Chronic Exposure to Uranium from Gestation: Effects on Behavior and Neurogenesis in Adulthood
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Exposure
2.2. Behavioral Tasks
2.3. Morphological Analysis and Neurogenesis
2.3.1. BrdU (5-bromo-2′deoxyuridine) Injection Protocol
2.3.2. Tissue Preparation
2.3.3. Double Immunohistochemical Labeling for BrdU/NeuN
2.3.4. Quantification and Image Analysis
2.4. Uranium Levels in Tissues
2.5. Statistical Analysis
3. Results and Discussion
3.1. General Health Parameters and U Concentrations in Tissues
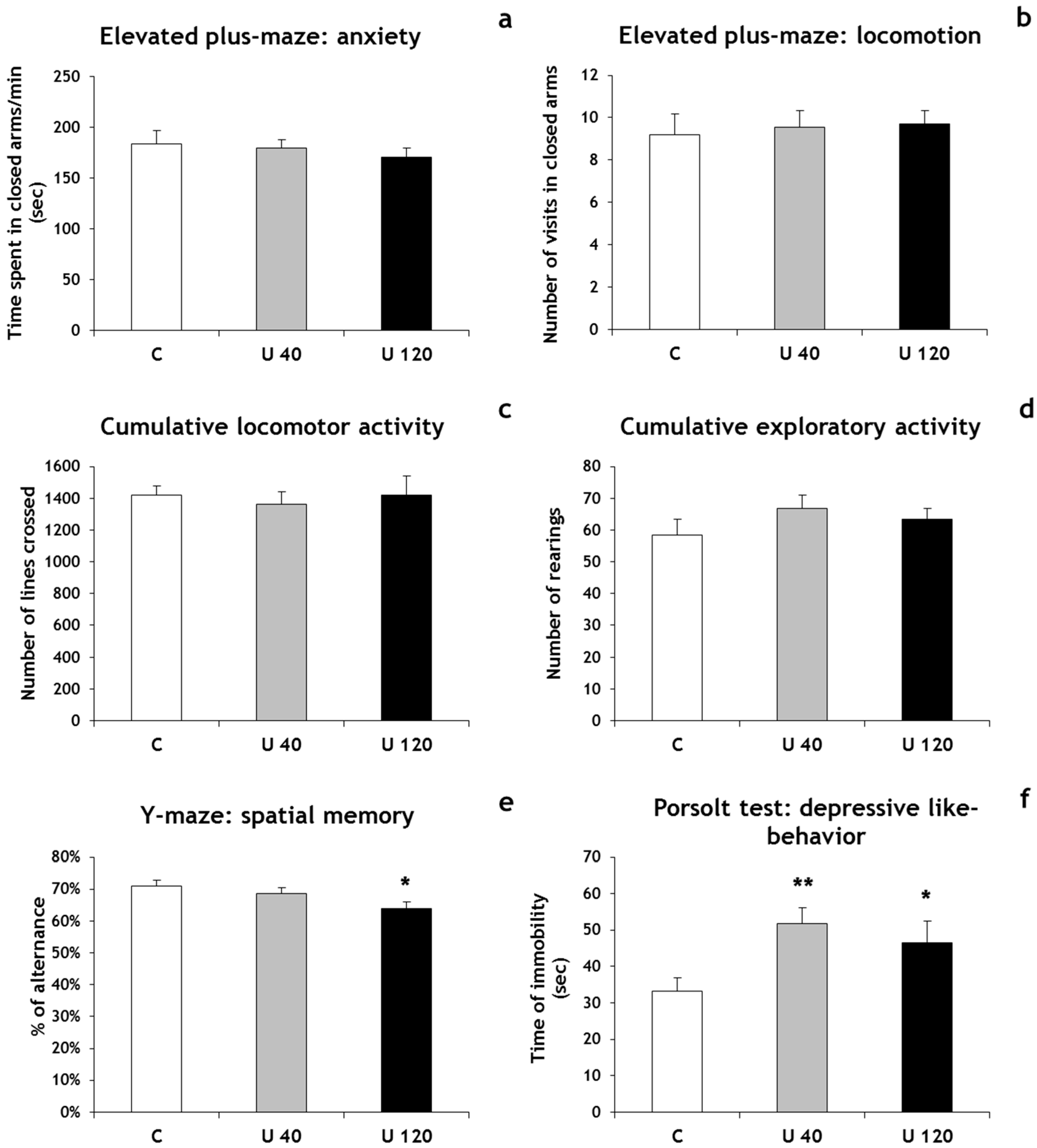
3.2. Behavior
3.3. Hippocampal Morphology and Neurogenesis
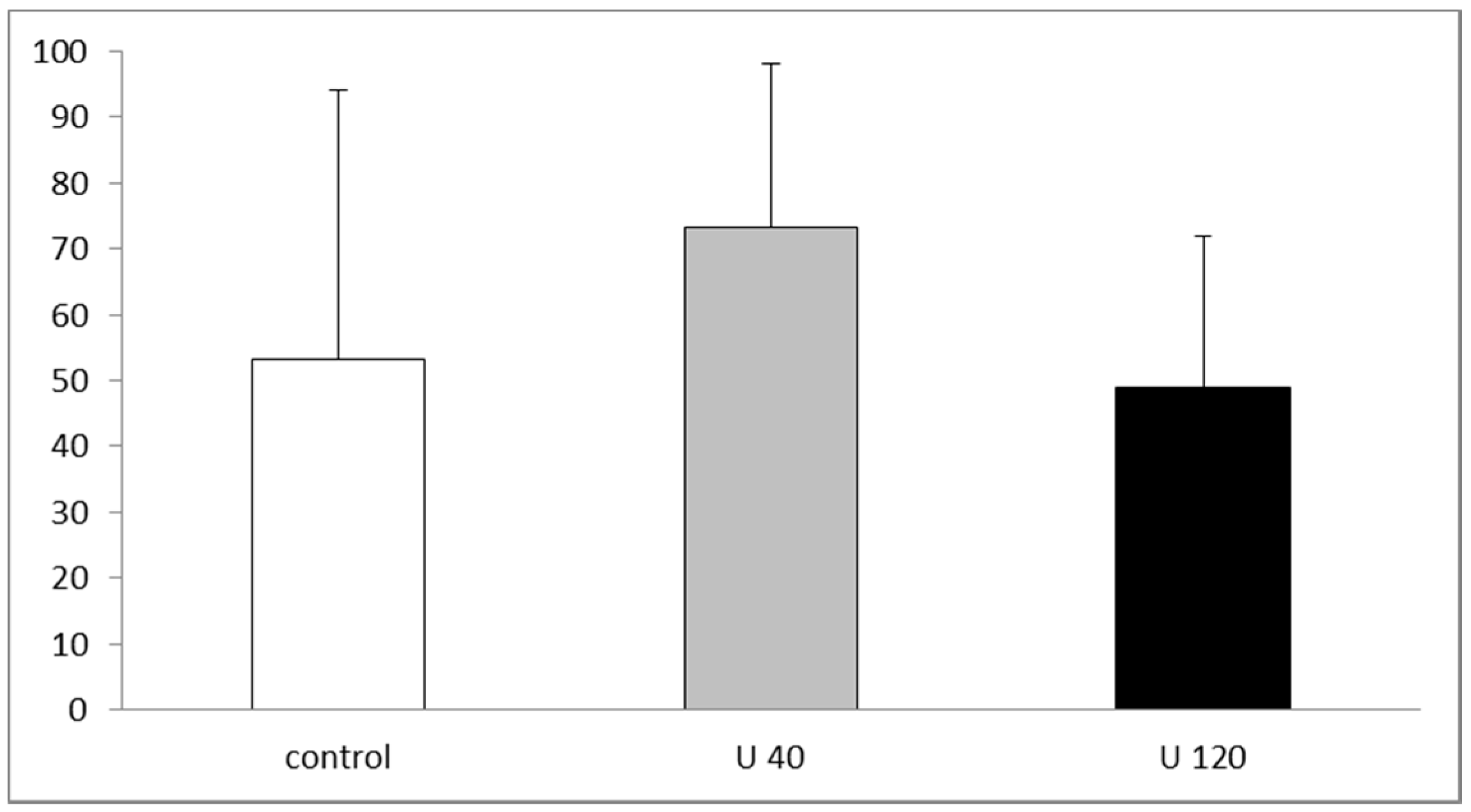
3.3.1. Hippocampal Adult Neurogenesis Is Normal in U-Exposed Rats
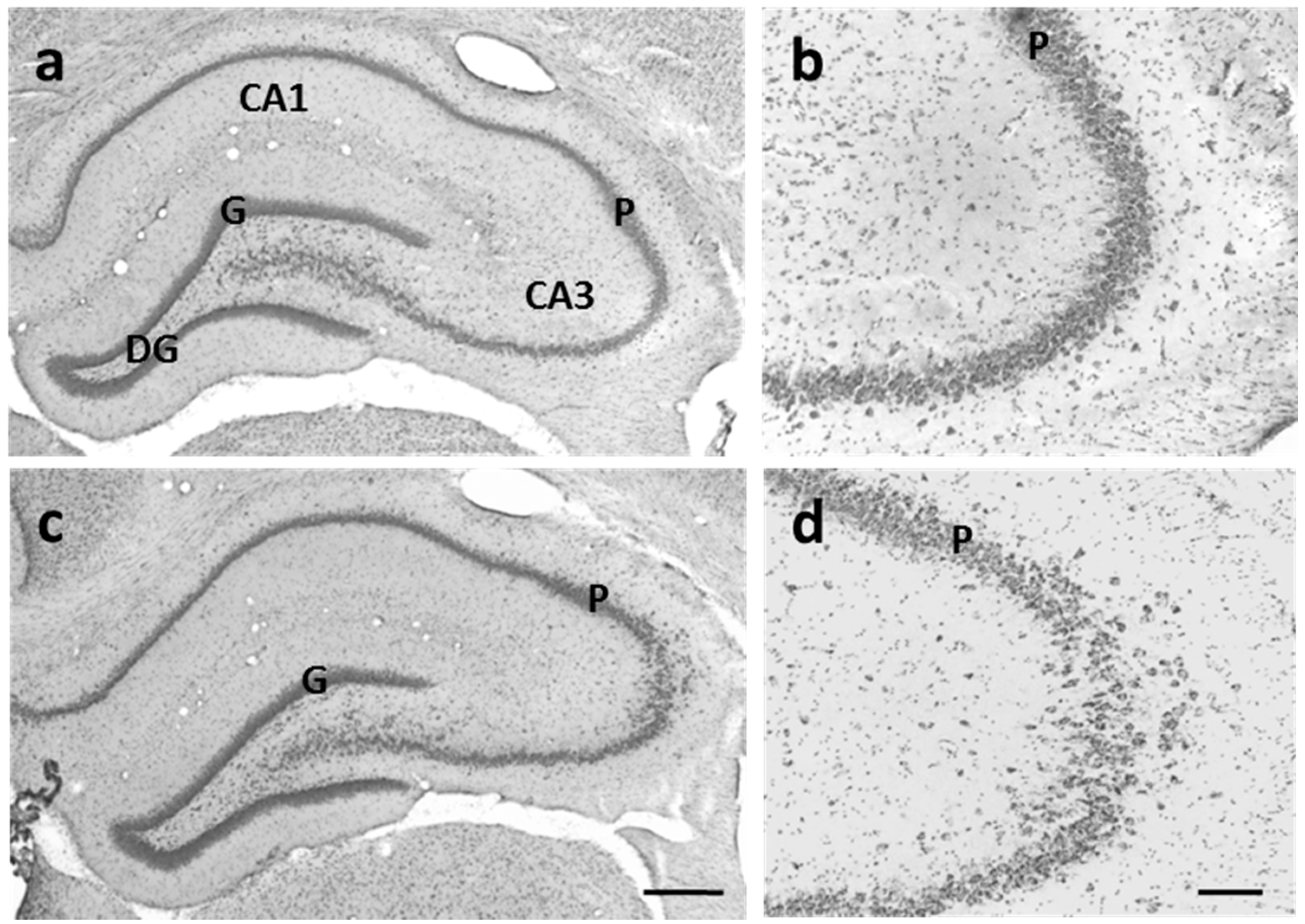
3.3.2. CA3 Pyramidal Cells are Dispersed in U-Exposed Rats
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Uranium; Public Health Service: Atlanta, GA, USA, 2013.
- WHO. Uranium in Drinking Water; World Health Organisation: Geneva, Switzerland, 2012. [Google Scholar]
- Craft, E.; Abu-Qare, A.; Flaherty, M.; Garofolo, M.; Rincavage, H.; Abou-Donia, M. Depleted and natural uranium: Chemistry and toxicological effects. J. Toxicol. Environ. Health Part B Crit. Rev. 2004, 7, 297–317. [Google Scholar] [CrossRef] [PubMed]
- Dinocourt, C.; Legrand, M.; Dublineau, I.; Lestaevel, P. The neurotoxicology of uranium. Toxicology 2015, 337, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Lestaevel, P.; Dhieux, B.; Delissen, O.; Benderitter, M.; Aigueperse, J. Uranium modifies or not behavior and antioxidant status in the hippocampus of rats exposed since birth. J. Toxicol. Sci. 2015, 40, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Lestaevel, P.; Bensoussan, H.; Dhieux, B.; Delissen, O.; Vacher, C.M.; Dublineau, I.; Voisin, P.; Taouis, M. Cerebral cortex and hippocampus respond differently after post-natal exposure to uranium. J. Toxicol. Sci. 2013, 38, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Briner, W.; Abboud, B. Behavior of Juvenile Mice Chronically Exposed to Depleted Uranium. In Proceedings of the 7th Symposium Metal Ions in Biology and Medicine, 7th ed.; Khassanova, L., Collery, P., Maymard, I., Khassanova, Z., Etienne, J.C., Eds.; John Libby Eurotext: Paris, France, 2002; pp. 353–356. [Google Scholar]
- Houpert, P.; Frelon, S.; Monleau, M.; Bussy, C.; Chazel, V; Paquet, F. Heterogeneous accumulation of uranium in the brain of rats. Radiat. Prot. Dosim. 2007, 127, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Albina, M.L.; Bellés, M.; Linares, V.; Sánchez, D.J.; Domingo, J.L. Restraint stress does not enhance the uranium-induced developmental and behavioral effects in the offspring of uranium-exposed male rats. Toxicology 2005, 215, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Legrand, M.; Elie, C.; Stefani, J.; Florès, N.; Culeux, C.; Delissen, O.; Ibanez, C.; Lestaevel, P.; Eriksson, P.; Dinocourt, C. Cell proliferation and cell death are disturbed during prenatal and postnatal brain development after uranium exposure. Neurotoxicology 2016, 52, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Legrand, M.; Lam, S.; Anselme, I.; Gloaguen, C.; Ibanez, C.; Eriksson, P.; Lestaevel, P.; Dinocourt, C. Exposure to depleted uranium during development affects neuronal differentiation in the hippocampal dentate gyrus and induces depressive-like behavior in offspring. Neurotoxicology 2016, 57, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Pothion, S.; Bizot, J.C.; Trovero, F.; Belzung, C. Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav. Brain. Res. 2004, 155, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Bourin, M.; Chenu, F.; Ripoll, N.; David, D.J. A proposal of decision tree to screen putative antidepressants using forced swim and tail suspension tests. Behav. Brain. Res. 2005, 164, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Germain, J.; Bruel-Jungerman, E.; Grannec, G.; Denis, C.; Lepousez, G.; Giros, B.; Francis, F.; Nosten-Bertrand, M. Doublecortin knockout mice show normal hippocampal-dependent memory despite CA3 lamination defects. PLoS ONE 2013, 8, e74992. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L. Reproductive and developmental toxicity of natural and depleted uranium: A review. Reprod. Toxicol. 2001, 15, 603–609. [Google Scholar] [CrossRef]
- Briner, W.E. The evolution of depleted uranium as an environmental risk factor: Lessons from other metals. Int. J. Environ. Res. Public Health 2006, 3, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Warner-Schmidt, J.L.; Duman, R.S. Hippocampal neurogenesis: Opposing effects of stress and antidepressant treatment. Hippocampus 2006, 16, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Dinocourt, C.; Stefani, J.; Elie, C.; Lestaevel, P.; Dublineau, I.; Gourmelon, P. Reduced carbachol-induced beta/gamma oscillations in CA3 region of hippocampus after post-natal contamination of uranium in adult rat. Neurosciences 2014. [Google Scholar]
- Lestaevel, P.; Grison, S.; Favé, G.; Elie, C.; Dhieux, B.; Martin, J.C.; Tack, K.; Souidi, M. Assessment of the Central Effects of Natural Uranium via Behavioural Performances and the Cerebrospinal Fluid Metabolome. Neural Plast. 2016. [Google Scholar] [CrossRef] [PubMed]
| Control | U 40 | U 120 | ||
|---|---|---|---|---|
| Health indicators | Weight (g) | 336.6 ± 31.7 | 343.0 ± 30.8 | 352.4 ± 40.1 |
| (n = 16/group) | Food (g/day/rat) | 27.8 ± 0.7 | 28.1 ± 0.4 | 28.0 ± 0.5 |
| Water (mL/day/rat) | 27.7 ± 0.7 | 28.0 ± 1.2 | 28.7 ± 1.0 | |
| U concentration | Kidneys (ng/g) | 6.5 ± 0.7 | 193.3 ± 21.8 * | 584.5 ± 73.3 * |
| (n = 8/group) | Brain (ng/g) | 0.10 ± 0.02 | 0.20 ± 0.02 * | 0.67 ± 0.13 * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinocourt, C.; Culeux, C.; Legrand, M.; Elie, C.; Lestaevel, P. Chronic Exposure to Uranium from Gestation: Effects on Behavior and Neurogenesis in Adulthood. Int. J. Environ. Res. Public Health 2017, 14, 536. https://doi.org/10.3390/ijerph14050536
Dinocourt C, Culeux C, Legrand M, Elie C, Lestaevel P. Chronic Exposure to Uranium from Gestation: Effects on Behavior and Neurogenesis in Adulthood. International Journal of Environmental Research and Public Health. 2017; 14(5):536. https://doi.org/10.3390/ijerph14050536
Chicago/Turabian StyleDinocourt, Céline, Cécile Culeux, Marie Legrand, Christelle Elie, and Philippe Lestaevel. 2017. "Chronic Exposure to Uranium from Gestation: Effects on Behavior and Neurogenesis in Adulthood" International Journal of Environmental Research and Public Health 14, no. 5: 536. https://doi.org/10.3390/ijerph14050536





