Cu Isotopic Composition in Surface Environments and in Biological Systems: A Critical Review
Abstract
:1. Introduction
2. Analytical Methodologies
2.1. Cu Separation from Sample Matrix
2.2. Cu Isotope Analysis
2.3. Mass Bias Correction
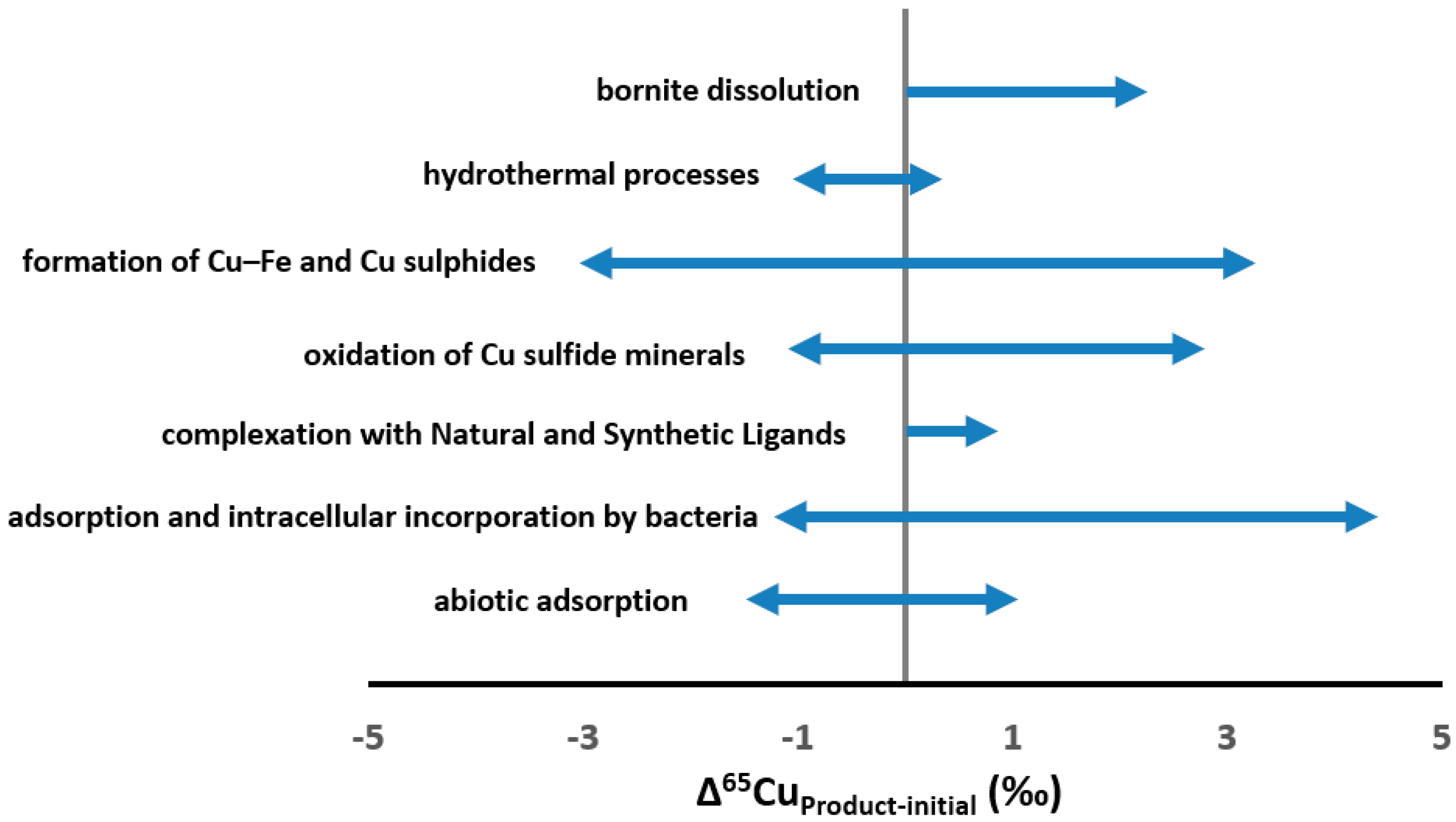
3. Cu Isotopic Fractionation during Low Temperature Processes Revealed by Experimental Studies
4. Cu Isotope Compositions in Earth Surface Reservoirs
4.1. Solid Reservoirs on the Earth Surface
4.1.1. Soil Weathering Profiles
4.1.2. Lithosphere
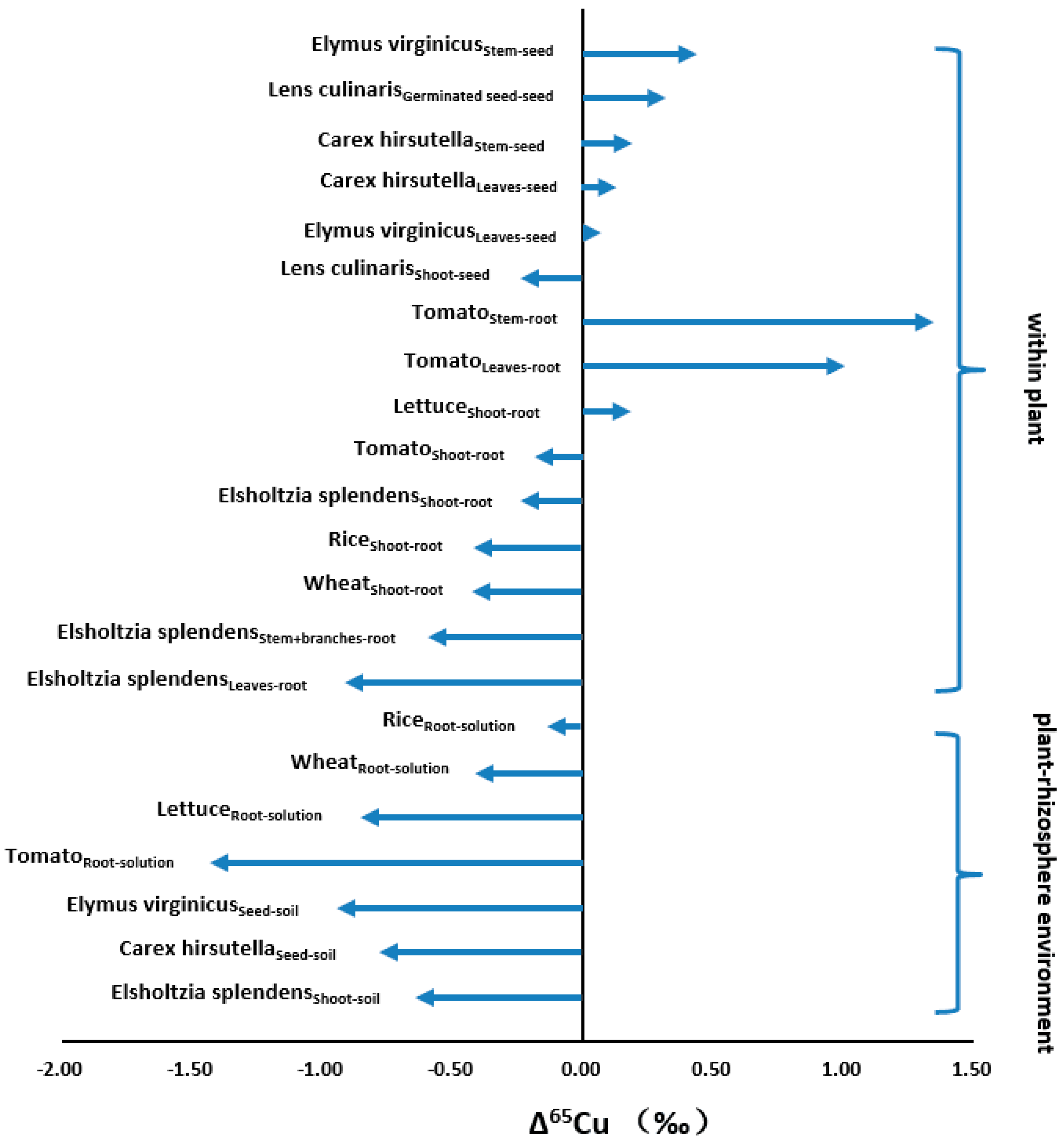
4.2. Biosphere
4.3. Hydrosphere
4.4. Atmosphere
5. Animals and Human Beings
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Semeniuk, D.M.; Cullen, J.T.; Johnson, W.K.; Gagnon, K.; Ruth, T.J.; Maldonado, M.T. Plankton copper requirements and uptake in the subarctic northeast pacific ocean. Deep Sea Res. Part I 2009, 56, 1130–1142. [Google Scholar] [CrossRef]
- De Oliveira Filho, E.C.; Lopes, R.M.; Paumgartten, F.J. Comparative study on the susceptibility of freshwater species to copper-based pesticides. Chemosphere 2004, 56, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Guasch, H. Effects of chronic copper exposure on fluvial systems: Linking structural and physiological changes of fluvial biofilms with the in-stream copper retention. Sci. Total Environ. 2009, 407, 5274–5282. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.; Inghram, M. The isotopic composition of meteoritic copper. Phys. Rev. 1947, 72, 347. [Google Scholar] [CrossRef]
- Chi Fru, E.; Rodríguez, N.P.; Partin, C.A.; Lalonde, S.V.; Andersson, P.; Weiss, D.J.; El Albani, A.; Rodushkin, I.; Konhauser, K.O. Cu isotopes in marine black shales record the great oxidation event. Proc. Natl. Acad. Sci. USA 2016, 113, 4941–4946. [Google Scholar] [CrossRef] [PubMed]
- Maréchal, C.N.; Télouk, P.; Albarède, F. Precise analysis of copper and zinc isotopic compositions by plasma-source mass spectrometry. Chem. Geol. 1999, 156, 251–273. [Google Scholar] [CrossRef]
- Zhu, X.K.; O’Nions, R.K.; Guo, Y.; Belshaw, N.S.; Rickard, D. Determination of natural Cu-isotope variation by plasma-source mass spectrometry: Implications for use as geochemical tracers. Chem. Geol. 2000, 163, 139–149. [Google Scholar] [CrossRef]
- Albarède, F. The stable isotope geochemistry of copper and zinc. Rev. Mineral. Geochem. 2004, 55, 409–427. [Google Scholar] [CrossRef]
- Balliana, E.; Aramendia, M.; Resano, M.; Barbante, C.; Vanhaecke, F. Copper and tin isotopic analysis of ancient bronzes for archaeological investigation: Development and validation of a suitable analytical methodology. Anal. Bioanal. Chem. 2013, 405, 2973–2986. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Zhou, L.; Gao, S.; Zhang, T.; Feng, L.; Yang, L. Use of Ga for mass bias correction for the accurate determination of copper isotope ratio in the NISTSRM3114 Cu standard and geological samples by MC-ICP-MS. J. Anal. At. Spectrom. 2015, 31, 280–287. [Google Scholar] [CrossRef]
- Larner, F.; Rehkämper, M.; Coles, B.J.; Kreissig, K.; Weiss, D.J.; Sampson, B.; Unsworth, C.; Strekopytov, S. A new separation procedure for Cu prior to stable isotope analysis by MC-ICP-MS. J. Anal. At. Spectrom. 2011, 26, 1627. [Google Scholar] [CrossRef]
- Petit, J.C.; Taillez, A.; Mattielli, N. A case study of spectral and non-spectral interferences on copper isotope measurements by multi-collector ICP-MS (wet plasma). Geostand. Geoanal. Res. 2013, 37, 319–335. [Google Scholar] [CrossRef]
- Yuan, H.; Yuan, W.; Bao, Z.; Chen, K.; Huang, F.; Liu, S. Development of two new copper isotope standard solutions and their copper isotopic compositions. Geostand. Geoanal. Res. 2017, 41, 77–84. [Google Scholar] [CrossRef]
- Zhu, X.K.; Guo, Y.; Williams, R.J.P.; O’Nions, R.K.; Matthews, A.; Belshaw, N.S.; Canters, G.W.; Waal, E.C.D.; Weser, U.; Burgess, B.K. Mass fractionation processes of transition metal isotopes. Earth Planet. Sci. Lett. 2002, 200, 47–62. [Google Scholar] [CrossRef]
- Vance, D.; Matthews, A.; Keech, A.; Archer, C.; Hudson, G.; Pett-Ridge, J.; Chadwick, O.A. The behaviour of Cu and Zn isotopes during soil development: Controls on the dissolved load of rivers. Chem. Geol. 2016, 445, 36–53. [Google Scholar] [CrossRef]
- Albarede, F.; Telouk, P.; Balter, V.; Bondanese, V.P.; Albalat, E.; Oger, P.; Bonaventura, P.; Miossec, P.; Fujii, T. Medical applications of the Cu, Zn, and S isotope effects. Metallomics 2016, 8, 1056–1070. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gaillrdet, J.; Louvat, P. Multi-isotopic (Zn, Cu) approach for anthropogenic contamination of suspended sediments of the Seine River, France. Geochim. Cosmochim. Acta Suppl. 2008, 72, 155. [Google Scholar] [CrossRef]
- Li, S.-Z.; Zhu, X.-K.; Wu, L.-H.; Luo, Y.-M. Cu isotopic compositions in elsholtzia splendens: Influence of soil condition and growth period on Cu isotopic fractionation in plant tissue. Chem. Geol. 2016, 444, 49–58. [Google Scholar] [CrossRef]
- Takano, S.; Tanimizu, M.; Hirata, T.; Sohrin, Y. Isotopic constraints on biogeochemical cycling of copper in the ocean. Nat. Commun. 2014, 5, 5663. [Google Scholar] [CrossRef] [PubMed]
- Thapalia, A.; Borrok, D.M.; Van Metre, P.C.; Musgrove, M.; Landa, E.R. Zn and Cu isotopes as tracers of anthropogenic contamination in a sediment core from an urban lake. Environ. Sci. Technol. 2010, 44, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.; Wilson, D.L.; Mathur, R. Tracing the source of native copper mineral specimens with copper isotope values. Rocks Miner. 2016, 352–356. [Google Scholar] [CrossRef]
- Moynier, F.; Vance, D.; Fujii, T.; Savage, P. The isotope geochemistry of zinc and copper. Rev. Mineral. Geochem. 2017, 82, 543–600. [Google Scholar] [CrossRef]
- Koster, H.M. Die bestimmung von kupfer(II), eisen(III) und zink(II) mittels atomabsorption bei der chemischen gesteinsanalyse nach abtrennung der losungspartner am cl-be-ladenen anionenaustauscher dowex 1 × 8 oder amberlite cg 400-1. Neues Jahrb. Miner. Abh. 1973, 119, 145–154. [Google Scholar]
- Van der Walt, T.N.; Strelow, F.W.E.; Verheij, R. The influence of crosslinkage on the distribution coefficients and anion exchange behaviour of some elements in hydrochloric acid. Solvent Extr. Ion Exch. 1985, 3, 723–740. [Google Scholar] [CrossRef]
- Bermin, J.; Vance, D.; Archer, C.; Statham, P.J. The determination of the isotopic composition of Cu and Zn in seawater. Chem. Geol. 2006, 226, 280–297. [Google Scholar] [CrossRef]
- Takano, S.; Tanimizu, M.; Hirata, T.; Sohrin, Y. Determination of isotopic composition of dissolved copper in seawater by multi-collector inductively coupled plasma mass spectrometry after pre-concentration using an ethylenediaminetriacetic acid chelating resin. Anal. Chim. Acta 2013, 784, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Moeller, K.; Schoenberg, R.; Pedersen, R.B.; Weiss, D.; Dong, S. Calibration of the new certified reference materials ERM-AE633 and ERM-AE647 for copper and IRMM-3702 for zinc isotope amount ratio determinations. Geostand. Geoanal. Res. 2012, 36, 177–199. [Google Scholar] [CrossRef]
- Liu, S.A.; Li, D.; Li, S.; Teng, F.Z.; Ke, S.; He, Y.; Lu, Y. High-precision copper and iron isotope analysis of igneous rock standards by MC-ICP-MS. J. Anal. At. Spectrom. 2013, 29, 122–133. [Google Scholar] [CrossRef]
- Mason, T.F.D.; Weiss, D.J.; Horstwood, M.; Parrish, R.R.; Russell, S.S.; Mullane, E.; Coles, B.J. High-precision Cu and Zn isotope analysis by plasma source mass spectrometry. J. Anal. At. Spectrom. 2004, 19, 218–226. [Google Scholar] [CrossRef]
- Archer, C.; Vance, D. Mass discrimination correction in multiple-collector plasma source mass spectrometry: An example using Cu and Zn isotopes. J. Anal. At. Spectrom. 2004, 19, 656–665. [Google Scholar] [CrossRef]
- Little, S.; Sherman, D.; Vance, D.; Hein, J. Molecular controls on Cu and Zn isotopic fractionation in Fe-Mn crusts. Earth Planet. Sci. Lett. 2014, 396, 213–222. [Google Scholar] [CrossRef]
- Mason, T.F.D.; Weiss, D.J.; Chapman, J.B.; Wilkinson, J.J.; Tessalina, S.G.; Spiro, B.; Horstwood, M.S.A.; Spratt, J.; Coles, B.J. Zn and Cu isotopic variability in the alexandrinka volcanic-hosted massive sulphide (VHMS) ore deposit, Urals, Russia. Chem. Geol. 2005, 221, 170–187. [Google Scholar] [CrossRef]
- Peel, K.; Weiss, D.; Chapman, J.; Arnold, T.; Coles, B. A simple combined sample-standard bracketing and inter-element correction procedure for accurate and precise Zn and Cu isotope ratio measurements. J. Anal. At. Spectrom. 2008, 23, 103–110. [Google Scholar] [CrossRef]
- Chen, J.B.; Louvat, P.; Gaillardet, J.; Birck, J.L. Direct separation of Zn from dilute aqueous solutions for isotope composition determination using multi-collector ICP-MS. Chem. Geol. 2009, 259, 120–130. [Google Scholar] [CrossRef]
- Chen, J.; Hintelmann, H.; Dimock, B. Chromatographic pre-concentration of Hg from dilute aqueous solutions for isotopic measurement by MC-ICP-MS. J. Anal. At. Spectrom. 2010, 74, A169. [Google Scholar] [CrossRef]
- Yang, L. Accurate and precise determination of isotopic ratios by MC-ICP-MS: A review. Mass Spectrom. Rev. 2009, 28, 990–1011. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Moynier, F.; Abe, M.; Nemoto, K.; Albarède, F. Copper isotope fractionation between aqueous compounds relevant to low temperature geochemistry and biology. Geochim. Cosmochim. Acta 2013, 110, 29–44. [Google Scholar] [CrossRef]
- Ryan, B.M.; Kirby, J.K.; Degryse, F.; Scheiderich, K.; Mclaughlin, M.J. Copper isotope fractionation during equilibration with natural and synthetic ligands. Environ. Sci. Technol. 2014, 48, 8620–8626. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, S.; Butler, I.; Halicz, L.; Rickard, D.; Oldroyd, A.; Matthews, A. Experimental study of the copper isotope fractionation between aqueous Cu(ii) and covellite, Cus. Chem. Geol. 2004, 209, 259–269. [Google Scholar] [CrossRef]
- Bigalke, M.; Weyer, S.; Wilcke, W. Copper isotope fractionation during complexation with insolubilized humic acid. Environ. Sci. Technol. 2010, 44, 5496–5502. [Google Scholar] [CrossRef] [PubMed]
- Fulda, B.; Voegelin, A.; Maurer, F.; Christl, I.; Kretzschmar, R. Copper redox transformation and complexation by reduced and oxidized soil humic acid. 1. X-ray absorption spectroscopy study. Environ. Sci. Technol. 2013, 47, 10903–10911. [Google Scholar] [CrossRef] [PubMed]
- Mathur, R.; Munk, L.A.; Townley, B.; Gou, K.Y.; Gómez Miguélez, N.; Titley, S.; Chen, G.G.; Song, S.; Reich, M.; Tornos, F.; et al. Tracing low-temperature aqueous metal migration in mineralized watersheds with Cuisotope fractionation. Appl. Geochem. 2014, 51, 109–115. [Google Scholar] [CrossRef]
- Mathur, R.; Ruiz, J.; Titley, S.; Liermann, L.; Buss, H.; Brantley, S. Cu isotopic fractionation in the supergene environment with and without bacteria. Geochim. Cosmochim. Acta 2005, 69, 5233–5246. [Google Scholar] [CrossRef]
- Navarrete, J.U.; Borrok, D.M.; Viveros, M.; Ellzey, J.T. Copper isotope fractionation during surface adsorption and intracellular incorporation by bacteria. Geochim. Cosmochim. Acta 2011, 75, 784–799. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, N.P.; Khoshkhoo, M.; Sandström, Å.; Rodushkin, I.; Alakangas, L.; Öhlander, B. Isotopic signature of Cu and Fe during bioleaching and electrochemical leaching of a chalcopyrite concentrate. Int. J. Miner. Process. 2015, 134, 58–65. [Google Scholar] [CrossRef]
- Sherman, D.M. Equilibrium isotopic fractionation of copper during oxidation/reduction, aqueous complexation and ore-forming processes: Predictions from hybrid density functional theory. Geochim. Cosmochim. Acta 2013, 118, 85–97. [Google Scholar] [CrossRef]
- Wall, A.J.; Mathur, R.; Post, J.E.; Heaney, P.J. Cu isotope fractionation during bornite dissolution: An in situ x-ray diffraction analysis. Ore Geol. Rev. 2011, 42, 62–70. [Google Scholar] [CrossRef]
- Fernandez, A.; Borrok, D.M. Fractionation of Cu, Fe, and Zn isotopes during the oxidative weathering of sulfide-rich rocks. Chem. Geol. 2009, 264, 1–12. [Google Scholar] [CrossRef]
- Kimball, B.E.; Mathur, R.; Dohnalkova, A.C.; Wall, A.J.; Runkel, R.L.; Brantley, S.L. Copper isotope fractionation in acid mine drainage. Geochim. Cosmochim. Acta 2009, 73, 1247–1263. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Viers, J.; Emnova, E.E.; Kompantseva, E.I.; Freydier, R. Copper isotope fractionation during its interaction with soil and aquatic microorganisms and metal oxy(hydr)oxides: Possible structural control. Geochim. Cosmochim. Acta 2008, 72, 1742–1757. [Google Scholar] [CrossRef]
- Seo, J.H.; Lee, S.K.; Lee, I. Quantum chemical calculations of equilibrium copper(I) isotope fractionations in ore-forming fluids. Chem. Geol. 2007, 243, 225–237. [Google Scholar] [CrossRef]
- Li, D.; Liu, S.A.; Li, S.; Li, D.; Liu, S.A. Copper isotope fractionation during adsorption onto kaolinite: Experimental approach and applications. Chem. Geol. 2015, 396, 74–82. [Google Scholar] [CrossRef]
- Pękala, M.; Asael, D.; Butler, I.B.; Matthews, A.; Rickard, D. Experimental study of Cu isotope fractionation during the reaction of aqueous Cu(II) with Fe(II) sulphides at temperatures between 40 and 200 °C. Chem. Geol. 2011, 289, 31–38. [Google Scholar] [CrossRef]
- Balistrieri, L.S.; Borrok, D.M.; Wanty, R.B.; Ridley, W.I. Fractionation of Cu and Zn isotopes during adsorption onto amorphous Fe(III) oxyhydroxide: Experimental mixing of acid rock drainage and ambient river water. Geochim. Cosmochim. Acta 2008, 72, 311–328. [Google Scholar] [CrossRef]
- Clayton, R.E.; Hudsonedwards, K.A.; Houghton, S.L. Isotopic effects during Cu sorption onto goethite. Geochim. Cosmochim. Acta Suppl. 2005, 69, A216. [Google Scholar] [CrossRef]
- Maher, K.C.; Jackson, S.; Mountain, B. Experimental evaluation of the fluid-mineral fractionation of Cu isotopes at 250 °C and 300 °C. Chem. Geol. 2011, 286, 229–239. [Google Scholar] [CrossRef]
- Mathur, R.; Schlitt, W. Identification of the dominant Cu ore minerals providing soluble copper at cañariaco, peru through Cu isotope analyses of batch leach experiments. Hydrometallurgy 2010, 101, 15–19. [Google Scholar] [CrossRef]
- Borrok, D.M.; Ridley, W.I.; Wanty, R.B. Isotopic fractionation of Cu and Zn during adsorption onto bacterial surfaces. Geochim. Cosmochim. Acta Suppl. 2008, 72, A99. [Google Scholar] [CrossRef]
- Bigalke, M.; Weyer, S.; Wilcke, W. Stable Cu isotope fractionation in soils during oxic weathering and podzolization. Geochim. Cosmochim. Acta 2011, 75, 3119–3134. [Google Scholar] [CrossRef]
- Weber, F.A.; Voegelin, A.; Kaegi, R.; Kretzschmar, R. Contaminant mobilization by metallic copper and metal sulphide colloids in flooded soil. Nat. Geosci. 2009, 2, 267–271. [Google Scholar] [CrossRef]
- Bigalke, M.; Kersten, M.; Weyer, S.; Wilcke, W. Isotopes trace biogeochemistry and sources of Cu and Zn in an intertidal soil. Soil Sci. Soc. Am. J. 2013, 77, 680–691. [Google Scholar] [CrossRef]
- Bigalke, M.; Weyer, S.; Wilcke, W. Stable copper isotopes: A novel tool to trace copper behavior in hydromorphic soils. Soil Sci. Soc. Am. J. 2010, 74, 60–73. [Google Scholar] [CrossRef]
- Liu, S.-A.; Teng, F.-Z.; Li, S.; Wei, G.-J.; Ma, J.-L.; Li, D. Copper and iron isotope fractionation during weathering and pedogenesis: Insights from saprolite profiles. Geochim. Cosmochim. Acta 2014, 146, 59–75. [Google Scholar] [CrossRef]
- Kusonwiriyawong, C.; Bigalke, M.; Abgottspon, F.; Lazarov, M.; Wilcke, W. Response of cu partitioning to flooding: A δ65Cu approach in a carbonatic alluvial soil. Chem. Geol. 2016, 420, 69–76. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, S.-A.; Zhu, J.-M.; Li, S. Copper and zinc isotope fractionation during deposition and weathering of highly metalliferous black shales in central China. Chem. Geol. 2016, 445, 24–35. [Google Scholar] [CrossRef]
- Mathur, R.; Jin, L.; Prush, V.; Paul, J.; Ebersole, C.; Fornadel, A.; Williams, J.Z.; Brantley, S. Cu isotopes and concentrations during weathering of black shale of the marcellus formation, Huntingdoncounty, Pennsylvania (USA). Chem. Geol. 2012, 304–305, 175–184. [Google Scholar] [CrossRef]
- Chiaradia, M. Copper enrichment in arc magmas controlled by overriding plate thickness. Nat. Geosci. 2014, 7, 43–46. [Google Scholar] [CrossRef]
- Mirnejad, H.; Mathur, R.; Einali, M.; Dendas, M.; Alirezaei, S. A comparative copper isotope study of porphyry copper deposits in Iran. Geochem. Exp. Environ. Anal. 2010, 10, 413–418. [Google Scholar] [CrossRef]
- Gale, N.H.; Woodhead, A.P.; Stos-Gale, Z.A.; Walder, A.; Bowen, I. Natural variations detected in the isotopic composition of copper: Possible applications to archaeology and geochemistry. Int. J. Mass Spectrom. 1999, 184, 1–9. [Google Scholar] [CrossRef]
- Dan, A.; Matthews, A.; Bar-Matthews, M.; Halicz, L. Copper isotope fractionation in sedimentary copper mineralization (Timna Valley, Israel). Chem. Geol. 2007, 243, 238–254. [Google Scholar] [CrossRef]
- Graham, S.; Pearson, N.; Jackson, S.; Griffin, W.; O’Reilly, S.Y. Tracing Cu and Fe from source to porphyry: In situ determination of Cu and Fe isotope ratios in sulfides from the grasberg Cu–Au deposit. Chem. Geol. 2004, 207, 147–169. [Google Scholar] [CrossRef]
- Li, W.; Jackson, S.E.; Pearson, N.J.; Alard, O.; Chappell, B.W. The Cu isotopic signature of granites from the lachlan Fold Belt, SE Australia. Chem. Geol. 2009, 258, 38–49. [Google Scholar] [CrossRef]
- Song, S.; Mathur, R.; Ruiz, J.; Chen, D.; Allin, N.; Guo, K.; Kang, W. Fingerprinting two metal contaminants in streams with Cu isotopes near the dexing mine, China. Sci. Total Environ. 2015, 544, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, S.A.; Wörner, G.; Yu, H.; Xiao, Y. Copper isotope behavior during extreme magma differentiation and degassing: a case study on Laacher See phonolite tephra (East Eifel, Germany). Contrib. Mineral. Petrol. 2016, 171, 8–9. [Google Scholar] [CrossRef]
- Savage, P.S.; Moynier, F.; Chen, H.; Shofner, G.; Siebert, J.; Badro, J.; Puchtel, I. Copper isotope evidence for large-scale sulphide fractionation during earth’s differentiation. Geochem. Perspect. Lett. 2015, 1, 53–64. [Google Scholar] [CrossRef]
- Liu, S.A.; Huang, J.; Liu, J.; Wörner, G.; Yang, W.; Tang, Y.J.; Chen, Y.; Tang, L.; Zheng, J.; Li, S. Copper isotopic composition of the silicate earth. Earth Planet. Sci. Lett. 2015, 427, 95–103. [Google Scholar] [CrossRef]
- Bishop, M.C.; Moynier, F.; Weinstein, C.; Fraboulet, J.G.; Wang, K.; Foriel, J. The Cu isotopic composition of iron meteorites. Meteorit. Planet. Sci. 2012, 47, 268–276. [Google Scholar] [CrossRef]
- Herzog, G.; Moynier, F.; Albarède, F.; Berezhnoy, A. Isotopic and elemental abundances of copper and zinc in lunar samples, Zagami, Pele’s hairs, and a terrestrial basalt. Geochim. Cosmochim. Acta 2009, 73, 5884–5904. [Google Scholar] [CrossRef]
- Moynier, F.; Koeberl, C.; Beck, P.; Jourdan, F.; Telouk, P. Isotopic fractionation of Cu in tektites. Geochim. Cosmochim. Acta 2010, 74, 799–807. [Google Scholar] [CrossRef]
- Büchl, A.; Hawkesworth, C.J.; Ragnarsdottir, K.V.; Brown, D.R. Re-partitioning of Cu and Zn isotopes by modified protein expression. Geochem. Trans. 2008, 9, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Jaouen, K.; Balter, V.; Herrscher, E.; Lamboux, A.; Telouk, P.; Albarede, F. Fe and Cu stable isotopes in archeological human bones and their relationship to sex. Am. J. Phys. Anthropol. 2012, 148, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.; Sipkova, A.; Chrastny, V.; Stepanova, M.; Voldrichova, P.; Veselovsky, F.; Prechova, E.; Blaha, V.; Curik, J.; Farkas, J. Cu-Zn isotope constraints on the provenance of air pollution in central Europe: Using soluble and insoluble particles in snow and rime. Environ. Pollut. 2016, 218, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Little, S.H.; Vance, D.; Walker-Brown, C.; Landing, W.M. The oceanic mass balance of copper and zinc isotopes, investigated by analysis of their inputs, and outputs to ferromanganese oxide sediments. Geochim. Cosmochim. Acta 2014, 125, 673–693. [Google Scholar] [CrossRef]
- Babcsányi, I.; Imfeld, G.; Granet, M.; Chabaux, F. Copper stable isotopes to trace copper behavior in wetland systems. Environ. Sci. Technol. 2014, 48, 5520–5529. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.C.J.; Schäfer, J.; Coynel, A.; Blanc, G.; Deycard, V.N.; Derriennic, H.; Lanceleur, L.; Dutruch, L.; Bossy, C.; Mattielli, N. Anthropogenic sources and biogeochemical reactivity of particulate and dissolved Cu isotopes in the turbidity gradient of the garonne river (France). Chem. Geol. 2013, 359, 125–135. [Google Scholar] [CrossRef]
- Mathur, R.; Titley, S.; Barra, F.; Brantley, S.; Wilson, M.; Phillips, A.; Munizaga, F.; Maksaev, V.; Vervoort, J.; Hart, G. Exploration potential of Cu isotope fractionation in porphyry copper deposits. J. Geochem. Explor. 2009, 102, 1–6. [Google Scholar] [CrossRef]
- Mathur, R.; Munk, L.; Nguyen, M.; Gregory, M.; Annell, H.; Lang, J. Modern and paleofluid pathways revealed by Cu isotope compositions in surface waters and ores of the pebble porphyry cu-au-mo deposit, alaska. Econ. Geol. 2013, 108, 529–541. [Google Scholar] [CrossRef]
- El Azzi, D.; Viers, J.; Guiresse, M.; Probst, A.; Aubert, D.; Caparros, J.; Charles, F.; Guizien, K.; Probst, J.-L. Origin and fate of copper in a small mediterranean vineyard catchment: New insights from combined chemical extraction and δ65Cu isotopic composition. Sci. Total Environ. 2013, 463, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Vance, D.; Archer, C.; Bermin, J.; Perkins, J.; Statham, P.; Lohan, M.; Ellwood, M.; Mills, R. The copper isotope geochemistry of rivers and the oceans. Earth Planet. Sci. Lett. 2008, 274, 204–213. [Google Scholar] [CrossRef]
- Asael, D.; Matthews, A.; Bar-Matthews, M.; Harlavan, Y.; Segal, I. Tracking redox controls and sources of sedimentary mineralization using copper and lead isotopes. Chem. Geol. 2012, 310–311, 23–35. [Google Scholar] [CrossRef]
- Molnár, F.; Mänttäri, I.; O’Brien, H.; Lahaye, Y.; Pakkanen, L.; Johanson, B.; Käpyaho, A.; Sorjonen-Ward, P.; Whitehouse, M.; Sakellaris, G. Boron, sulphur and copper isotope systematics in the orogenic gold deposits of the archaean hattu schist belt, eastern finland. Ore Geol. Rev. 2016, 77, 133–162. [Google Scholar] [CrossRef]
- Huang, J.; Liu, S.A.; Gao, Y.; Xiao, Y.; Chen, S. Copper and Zinc isotope systematics of altered oceanic crust at IODP site 1256 in the eastern equatorial pacific. J. Geophys. Res. Solid Earth 2016, 121, 7086–7100. [Google Scholar] [CrossRef]
- Dekov, V.M.; Rouxel, O.; Asael, D.; Hålenius, U.; Munnik, F. Native cu from the oceanic crust: Isotopic insights into native metal origin. Chem. Geol. 2013, 359, 136–149. [Google Scholar] [CrossRef]
- Yang, M.J.; Yang, X.E.; Römheld, V. Growth and nutrient composition of elsholtziasplendensnakai under copper toxicity. J. Plant Nutr. 2002, 25, 1359–1375. [Google Scholar] [CrossRef]
- Jouvin, D.; Weiss, D.J.; Mason, T.F.; Bravin, M.N.; Louvat, P.; Zhao, F.; Ferec, F.; Hinsinger, P.; Benedetti, M.F. Stable isotopes of Cu and Zn in higher plants: Evidence for Cu reduction at the root surface and two conceptual models for isotopic fractionation processes. Environ. Sci. Technol. 2012, 46, 2652–2660. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, S.; Luo, Y.; Wu, L. Copper isotope fractionation by higher plants. Geochim. Cosmochim. Acta 2010, 74, A1234. [Google Scholar] [CrossRef]
- Ryan, B.M.; Kirby, J.K.; Degryse, F.; Harris, H.; McLaughlin, M.J.; Scheiderich, K. Copper speciation and isotopic fractionation in plants: Uptake and translocation mechanisms. New Phytol. 2013, 199, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, C.; Moynier, F.; Wang, K.; Paniello, R.; Foriel, J.; Catalano, J.; Pichat, S. Isotopic fractionation of Cu in plants. Chem. Geol. 2011, 286, 266–271. [Google Scholar] [CrossRef]
- Ilina, S.M.; Viers, J.; Lapitsky, S.A.; Mialle, S.; Mavromatis, V.; Chmeleff, J.; Brunet, P.; Alekhin, Y.V.; Isnard, H.; Pokrovsky, O.S. Stable (Cu, Mg) and radiogenic (Sr, Nd) isotope fractionation in colloids of boreal organic-rich waters. Chem. Geol. 2013, 342, 63–75. [Google Scholar] [CrossRef]
- Rodríguez, N.P.; Engström, E.; Rodushkin, I.; Nason, P.; Alakangas, L.; Öhlander, B. Copper and iron isotope fractionation in mine tailings at the laver and kristineberg mines, northern Sweden. Appl. Geochem. 2013, 32, 204–215. [Google Scholar] [CrossRef]
- Komjarova, I.; Bury, N.R. Evidence of common cadmium and copper uptake routes in ZebrafishDaniorerio. Environ. Sci. Technol. 2014, 48, 12946–12951. [Google Scholar] [CrossRef] [PubMed]
- Balter, V.; Lamboux, A.; Zazzo, A.; Telouk, P.; Leverrier, Y.; Marvel, J.; Moloney, A.P.; Monahan, F.J.; Schmidt, O.; Albarede, F. Contrasting Cu, Fe, and Zn isotopic patterns in organs and body fluids of mice and sheep, with emphasis on cellular fractionation. Metallomics 2013, 5, 1470–1482. [Google Scholar] [CrossRef] [PubMed]
- Jaouen, K.; Gibert, M.; Lamboux, A.; Telouk, P.; Fourel, F.; Albarede, F.; Alekseev, A.N.; Crubezy, E.; Balter, V. Is aging recorded in blood Cu and Zn isotope compositions? Metallomics 2013, 5, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Van Heghe, L.; Engström, E.; Rodushkin, I.; Cloquet, C.; Vanhaecke, F. Isotopic analysis of the metabolically relevant transition metals Cu, Fe and Zn in human blood from vegetarians and omnivores using multi-collector icp-mass spectrometry. J. Anal. At. Spectrom. 2012, 27, 1327. [Google Scholar] [CrossRef]
- Albarede, F.; Telouk, P.; Lamboux, A.; Jaouen, K.; Balter, V. Isotopic evidence of unaccounted for Fe and Cu erythropoietic pathways. Metallomics 2011, 3, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Balter, V.; da Costa, A.N.; Bondanese, V.P.; Jaouen, K.; Lamboux, A.; Sangrajrang, S.; Vincent, N.; Fourel, F.; Télouk, P.; Gigou, M. Natural variations of copper and sulfur stable isotopes in blood of hepatocellular carcinoma patients. Proc. Natl. Acad. Sci. USA 2015, 112, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Télouk, P.; Puisieux, A.; Fujii, T.; Balter, V.; Bondanese, V.P.; Morel, A.-P.; Clapisson, G.; Lamboux, A.; Albarede, F. Copper isotope effect in serum of cancer patients. A pilot study. Metallomics 2015, 7, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Jaouen, K.; Balter, V. Menopause effect on blood Fe and Cu isotope compositions. Am. J. Phys. Anthropol. 2014, 153, 280–285. [Google Scholar] [CrossRef] [PubMed]

| Material | δ65Cu Variation (‰) | Average δ65Cu (‰, 2SD) | References |
|---|---|---|---|
| Healthy women serum | −0.80~0.04 | −0.28 ± 0.41 | [105] |
| Healthy men serum | −0.64~0.06 | −0.28 ± 0.40 | |
| Healthy womenerythrocytes | −0.04~0.80 | 0.46 ± 0.47 | |
| Healthy menerythrocytes | 0.23~0.91 | 0.67 ± 0.36 | |
| Healthy womentotal blood | −0.52~0.32 | 0.00 ± 0.41 | |
| Healthy mentotal blood | −0.21~0.43 | 0.16 ± 0.33 | |
| Cancer patients (HCC) serum | −0.66~0.47 | −0.02 ± 0.54 | [106] |
| Control group serum | −0.39~0.38 | 0.10 ± 0.45 | |
| Cancer patients (HCC) red blood cell | −0.07~0.92 | 0.51 ± 0.56 | |
| Control group red blood cell | 0.57~1.24 | 0.88 ± 0.44 | |
| Breast cancer patients serum | −1.45~0.12 | −0.51 ± 0.52 | [107] |
| Colorectal cancer patients serum | −0.65~0.04 | −0.29 ± 0.30 | |
| Aging men blood | 0.68 ± 0.49 | [108] | |
| Control group (Young men) | 0.67 ± 0.36 | ||
| Postmenopausal women | 0.71 ± 0.54 | ||
| Control group (Premenopausal women) | 0.43 ± 0.48 | ||
| Liver | −0.26 ± 0.22 | ||
| Vegetarian female blood | −0.75~−0.29 | −0.51 ± 0.46 | [104] |
| Vegetarian male blood | −0.22~0.23 | −0.07 ± 0.52 | |
| Omnivorous female blood | −0.14~0.17 | −0.02 ± 0.34 | |
| Omnivorous male blood | −0.28~0.09 | −0.05 ± 0.41 | |
| Russian and Yakut blood | −1.37~−0.22 | −0.68 ± 0.62 | [81] |
| Archeological women bones | −0.20 ± 0.25 | [103] | |
| Archeological men bones | −0.11 ± 0.16 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Chen, J.; Zhang, T. Cu Isotopic Composition in Surface Environments and in Biological Systems: A Critical Review. Int. J. Environ. Res. Public Health 2017, 14, 538. https://doi.org/10.3390/ijerph14050538
Wang Z, Chen J, Zhang T. Cu Isotopic Composition in Surface Environments and in Biological Systems: A Critical Review. International Journal of Environmental Research and Public Health. 2017; 14(5):538. https://doi.org/10.3390/ijerph14050538
Chicago/Turabian StyleWang, Zhuhong, Jiubin Chen, and Ting Zhang. 2017. "Cu Isotopic Composition in Surface Environments and in Biological Systems: A Critical Review" International Journal of Environmental Research and Public Health 14, no. 5: 538. https://doi.org/10.3390/ijerph14050538





