Barnard, R.; Joubert, B.; Cloete, M.; et al. The continuum of fetal alcohol spectrum disorders in a community in South Africa: Prevalence and characteristics in a fifth sample. Drug Alcohol Depend. 2016, 168, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, D.L.; Gossage, J.P.; Adnams, C.M.; Jones, K.L.; Robinson, L.K.; Hoyme, H.E.; Snell, C.; Khaole, N.; Asante, K.O.; Findlay, R.; et al. Fetal Alcohol Syndrome epidemiology in a South African community: A second study of a very high prevalence area. J. Stud. Alcohol 2005, 66, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Oliver, L.; Urban, M.; Chersich, M.; Temmerman, M.; Vijoen, D.L. Burden of fetal alcohol syndrome in a rural West Coast area of South Africa. S. Afr. Med. J. 2013, 103, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.; Chersich, M.; Fourie, L.A.; Chetty, C.; Olivier, L.; Viljoen, D.L. Fetal alcohol syndrome among Grade 1 schoolchildren in Northern Cape Province: Prevalence and risk factors. S. Afr. Med. J. 2008, 98, 877–880. [Google Scholar] [PubMed]
- Urban, M.F.; Olivier, L.; Viljoen, D.L.; Lombard, C.; Louw, J.G.; Drotsky, L.M.; Temmerman, M.; Chersich, M.F. Prevalence of fetal alcohol syndrome in a South African city with a predominantly Black African population. Alcohol. Clin. Exp. Res. 2015, 39, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, D.; Hymbaugh, D. Fetal Alcohol syndrome—South Africa, 2001. MMWR 2003, 52, 660–662. [Google Scholar]
- May, P.A.; Brooke, L.E.; Gossage, J.P.; Snell, C.; Hendricks, L.; Croxford, J.; Marais, A.S.; Viljoen, D.L. Maternal risk factors for Fetal Alcohol Syndrome in the Western Cape Province of South Africa: A population-based study. Am. J. Public Health 2005, 95, 1190–1199. [Google Scholar] [CrossRef] [PubMed]
- May, P.A.; Gossage, J.P.; Marais, A.S.; Hendricks, L.S.; Snell, C.L.; Tabachnick, B.G.; Stellavato, C.; Buckley, D.G.; Brooke, L.E.; Viljoen, D.L. Maternal risk factors for fetal alcohol syndrome and partial fetal alcohol syndrome in South Africa: A third study. Alcohol. Clin. Exp. Res. 2008, 32, 738–753. [Google Scholar] [CrossRef] [PubMed]
- May, P.A.; Blankenship, J.; Marais, A.S.; Gossage, J.P.; Kalberg, W.O.; Joubert, B.; Cloete, M.; Barnard, R.; de Vries, M.; Hasken, J.; et al. Maternal alcohol consumption producing fetal alcohol spectrum disorders (FASD): Quantity, frequency, and timing of drinking. Drug Alcohol Depend. 2013, 133, 502–512. [Google Scholar] [CrossRef] [PubMed]
- May, P.A.; Hamrick, K.J.; Corbin, K.D.; Hasken, J.; Marais, A.S.; Brooke, L.E.; Blankenship, J.; Hoyme, H.E.; Gossage, J.P. Dietary intake, nutrition, and fetal alcohol spectrum disorders in the Western Cape Province of South Africa. Reprod. Toxicol. 2014, 46, 31–39. [Google Scholar] [CrossRef] [PubMed]
- May, P.A.; Hamrick, K.J.; Corbin, K.D.; Hasken, J.M.; Marais, A.S.; Blankenship, J.; Hoyme, H.E.; Gossage, J.P. Maternal nutritional status as a contributing factor for the risk of fetal alcohol spectrum disorders. Reprod. Toxicol. 2016, 59, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, D.; Croxford, J.; Gossage, J.P.; Kodituwakku, P.W.; May, P.A. Characteristics of mothers of children with fetal alcohol syndrome in the Western Cape Province of South Africa: A case control study. J. Stud. Alcohol. 2002, 63, 6–17. [Google Scholar] [PubMed]
- May, P.A.; de Vries, M.M.; Marais, A.S.; Kalberg, W.O.; Adnams, C.M.; Hasken, J.M.; Tabachnick, B.; Robinson, L.K.; Manning, M.A.; Jones, K.L.; et al. The continuum of fetal alcohol spectrum disorders in four rural communities in South Africa: Prevalence and characteristics. Drug Alcohol Depend. 2016, 159, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Hoyme, H.E.; Hoyme, D.B.; Elliott, A.J.; Blankenship, J.; Kalberg, W.O.; Buckley, D.; Abdul-Rahman, O.; Adam, M.P.; Robinson, L.K.; Manning, M.A.; et al. A South African mixed race lip/philtrum guide for diagnosis of fetal alcohol spectrum disorders. Am. J. Med. Genet. A 2015, 167A, 752–755. [Google Scholar] [CrossRef] [PubMed]
- May, P.A.; Tabachnick, B.G.; Gossage, J.P.; Kalberg, W.O.; Marais, A.S.; Robinson, L.K.; Manning, M.A.; Buckley, D.; Hoyme, H.E. Maternal risk factors predicting child physical characteristics and dysmorphology in Fetal Alcohol Syndrome and Partial Fetal Alcohol Syndrome. Drug Alcohol Depend. 2011, 119, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, T.M.; Rescorla, L.A. Manual for the ASEBA School-Age Forms and Profiles; University of Vermont, Research Center for Children, Youth & Families: Burlington, VT, USA, 2001. [Google Scholar]
- Bishop, D.V.M. Test of Reception of Grammar (TROG), 2nd ed.; University of Manchester: Manchester, UK, 1989. [Google Scholar]
- Raven, J. Manual for Raven’s Progressive Matrices and Vocabulary Scales. Research Supplement No.1: The 1979 British Standardisation of the Standard Progressive Matrices and Mill Hill Vocabulary Scales, together with Comparative Data from Earlier Studies in the UK, US, Canada, Germany and Ireland; Harcourt Assessment: San Antonio, TX, USA, 1981. [Google Scholar]
- Wechsler, D. Wechsler Intelligence Scale for Children, 3rd ed.; Psychological Corporation: San Antonio, TX, USA, 2003. [Google Scholar]
- Sobell, L.C.; Agrawal, S.; Annis, H.; Ayala-Velazquez, H.; Echeverria, L.; Leo, G.I.; Rybakowski, J.K.; Sandahl, C.; Saunders, B.; Thomas, S.; et al. Cross-cultural evaluation of two drinking assessment instruments: Alcohol timeline followback and inventory of drinking situations. Subst. Use Misuse 2001, 36, 313–331. [Google Scholar] [CrossRef] [PubMed]
- Sobell, L.C.; Sobell, M.B.; Leo, G.I.; Cancilla, A. Reliability of a timeline method: Assessing normal drinker’s reports of recent drinking and a comparative evaluation across several populations. Br. J. Addict. 1998, 83, 393–402. [Google Scholar] [CrossRef]
- Jacobson, S.W.; Staton, M.E.; Molteno, C.D.; Burden, M.J.; Fuller, D.S.; Hoyme, H.E.; Robinson, L.K.; Khaole, N.; Jacobson, J.L. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol. Clin. Exp. Res. 2008, 32, 365–372. [Google Scholar] [CrossRef] [PubMed]
- King, A.C. Enhancing the self-report of alcohol consumption in the community: Two questionnaire formats. Am. J. Public Health 1994, 84, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Marais, A.S.; de Vries, M.M.; Hasken, J.M.; Marsden, D.; Seedat, S.; Parry, C.D.H.; May, P.A. Comparing self-reported drinking history with phosphatidyelethanol (PEth) and Ethyl Glucuronide (EtG) values of pregnant woman recruited at antenatal clinics. Alcohol. Clin. Exp. Res. 2016, 40, 46A. [Google Scholar]
- Microsoft. Microsoft Excel [Computer Program]; Excel 2010; Microsoft: Redmond, WA, USA, 2010. [Google Scholar]
- IBM. IBM SPSS Statistics for Windows [Computer Program]; Version 24.0; IBM Co.: Armonk, NY, USA, 2015. [Google Scholar]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, 6th ed.; Pearson: Boston, MA, USA, 2013. [Google Scholar]
- Abell, K.; May, W.; May, P.A.; Kalberg, W.O.; Hoyme, H.E.; Robinson, L.K.; Manning, M.A.; Jones, K.L.; Abdul-Rahman, O. Fetal alcohol spectrum disorders and assessment of maxillary and mandibular arc measurements. Am. J. Med. Genet. A 2016, 170, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- May, P.A.; Hasken, J.M.; Blankenship, J.; Marais, A.S.; Joubert, B.; Cloete, M.; de Vries, M.M.; Barnard, R.; Botha, I.; Roux, S.; et al. Breastfeeding and maternal alcohol use: Prevalence and effects on child outcomes and fetal alcohol spectrum disorders. Reprod. Toxicol. 2016, 63, 13–21. [Google Scholar] [CrossRef] [PubMed]
- May, P.A.; Tabachnick, B.; Gossage, J.P.; Kalberg, W.O.; Marais, A.S.; Robinson, L.K.; Manning, M.A.; Blankenship, J.; Buckley, D.; Hoyme, H.E.; et al. Maternal factors predicting cognitive and behavioral characteristics of children with fetal alcohol spectrums disorders. J. Dev. Behav. Pediatr. 2013, 34, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Czarnecki, D.M.; Russell, M.; Cooper, M.L.; Salter, D. Five-year reliability of self-reported alcohol consumption. J. Stud. Alcohol. 1990, 51, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Hannigan, J.H.; Chiodo, L.M.; Sokol, R.M.; Janisse, J.; Ager, J.W.; Greenwalk, M.K.; Delaney Black, V. A 14-year retrospective maternal report of alcohol consumption in pregnancy predicts pregnancy and teen outcomes. Alcohol 2010, 44, 583–594. [Google Scholar] [CrossRef] [PubMed]
| All Children 1 | Children with FAS (n = 129) | Children with Partial FAS (n = 100) | Children with ARND (n = 55) | Randomly-Selected Normal Controls (n = 104) | Statistical Test | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex (% male) | 53.6 | 52.7 | 44.0 | 52.7 | 54.3 | χ2 = 2.630 | 0.452 | ||||
| Age (months)—Mean (Standard Deviation (SD)) | 84.2 (9.1) | 86.5 | (10.1) | 85.1 | (8.7) | 85.4 | (9.0) | 83.6 | (10.7) | F = 1.644 | 0.179 |
| Height (cm)—Mean (SD) | 118.3 (20.6) | 112.0 | (5.9) | 116.1 | (6.0) | 114.7 | (4.8) | 118.4 | (6.0) | F = 52.681 | <0.001 a,b,c,e,f |
| Weight (kg)—Mean (SD) | 17.2 (21.2) | 17.5 | (2.7) | 19.9 | (2.8) | 18.5 | (2.5) | 21.3 | (4.0) | F = 43.675 | <0.001 a,b,c,d,e,f |
| Child’s BMI—Mean (SD) | 14.6 (1.6) | 13.9 | (1.3) | 14.7 | (1.2) | 14.3 | (2.6) | 15.1 | (1.8) | F = 10.947 | <0.001 a,c |
| BMI Percentile—Mean (SD) | 27.3 (25.5) | 15.0 | (20.1) | 29.9 | (25.4) | 16.3 | (18.8) | 35.8 | (28.3) | F = 18.590 | <0.001 a,c,d,f |
| OFC (cm)—Mean (SD) | -- | 48.7 | (1.2) | 50.9 | (1.2) | 49.1 | (1.2) | 51.4 | (1.4) | F = 91.713 | <0.001 a,c,d,e,f |
| <3rd centile (%) | -- | 70.5 | 10.0 | 56.4 | 5.7 | F = 148.766 | <0.001 | ||||
| <10th centile (%) | -- | 100.0 | 24.0 | 92.7 | 14.3 | F = 247.530 | <0.001 | ||||
| PFL centile—Mean (SD) | -- | 7.3 | (11.6) | 13.6 | (15.3) | 25.7 | (14.3) | 27.6 | (15.5) | F = 48.925 | <0.001 a,b,c,d,e |
| Smooth Philtrum 2 (%) | -- | 76.7 | 79.0 | 18.2 | 26.7 | χ2 = 112.305 | <0.001 | ||||
| Narrow Vermilion 2 (%) | -- | 87.6 | 80.0 | 18.2 | 19.0 | χ2 = 166.675 | <0.001 | ||||
| Total Dysmorphology Score—Mean (SD) | -- | 17.5 | (3.7) | 12.3 | (2.9) | 9.5 | (3.1) | 6.4 | (3.3) | F = 225.696 | <0.001 a,b,c,d,e,f |
| Children with FAS (n = 129) | Children with Partial FAS (n = 100) | Children with ARND (n = 55) | Randomly-Selected Normal Controls (n = 104) | Test Score | p f | |
|---|---|---|---|---|---|---|
| Maxillary Arc (cm) | 23.1 (1.2) | 23.9 (0.9) | 23.6 (0.9) | 24.1 (1.3) | F = 19.187 | <0.001 a,b,c,d,e |
| Mandibular Arc (cm) | 24.1 (1.3) | 25.1 (1.0) | 24.6 (1.1) | 25.2 (1.5) | F = 17.951 | <0.001 a,c,d,e |
| Inner canthal distance (centile) | 53.7 (22.5) | 59.3 (20.6) | 51.9 (19.7) | 60.0 (22.9) | F = 2.950 | <0.001 |
| Inter-pupillary distance (centile) | 44.7 (25.1) | 54.1 (25.2) | 53.1 (26.0) | 58.5 (25.9) | F = 5.992 | <0.001 a,c |
| Hypoplastic midface (%) | 76.7 | 61.0 | 41.8 | 20.8 | χ2 = 31.398 | <0.001 |
| “Railroad” track ears (%) | 53.3 | 20.0 | 13.3 | 13.3 | χ2 = 10.429 | 0.015 |
| Ptosis (%) | 17.8 | 5.1 | 3.6 | 2.9 | χ2 = 21.911 | <0.001 |
| Camptodactyly (%) | 22.5 | 3.0 | 5.5 | 5.7 | χ2 = 29.638 | <0.001 |
| Altered palmar creases (%) | 45.7 | 32.0 | 27.3 | 22.9 | χ2 = 15.084 | 0.002 |
| Anteverted nostrils (%) | 19.4 | 24.0 | 3.6 | 14.3 | χ2 = 11.518 | 0.009 |
| Children with FAS (n = 125) | Children with Partial FAS (n = 99) | Children with ARND (n = 55) | Randomly-Selected Normal Controls (n = 101) | F | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | Mean | (SD) | Mean | (SD) | |||
| Verbal Ability ^ (percentile scores) | 9.0 | (11.6) | 13.6 | (18.1) | 8.3 | (11.6) | 23.6 | (21.2) | F = 17.567 | <0.001 b,d,e |
| Non-verbal Ability + (percentile scores) | 12.6 | (12.8) | 20.0 | (18.2) | 10.9 | (7.6) | 28.1 | (23.1) | F = 19.691 | <0.001 a,b,c,d,e |
| WISC-IV Digit-Span Scaled Score † | 4.6 | (2.8) | 5.7 | (2.8) | 4.3 | (2.7) | 6.9 | (2.7) | F = 16.139 | <0.001 a,b,c,d,e |
| Teacher Report Form (TRF) Total Problem Score | 34.5 | (24.9) | 29.8 | (25.7) | 41.9 | (32.2) | 19.9 | (19.5) | F = 11.063 | <0.001 b,d,e |
| TRF Inattention Score | 17.9 | (12.0) | 14.2 | (11.5) | 18.9 | (12.3) | 9.7 | (8.9) | F = 12.807 | <0.001 b,d,e |
| Mothers of | Statistical Test | p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Children with FAS (n = 118) | Children with Partial FAS (n = 91) | Children with ARND (n = 55) | Randomly-Selected Normal Controls (n = 100) | ||||||||
| Alcohol Consumption Variables | |||||||||||
| Current drinker (% Yes) | 50.8 | 34.1 | 52.9 | 27.6 | χ2 = 16.829 | <0.001 | |||||
| Drank before index pregnancy (% Yes) | 84.6 | 69.1 | 100.0 | 47.0 | χ2 = 63.450 | <0.001 | |||||
| Drank during index pregnancy (% Yes) | 84.9 | 69.8 | 100.0 | 45.0 | χ2 = 69.170 | <0.001 | |||||
| Average # drinks per day during pregnancy | 5.9 (5.8) | 2.9 (4.3) | 6.1 (5.9) | 1.7 (3.4) | F = 17.599 | <0.001 a,c,d,f | |||||
| Average # of drinking days during pregnancy | 1.7 (1.6) | 0.8 (1.0) | 1.5 (1.4) | 0.4 (0.7) | F = 23.910 | <0.001 a,c,d,e,f | |||||
| Consumed 3 drinks or more per occasion during pregnancy (%) ^ | 73.7 | 53.8 | 94.2 | 34.0 | χ2 = 64.450 | <0.001 | |||||
| Consumed 5 drinks or more per occasion during pregnancy (%) ^ | 65.3 | 39.6 | 57.7 | 26.0 | χ2 = 37.909 | <0.001 | |||||
| Alcohol Use by Trimester | |||||||||||
| Drank during 1st trimester (% Yes) | 81.0 | 67.0 | 100.0 | 43.0 | χ2 = 66.045 | <0.001 | |||||
| Binged 3+ (%) ^ | 72.2 | 53.8 | 92.2 | 31.3 | χ2 = 63.795 | <0.001 | |||||
| Binged 5+ (%) ^ | 63.5 | 38.5 | 54.9 | 23.2 | χ2 = 38.449 | <0.001 | |||||
| Average # of drinks per drinking day 1 | 8.4 (5.4) | 6.6 (4.8) | 7.9 (7.1) | 5.7 (3.9) | F = 3.655 | 0.013 c | |||||
| # of drinking days per week 1 | 2.4 (1.5) | 1.8 (1.2) | 1.8 (1.3) | 1.5 (0.7) | F = 6.087 | 0.001 a,c | |||||
| Drank during 2nd trimester (% Yes) | 71.4 | 45.2 | 83.3 | 25.0 | χ2 = 70.927 | <0.001 | |||||
| Binged 3+ (%) ^ | 58.3 | 35.2 | 74.5 | 18.2 | χ2 = 58.529 | <0.001 | |||||
| Binged 5+ (%) ^ | 53.0 | 25.3 | 41.2 | 14.1 | χ2 = 40.526 | <0.001 | |||||
| Average # of drinks per drinking day 1 | 8.5 (5.7) | 6.7 (5.3) | 7.4 (6.3) | 6.6 (4.8) | F = 1.241 | 0.296 | |||||
| # of drinking days per week 1 | 2.4 (1.4) | 1.8 (1.4) | 1.9 (1.4) | 1.7 (0.8) | F = 2.955 | 0.034 c | |||||
| Drank during 3rd trimester (% Yes) | 60.6 | 24.5 | 59.3 | 13.1 | χ2 = 70.627 | <0.001 | |||||
| Binged 3+ (%) ^ | 50.9 | 16.5 | 47.1 | 11.0 | χ2 = 55.713 | <0.001 | |||||
| Binged 5+ (%) ^ | 44.8 | 12.1 | 29.4 | 10.0 | χ2 = 45.421 | <0.001 | |||||
| Average # of drinks per drinking day 1 | 8.9 (5.5) | 7.5 (6.8) | 7.3 (6.6) | 8.1 (5.6) | F = 0.687 | 0.562 | |||||
| # of drinking days per week 1 | 2.5 (1.5) | 1.7 (1.4) | 2.1 (1.6) | 2.0 (0.7) | F = 2.325 | 0.078 | |||||
| Tobacco and Use of Other Drugs | |||||||||||
| Other Drug Use in lifetime (%) | 7.2 | 11.6 | 7.4 | 12.0 | χ2 = 2.162 | 0.529 | |||||
| Other Drug Use during pregnancy (%) | 2.5 | 5.4 | 1.8 | 2.1 | χ2 = 2.470 | 0.481 | |||||
| Used tobacco during index pregnancy (%) | 75.0 | 53.2 | 65.5 | 32.7 | χ2 = 42.221 | <0.001 | |||||
| Current smoker, smoked within week (%) | 83.9 | 70.2 | 76.7 | 15.9 | χ2 = 4.125 | 0.248 | |||||
| Total # of grams of tobacco used per week (each cigarette =1 g) | 19.1 (20.9) | 16.8 (29.3) | 17.3 (22.0) | 19.1 (31.8) | F = 0.180 | 0.910 | |||||
| Demographics | |||||||||||
| Age at pregnancy (year)—Mean (SD) | 29.1 | (6.4) | 27.1 | (7.2) | 26.0 | (6.9) | 25.1 | (7.3) | F = 6.186 | <0.001 b,c | |
| Height (cm)—Mean (SD) | 155.2 | (6.2) | 156.6 | (6.1) | 157.8 | (7.9) | 158.1 | (6.5) | F = 3.682 | 0.012 c | |
| Weight (kg)—Mean (SD) | 58.9 | (17.1) | 66.7 | (16.5) | 64.2 | (15.8) | 73.4 | (17.5) | F = 12.518 | <0.001 a,c,f | |
| Body Mass Index (BMI)—Mean (SD) | 24.4 | (6.9) | 27.2 | (6.8) | 25.7 | (6.4) | 29.4 | (6.8) | F = 9.419 | <0.001 a,c,f | |
| Occipitofrontal circumference (OFC)—Mean (SD) | 54.7 | (2.1) | 55.0 | (1.9) | 55.0 | (1.6) | 55.5 | (2.0) | F = 3.005 | 0.031 c | |
| Gravidity—Mean (SD) | 3.8 | (1.6) | 3.3 | (1.7) | 3.1 | (1.5) | 2.9 | (1.4) | F = 6.355 | <0.001 b,c | |
| Parity—Mean (SD) | 3.3 | (1.5) | 3.1 | (1.6) | 2.7 | (1.2) | 2.6 | (1.4) | F = 5.202 | 0.002 b,c | |
| Miscarriages—Mean (SD) | 0.3 | (0.7) | 0.2 | (0.5) | 0.2 | (0.5) | 0.2 | (0.4) | F = 1.710 | 0.164 | |
| Stillbirths—Mean (SD) | 0.09 | (0.3) | 0.04 | (0.5) | 0.05 | (0.2) | 0.06 | (0.3) | F = 0.557 | 0.644 | |
| Breastfed index child (% Yes) | 89.0 | 93.5 | 81.8 | 88.8 | χ2 = 4.780 | 0.189 | |||||
| Duration of breastfeeding (months)—Mean (SD) | 20.2 | (19.2) | 20.3 | (21.3) | 22.4 | (21.4) | 21.5 | (19.8) | F = 0.180 | 0.910 | |
| Maternal education (years)—Mean (SD) | 6.8 | (3.4) | 8.1 | (3.1) | 8.6 | (3.2) | 9.3 | (2.7) | F = 11.771 | <0.001 a,b,c,e | |
| Residence during pregnancy (% Rural) | 51.3 | 49.5 | 45.5 | 32.0 | χ2 = 9.463 | 0.024 | |||||
| Income (Rand per week)—Mean (SD) | 818 | (586) | 945 | (546) | 829 | (534) | 1406 | (2669) | F = 3.349 | 0.019 | |
| Child Trait | Mothers Reported Drinking during Pregnancy | Drinks per Drinking Day during Pregnancy (log) | Drinks per Week during Pregnancy (log) | 3 or More Drinks per Occasion during Pregnancy | 5 or More Drinks per Occasion during Pregnancy | Paternal Drinking Problem e | |
|---|---|---|---|---|---|---|---|
| Verbal ability a (log) | Partial r | −0.134 | −0.148 | −0.140 | −0.083 | −0.099 | −0.005 |
| p f | 0.007 | 0.003 | 0.005 | 0.096 | 0.047 | 0.927 | |
| n | 397 | 396 | 398 | 399 | 399 | 344 | |
| Non-verbal ability b (log) | Partial r | −0.151 | −0.145 | −0.146 | −0.110 | −0.087 | −0.055 |
| p f | 0.002 | 0.004 | 0.003 | 0.027 | 0.081 | 0.304 | |
| n | 399 | 397 | 399 | 400 | 400 | 345 | |
| WISC-IV Digit Span c | Partial r | −0.151 | −0.164 | −0.168 | −0.098 | −0.106 | −0.069 |
| p f | 0.002 | 0.001 | 0.001 | 0.048 | 0.032 | 0.198 | |
| n | 397 | 397 | 399 | 400 | 400 | 345 | |
| Behavior problems d (sqrt) | Partial r | 0.222 | 0.178 | 0.176 | 0.191 | 0.148 | 0.038 |
| p f | <0.001 | <0.001 | <0.001 | <0.001 | 0.003 | 0.175 | |
| n | 402 | 401 | 403 | 404 | 404 | 348 | |
| Inattention problems d (sqrt) | Partial r | 0.219 | 0.165 | 0.166 | 0.175 | 0.149 | 0.026 |
| p f | <0.001 | 0.001 | 0.001 | <0.001 | 0.002 | 0.624 | |
| n | 401 | 400 | 402 | 403 | 403 | 348 | |
| Head circumference | Partial r | −0.224 | −0.256 | −0.255 | −0.206 | −0.217 | −0.028 |
| p f | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.596 | |
| n | 410 | 409 | 411 | 412 | 412 | 355 | |
| Dysmorphology score | Partial r | 0.186 | 0.193 | 0.215 | 0.168 | 0.186 | −0.001 |
| p f | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | 0.993 | |
| n | 408 | 407 | 409 | 412 | 410 | 353 | |
| Oversample of Children ≤25th Centile on Height, Weight, or OFC | Random Sample Rate of FASD Diagnoses and Estimated Cases in the Non-Consented Children | Combined Rate from Cases in Consented Sample (n = 1088) and Estimated Cases in Non-Consented Sample (n = 365) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (a) Total Cases Diagnosed n | Low Estimated Rate: School Enrollment Rate 1 (n = 1448) | Consented Student Rate 2 (n = 1088) | Cases Found among Randomly-Selected Controls n | Proportion of FASD Cases in Random Sample (n = 276) | (b) Estimated Cases in Non-Consented Sample (n = 365) | Rate of FASD from Random Sample Only 3 | 95% CI | (a + b) Total Estimated Cases (n = 399) | High Estimated Rate: Estimated Rate for All Enrolled Students 4 | 95% CI | |
| FAS | 129 | 89.1 | 118.6 | 44 | 0.1594 | 58 | 159.4 | 116.2 to 202.6 | 187 | 129.1 | 111.8 to 146.4 |
| PFAS | 100 | 69.1 | 91.9 | 28 | 0.1014 | 37 | 101.4 | 65.8 to 137.1 | 137 | 94.6 | 79.5 to 109.7 |
| ARND | 55 | 37.9 | 50.6 | 15 | 0.0543 | 20 | 54.3 | 27.6 to 81.1 | 75 | 51.8 | 40.4 to 63.2 |
| Total FASD | 284 | 196.1 | 261.0 | 87 | 0.3152 | 115 | 315.2 | 260.4 to 370.0 | 399 | 275.5 | 252.5 to 298.6 |
| Sample 1 (Initiated in 2009) [33] | Sample 2 (Initiated in 2011) | Sample 1 Case Ratio: Cases per FAS Case | Sample 2 Case Ratio: Cases per FAS Case | |
|---|---|---|---|---|
| FAS | 92.7–127.0 | 89.1–129.1 | - | - |
| PFAS | 58.4–86.2 | 69.1–94.6 | 0.68 | 0.73 |
| ARND | 31.6–45.6 | 37.9–51.8 | 0.36 | 0.40 |
| Total FASD | 182.7–258.9 | 196.1–275.5 | 1.04 | 1.13 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
May, P.A.; De Vries, M.M.; Marais, A.-S.; Kalberg, W.O.; Buckley, D.; Adnams, C.M.; Hasken, J.M.; Tabachnick, B.; Robinson, L.K.; Manning, M.A.; et al. Replication of High Fetal Alcohol Spectrum Disorders Prevalence Rates, Child Characteristics, and Maternal Risk Factors in a Second Sample of Rural Communities in South Africa. Int. J. Environ. Res. Public Health 2017, 14, 522. https://doi.org/10.3390/ijerph14050522
May PA, De Vries MM, Marais A-S, Kalberg WO, Buckley D, Adnams CM, Hasken JM, Tabachnick B, Robinson LK, Manning MA, et al. Replication of High Fetal Alcohol Spectrum Disorders Prevalence Rates, Child Characteristics, and Maternal Risk Factors in a Second Sample of Rural Communities in South Africa. International Journal of Environmental Research and Public Health. 2017; 14(5):522. https://doi.org/10.3390/ijerph14050522
Chicago/Turabian StyleMay, Philip A., Marlene M. De Vries, Anna-Susan Marais, Wendy O. Kalberg, David Buckley, Colleen M. Adnams, Julie M. Hasken, Barbara Tabachnick, Luther K. Robinson, Melanie A. Manning, and et al. 2017. "Replication of High Fetal Alcohol Spectrum Disorders Prevalence Rates, Child Characteristics, and Maternal Risk Factors in a Second Sample of Rural Communities in South Africa" International Journal of Environmental Research and Public Health 14, no. 5: 522. https://doi.org/10.3390/ijerph14050522






