Geographical Environment Factors and Risk Assessment of Tick-Borne Encephalitis in Hulunbuir, Northeastern China
Abstract
:1. Introduction
- (1)
- The topographic factors include elevation, slope, and aspect. These factors play important roles in the distribution of ticks and their hosts by affecting the reallocation of the hydrothermal combination. Merler used classification tree method to analyze the distribution of Ixodes ricinus in Trentino, Italian Alps, and concluded that the most important factors determining the distribution of the ticks are the altitude and geological environment [13]. Toomer used the digital elevation model (DEM) and other factors to simulate the general distribution of Pan-African ticks [14]. Randolph et al. used the DEM and Land Surface Temperature (LST) as predictive variables and reported that the distribution of five tick aggregation places in central Europe and around the Baltic Ocean is closely related to the DEM [15]. Materna et al. mentioned in their study that the density of ticks in small-scale research is highly impacted by the aspect [16].
- (2)
- Climate factors, such as temperature, light duration, and rainfall, determine the living range of hosts and vectors to a certain extent, which affects the distribution of natural foci. Lindgren suggested that more ticks might survive in a mild winter in host and reservoir animals [17]. Due to an early arrival of the spring and/or late arrival of the next winter, ticks will be active for an extended period. Eisen reported that the tick density is closely related to the daily maximum temperatures [18]. Süss et al. proposed that an increase in the temperature up to a certain level causes the acceleration and extension of the developmental cycle of the ticks, increase in the egg production and population density, and shift of the risk areas [19]. Kahl et al. concluded that the relative humidity (RH) affects the life circle of ticks due to the transformation and absorption of water vapor in half-saturated air [20].
- (3)
- The vegetation can provide a suitable living environment for ticks and their vectors. The density of ticks correlates to the type and structure of the forest; it is the highest in mixed and deciduous forests [15]. Jackson reported that the Lyme disease incidences in 12 Maryland counties were the highest when the edge-contrast index of the forest–herbaceous edge reached 53% [21].
2. Material and Methods
2.1. Study Area
2.2. Data and Preprocessing
2.2.1. Disease Data
2.2.2. Geographic and Environmental Data
2.3. Methods
2.3.1. Spatial Autocorrelation
2.3.2. Spatial Regression
3. Results and Analysis
3.1. Descriptive Analysis
3.2. Spatial Autocorrelation
3.3. Global Regression Analysis
3.4. Local Regression Analysis
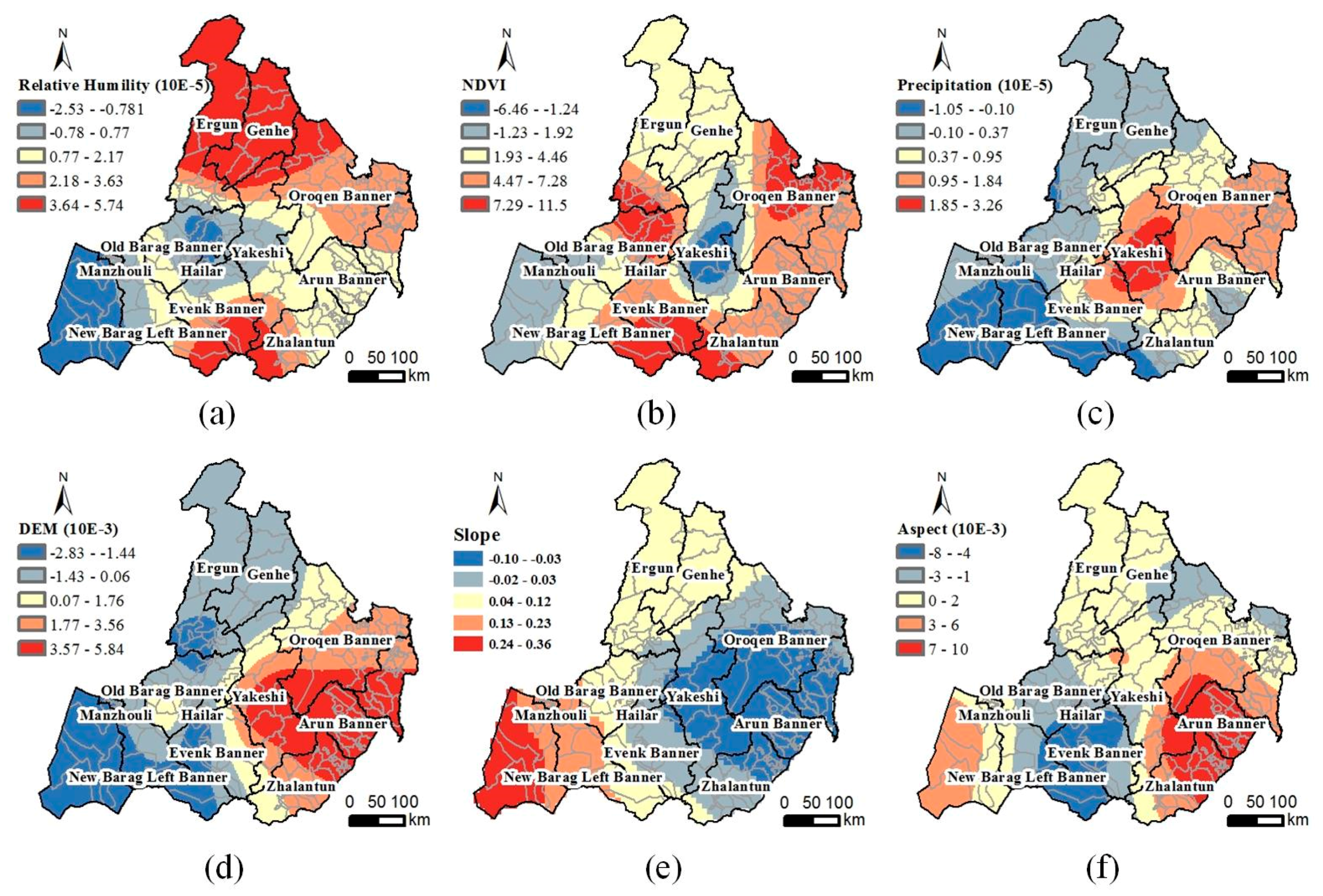
- (1)
- Relative humidity: The RH in the three northern counties and several southern counties and the TBE risk are positively correlated (Figure 6a). The TBE risk in these areas increases with the RH. The TBE risk in the New Barag Right Banner and the RH are negatively related. Regions with relatively high RH values always have a lower risk.
- (2)
- Vegetation index: Based on the spatial distribution of the NDVI coefficient in Figure 6b, the TBE risk is positively correlated with the NDVI in the Old Barag Banner, Oroqen Banner, and several southernmost towns. The TBE risk in these areas increases with increasing vegetation cover. In the middle Yakeshi County, especially in Wunu’er and Miandu, the TBE risk reaches the highest value while it is negatively correlated with the NDVI. No direct relationship between the increasing vegetation coverage and TBE risk was observed in these areas.
- (3)
- Precipitation: The TBE risks in southwestern Hulunbuir are negatively correlated with the precipitation (Figure 6c). The TBE risks in this area decrease with increasing precipitation. The TBE risks and precipitation in the center of the Yakeshi County are positively correlated. The risks increase with increasing precipitation.
- (4)
- DEM: The correlation between the TBE risks and DEM changes from negative to positive from west to east (Figure 6d). Areas with high elevation in western Hulunbuir exhibit less risk than the low regions. The effect of the DEM is the opposite in eastern Hulunbuir.
- (5)
- Slope: The correlation between the slope and TBE risk is negative in the east, while it is highly positive in the west. In western Hulunbuir, where most of the land cover is grassland, the TBE risk is a bit higher at a steep slope than in gentle areas. In contrast, the TBE risk in the broad farmland of eastern Hulunbuir is lower at a steep slope than in the gentle areas (Figure 6e).
- (6)
- Aspect: The TBE risk in northern Hulunbuir is slightly impacted by the change of aspect. In contrast, there are two different situations in the southern area. The TBE risk is negatively correlated with the aspect in the Evenk and New Barag Right banners, while they are positively correlated in the southeastern Arun Banner and Zhalantun County (Figure 6f).
4. Discussion
4.1. Endemic Seasonal Features of TBE
4.2. Predicted Risk Distribution
4.2.1. Central High-Risk Triangle Zone
4.2.2. Western Low-Risk Belt
4.2.3. Eastern Low-Risk Belt
4.3. Regression Models
5. Conclusions
- (1)
- The spatial autocorrelation results show that the distribution of the TBE risk in Hulunbuir was significantly autocorrelated from 2006 to 2013. The high-risk aggregation area gradually changes during the study period. The high-risk TBE aggregation area first extends from the northern part of the Great Khingan Range southward. The aggregation foci return back to the origin in 2011, and the high-risk aggregations continue to expand northward up to Moerdaoga, Ergun County. The statistical data show that the people in Hulunbuir more easily get infected with TBE in spring and summer. The prevalence of the patients has notable occupational and gender characteristics. Male workers inhabiting or working in forests more easily get infected.
- (2)
- The impact degree of the geographic and environmental factors on the TBE risk has the following descending order: temperature, RH, vegetation coverage, precipitation, and topographic information. The temperature and RH in Hulunbuir are strongly negatively correlated. In addition, the spatial distribution of the different coefficients of the variables in the local regression model show that the correlations between the TBE risk and geographic and environmental factors change depending on the spatial location.
- (3)
- The distribution of TBE risk in Hulunbuir was quite particular. Central high-risk region seemed to be a triangle area. The eastern and western belts are at low TBE risk. The high-risk triangle includes Ergun, Genhe, Oroqen Banner, and Yakeshi County. The TBE risk inside the triangle region increases from south to north. The most relevant factor in this triangle is the RH. The TBE risk in most parts of this triangle is positively correlated with NDVI, precipitation, and aspect and negatively correlated with slope; they are negatively correlated in the northern triangle. The local regression results provide a risk evaluation model and data support for the TBE prediction and control.
Acknowledgments
Authors Contribution
Conflicts of Interest
References
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.S.; Dautel, H.; Estrada-Peña, A.; Kahl, O.; Lindgren, E. Effects of Climate Change on Ticks and Tick-Borne Diseases in Europe. Interdiscip. Perspect. Infect. Dis. 2009, 2009, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zöldi, V.; Juhász, A.; Nagy, C.; Papp, Z.; Egyed, L. Tick-Borne Encephalitis and Lyme Disease in Hungary: The Epidemiological Situation Between 1998 and 2008. Vector Borne Zoonotic Dis. 2013, 13, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Jiang, Y.T.; Guo, C.S.; Guan, B.P. Some epidemiological characters of TBE natural foci in Jilin Province. Mil. Med. Sci. 1979, 1, 109–120. [Google Scholar]
- Yin, D.M.; Liu, R.Z. Review of Tick-borne Encephalitis in the forest area of Northeastern China. Chin. J. Epidemiol. 2000, 21, 387–389. [Google Scholar]
- Bi, W.M.; Deng, H.P.; Pu, X.Y. A regionalization study of natured epidemic-stricken areas of forest Encephalitis. J. Cap. Norm. Univ. Nat. Sci. Ed. 1997, 18, 100–107. [Google Scholar]
- Lu, Z.; Bröker, M.; Liang, G. Tick-Borne Encephalitis in Mainland China. Vector Borne Zoonotic Dis. 2008, 8, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.L.; Lu, Z.X.; Hu, L.M.; Zhao, Z.L.; Jin, X.T.; He, Y.X. Epidemiology survey of Russian Spring Summer Encephalitis in the Northeast area, China. J. Microbiol. 1996, 16, 19–22. [Google Scholar]
- Dong, J.H.; Zhu, J.H.; Yin, F.R. Epidemiological studies of Tick-borne Encephalitis and Lyme Disease in the forest area of Greater Khingan Range. Chin. Prev. Med. 2007, 8, 718–719. [Google Scholar]
- Rogers, D.J.; Randolph, S.E. Studying the global distribution of infectious diseases using GIS and RS. Nat. Rev. Microbiol. 2003, 1, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Hay, S.I.; Packer, M.J.; Rogers, D.J. The impact of remote sensing on the study and control of invertebrate intermediate hosts and vectors for disease. Int. J. Remote Sens. 1997, 18, 2899–2930. [Google Scholar] [CrossRef]
- Meade, M.S.; Florin, J.W.; Gesler, W.M. Medical Geography; Guiford Press: New York, NY, USA, 1988. [Google Scholar]
- Merler, S.; Furlanello, C.; Chemini, C.; Nicolini, G. Classification tree methods for analysis of mesoscale distribution of Ixodes ricinus (Acari: Ixodidae) in Trentino, Italian Alps. J. Med. Entomol. 1996, 33, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Toomer, J. Predicting Pan-African Tick Distributions Using Remotely Sensed Surrogates of Meteorological and Environmental Conditions; Undergraduate Project for FHS Biological Sciences; University of Oxford: Oxford, UK, 1997. [Google Scholar]
- Randolph, S.E. Ticks and tick-borne disease systems in space and from space. Adv. Parasitol. 2000, 47, 217–243. [Google Scholar] [PubMed]
- Materna, J.; Daniel, M.; Metelka, L.; Harčarik, J. The vertical distribution, density and the development of the tick Ixodes ricinus in mountain areas influenced by climate changes (The Krkonoše Mts., Czech Republic). Int. J. Med. Microbiol. 2008, 298, 25–37. [Google Scholar] [CrossRef]
- Lindgren, E. Climate and Tickborne Encephalitis. Ecol. Soc. 1998, 2, 5. [Google Scholar] [CrossRef]
- Eisen, L. Seasonal pattern of host-seeking activity by the human-biting adult life stage of Dermacentor andersoni (Acari: Ixodidae). J. Med. Entomol. 2007, 44, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Süss, J.; Klaus, C.; Gerstengarbe, F.; Werner, P.C. What Makes Ticks Tick? Climate Change, Ticks, and Tick-Borne Diseases. J. Travel Med. 2008, 15, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Kahl, O.; Knuelle, W. Wirtssuchaktivität der Schildzecke, Ixodes ricinus (Acari, Ixodidae) und ihre Durchseuchung mit Lyme-Spirochäten und dem Frühsommer-Meningoencepahlitis (FSME)-Virus in Berlin (West). Mitt. Dtsch. Ges. Allg. Angew. Entomol. 1988, 6, 223–225. [Google Scholar]
- Jackson, L.E. The Relationship of Land-Cover Pattern to Lyme Disease. Ph.D. Thesis, University of North Carolina, Chapel Hill, NC, USA, 2005. [Google Scholar]
- Sun, R.X.; Li, X.L.; Liu, K.; Yao, H.W.; Fang, L.Q.; Cao, W.C. Study on the distribution and environmental determinants of tick-borne encephalitis in northeastern China. J. Pathog. Biol. 2016, 11, 481–487. [Google Scholar]
- Feng, N. Tabulation on the Population Census of the People’s Republic of China by Township; China Statistics Press: Beijing, China, 2012. [Google Scholar]
- Wang, J.F.; Liao, Y.L.; Liu, X. Tutorial of Spatial Data Analysis; Science Press: Beijing, China, 2010; pp. 50–56. [Google Scholar]
- Hu, W.; Clements, A.; Williams, G.; Tong, S. Spatial analysis of notified dengue fever infections. Epidemiol. Infect. 2011, 139, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Ord, J.K.; Getis, A. Local spatial autocorrelation statistics: Distributional issues and an application. Geogr. Anal. 1995, 27, 286–306. [Google Scholar] [CrossRef]
- Brunsdon, C.; Fotheringham, A.S.; Charlton, M.E. Geographically Weighted Regression: A Method for Exploring Spatial Nonstationarity. Geogr. Anal. 1996, 4, 281–298. [Google Scholar] [CrossRef]
- Fotheringham, A.S.; Brunsdon, C.; Charlton, M.E. Geographically Weighted Regression: The Analysis of Spatially Varying Relationships; John Wiley & Sons: Chichester, UK, 2003. [Google Scholar]
- Ma, X.Y.; Peng, W.M.; Gao, X. Review on tick-borne encephalitis research. Chin. J. Virol. 2004, 2, 190–192. [Google Scholar]
- Wimberly, M.C.; Yabsley, M.J.; Baer, A.D.; Dugan, V.G.; Davidson, W.R. Spatial heterogeneity of climate and land-cover constraints on distributions of tick-borne pathogens. Glob. Ecol. Biogeogr. 2008, 17, 189–202. [Google Scholar] [CrossRef]
- Estradapeña, A. Geostatistics and remote sensing using NOAA-AVHRR satellite imagery as predictive tools in tick distribution and habitat suitability estimations for Boophilus microplus (Acari: Ixodidae) in South America. National Oceanographic and Atmosphere Administratio. Vet. Parasitol. 1999, 81, 73–82. [Google Scholar] [CrossRef]
- Cumming, G.S. Using habitat models to map diversity: Pan-African species richness of ticks (Acari: Ixodida). J. Biogeogr. 2000, 27, 425–440. [Google Scholar] [CrossRef]
- Li, Y.F.; Wang, J.L.; Gao, M.X. A review of geographical and environmental factor detection and risk prediction of natural focus diseases. Prog. Geogr. 2015, 34, 926–935. [Google Scholar]
- Bi, P.; Cameron, A.S.; Zhang, Y.; Parton, K.A. Weather and notified campylobacter infections in temperate and sub-tropical regions of Australia: An ecological study. J. Infect. 2008, 57, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Guo, W.D.; Fang, L.Q.; Li, C.P.; Bi, P.; Glass, G.E.; Jiang, J.F.; Sun, S.H.; Qian, Q.; Liu, W.; et al. Climate variability and hemorrhagic fever with renal syndrome transmission in Northeastern China. Environ. Health Perspect. 2010, 118, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.Y. Discussion of Application of Multiple Regression Analysis and Logistic Regression Analysis; Nanjing University of Information Science and Technology: Nanjing, China, 2008. [Google Scholar]
- Glass, G.E.; Schwartz, B.S.; Morgan, J.M.; Johnson, D.T.; Noy, P.M.; Israel, E. Environmental risk factors for Lyme disease identified with geographic information systems. Am. J. Public Health 1995, 85, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Fang, L.Q.; Jiang, J.F.; Hui, F.M.; Glass, G.E.; Yan, L.; Xu, Y.F.; Zhao, W.J.; Yang, H.; Liu, W.; et al. Predicting the risk of hantavirus infection in Beijing, People’s Republic of China. Am. J. Trop. Med. Hyg. 2009, 80, 678–683. [Google Scholar] [PubMed]
- Hu, Y. Spatial Model-Based Ecological Study on Geographical Health Events; China University of Geosciences: Beijing, China, 2012. [Google Scholar]
- Bayles, B.R.; Allan, B.F. Social-ecological factors deter mine spatial variation in human incidence of tick-borne ehrlichiosis. Epidemiol. Infect. 2014, 142, 1911–1924. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Teel, P.D.; Grant, W.E.; Schuster, G.; Pérez de León, A.A. Simulated interactions of white-tailed deer (Odocoileus virginianus), climate variation and habitat heterogeneity on southern cattle tick (Rhipicephalus (Boophilus) microplus) eradication methods in south Texas, USA. Ecol. Model. 2016, 342, 82–96. [Google Scholar] [CrossRef]
- Donaldson, T.G.; Pèrez de León, A.A.; Li, A.Y.; Castro-Arellano, I.; Wozniak, E.; Boyle, W.K.; Hargrove, R.; Wilder, H.K.; Kim, H.J.; Teel, P.D.; et al. Assessment of the geographic distribution of Ornithodoros turicata (Argasidae): Climate variation and host diversity. PLoS Negl. Trop. Dis. 2016, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Grant, W.E.; Teel, P.D.; Hamer, S.A. Tick-borne infectious agents in nature: Simulated effects of changes in host density on spatial-temporal prevalence of infected ticks. Ecol. Model. 2016, 323, 77–86. [Google Scholar] [CrossRef]






| Indices | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2006–2013 Average |
|---|---|---|---|---|---|---|---|---|---|
| Moran’s I | 0.156 | 0.113 | 0.092 | 0.089 | 0.110 | 0.106 | 0.079 | 0.115 | 0.144 |
| E(I) | −0.008 | −0.008 | −0.008 | −0.008 | −0.008 | −0.008 | −0.008 | −0.008 | −0.008 |
| Z-score | 3.205 | 2.085 | 1.791 | 1.707 | 2.169 | 2.003 | 1.485 | 2.081 | 2.655 |
| p-value | 0.001 | 0.037 | 0.073 | 0.088 | 0.030 | 0.045 | 0.137 | 0.037 | 0.008 |
| Elements | Aspect | Slope | DEM | EVI | NDVI | Prep | PF | SH | RH | Temp |
|---|---|---|---|---|---|---|---|---|---|---|
| Coefficients | 0.15 | 0.56 | 0.46 | 0.46 | 0.63 | 0.28 | 0.68 | −0.40 | 0.60 | −0.60 |
| p-value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Model Code | Factors | F-Value | p-Value | R² | Rc² |
|---|---|---|---|---|---|
| 1 | PF | 1.457E3 | 0.000 | 0.384 | 0.384 |
| 2 | PF, Slope | 916.339 | 0.000 | 0.440 | 0.439 |
| 3 | PF, Slope, Temp | 831.242 | 0.000 | 0.516 | 0.516 |
| 4 | PF, Slope, Temp, NDVI | 670.466 | 0.000 | 0.535 | 0.534 |
| 5 | PF, Slope, Temp, NDVI, RH | 609.865 | 0.000 | 0.566 | 0.566 |
| 6 | PF, Slope, Temp, NDVI, RH, EVI | 527.187 | 0.000 | 0.576 | 0.574 |
| 7 | Slope, Temp, NDVI, RH, EVI | 632.140 | 0.000 | 0.575 | 0.574 |
| 8 | Slope, Temp, NDVI, RH, EVI, Aspect | 529.974 | 0.000 | 0.577 | 0.576 |
| 9 | Slope, Temp, NDVI, RH, EVI, Aspect, DEM | 455.911 | 0.000 | 0.578 | 0.577 |
| 10 | Slope, Temp, NDVI, RH, EVI, Aspect, DEM, Prep | 401.706 | 0.000 | 0.580 | 0.578 |
| 11 | Slope, Temp, NDVI, RH, EVI, Aspect, DEM, Prep, PF | 358.195 | 0.000 | 0.580 | 0.579 |
| 12 | Slope, Temp, NDVI, RH, EVI, DEM, Prep, PF | 402.405 | 0.000 | 0.580 | 0.579 |
| Independent Variables | Coefficient | Standard Coefficient | t-Value | p-Value |
|---|---|---|---|---|
| Constant | 15.336 | 11.040 | 0.000 | |
| Slope (°) | 0.026 | 0.133 | 4.548 | 0.000 |
| DEM (km) | −0.207 | −0.064 | −2.337 | 0.020 |
| EVI | −3.978 | −0.379 | −7.723 | 0.000 |
| NDVI | 5.148 | 0.897 | 14.969 | 0.000 |
| PF (days) | −0.006 | −0.145 | −2.546 | 0.011 |
| Prep (mm) | 0.022 | 0.236 | 4.475 | 0.000 |
| Temp (°C) | −0.449 | −1.376 | −14.604 | 0.000 |
| RH (%) | −0.263 | −1.041 | −11.111 | 0.000 |
| Model ID | Involved Factors | R2 | Rc2 | AIC |
|---|---|---|---|---|
| 1 | DEM, Slope, Aspect, Prep, NDVI, RH | 0.98 | 0.99 | 7.55 |
| 2 | DEM, Slope, Aspect, Prep, EVI, RH | - | - | - |
| 3 | DEM, Slope, Aspect, Prep, NDVI, Temp | 0.87 | 0.88 | 56.85 |
| 4 | DEM, Slope, Aspect, Prep, EVI, Temp | 0.96 | 0.96 | 24.46 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, J.; Gao, M.; Fang, L.; Liu, C.; Lyu, X.; Bai, Y.; Zhao, Q.; Li, H.; Yu, H.; et al. Geographical Environment Factors and Risk Assessment of Tick-Borne Encephalitis in Hulunbuir, Northeastern China. Int. J. Environ. Res. Public Health 2017, 14, 569. https://doi.org/10.3390/ijerph14060569
Li Y, Wang J, Gao M, Fang L, Liu C, Lyu X, Bai Y, Zhao Q, Li H, Yu H, et al. Geographical Environment Factors and Risk Assessment of Tick-Borne Encephalitis in Hulunbuir, Northeastern China. International Journal of Environmental Research and Public Health. 2017; 14(6):569. https://doi.org/10.3390/ijerph14060569
Chicago/Turabian StyleLi, Yifan, Juanle Wang, Mengxu Gao, Liqun Fang, Changhua Liu, Xin Lyu, Yongqing Bai, Qiang Zhao, Hairong Li, Hongjie Yu, and et al. 2017. "Geographical Environment Factors and Risk Assessment of Tick-Borne Encephalitis in Hulunbuir, Northeastern China" International Journal of Environmental Research and Public Health 14, no. 6: 569. https://doi.org/10.3390/ijerph14060569





