Antibiotic Concentrations Decrease during Wastewater Treatment but Persist at Low Levels in Reclaimed Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Sample Size and Description
2.3. Extraction and Analysis of Antibiotic Concentrations
2.4. Statistical Analysis
3. Results and Discussion
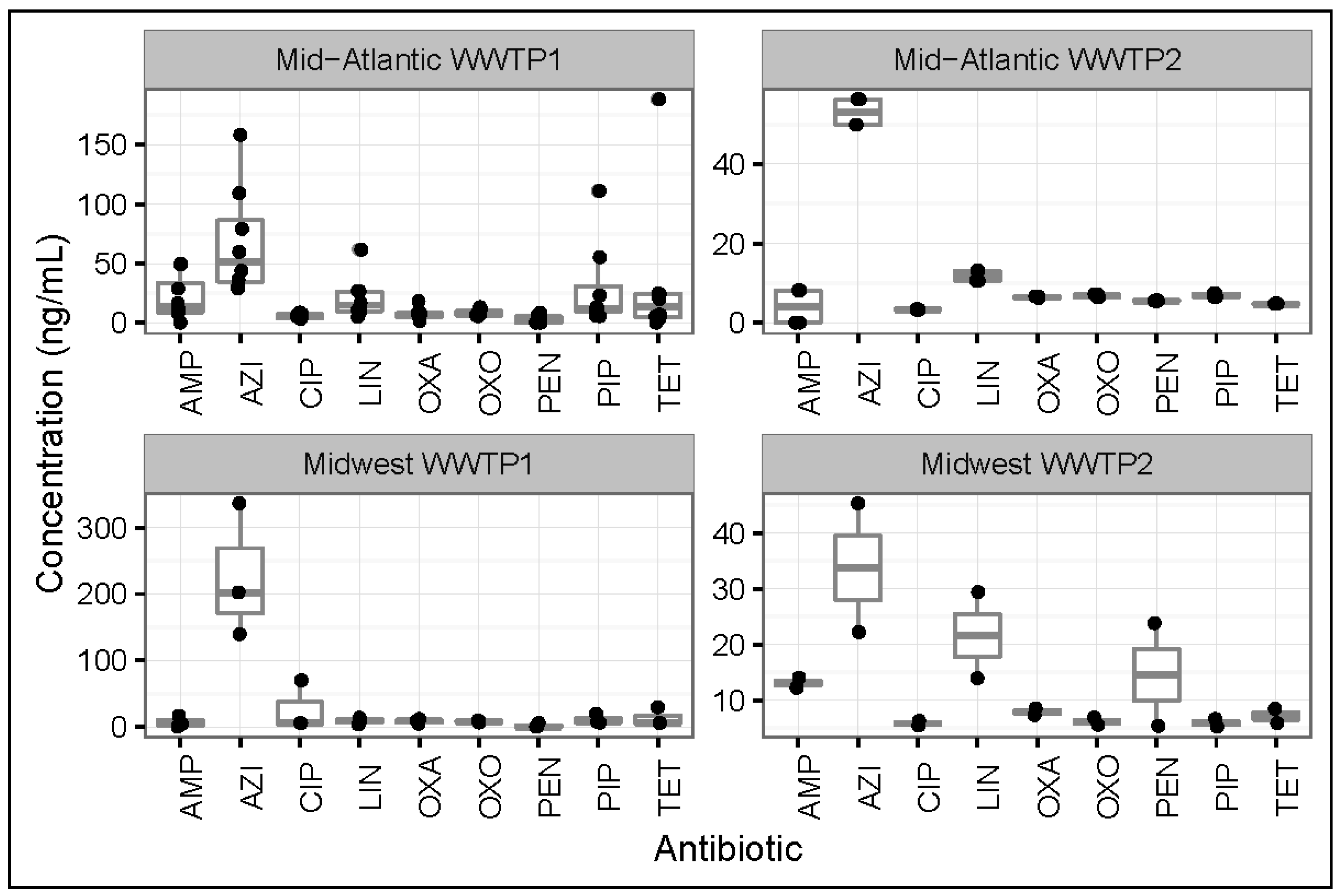
3.1. Antibiotic Concentrations in Influent Samples from All WWTPs
3.2. Antibiotic Concentrations in Effluent Samples from All WWTPs
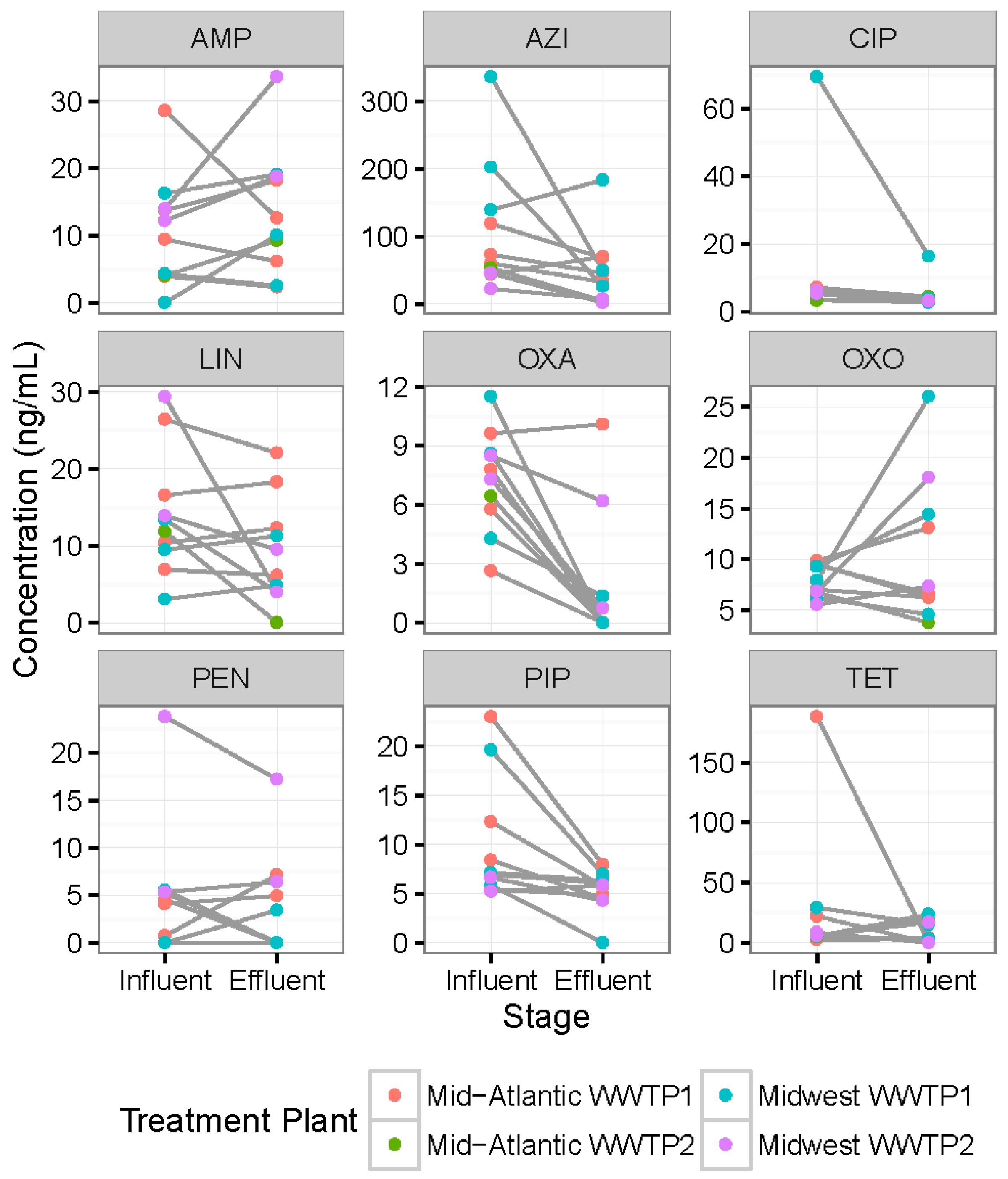
3.3. Differences in Antibiotic Concentrations between Same-Day Influent versus Effluent Samples
3.4. Regional Differences between Antibiotic Concentrations in Influents and Effluents
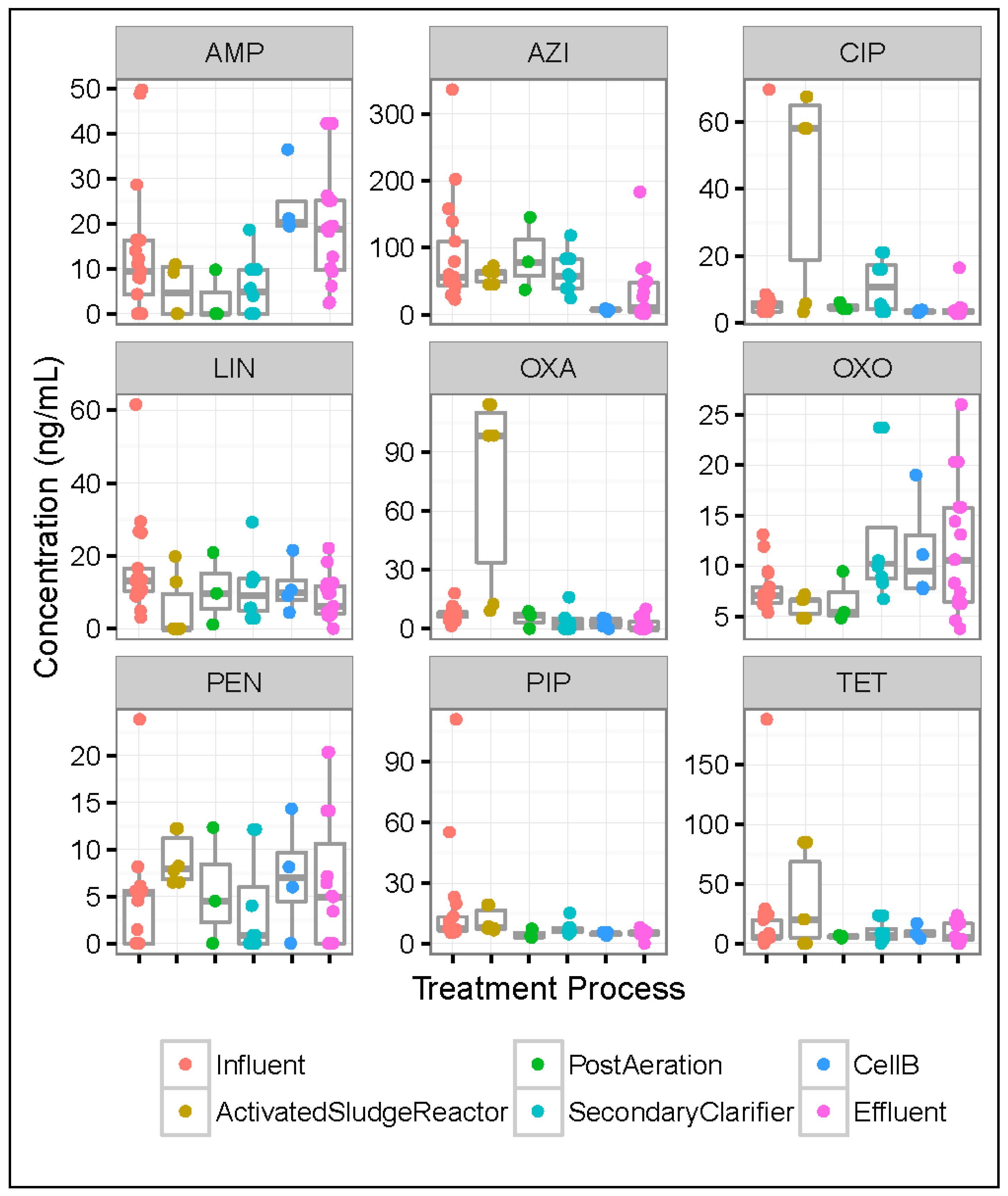
3.5. Differences in Antibiotic Concentrations across Wastewater Treatment Processes
3.6. Differences in Antibiotic Concentrations from Mid-Atlantic WWTP1 to Mid-Atlantic SI1
3.7. Limitations
3.8. Public Health Impacts and Future Research
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Disclaimer
References
- United States Environmental Protection Agency (EPA). US Environmental Protection Agency. 2012 Guidelines for Water Reuse; EPA: Washington, DC, USA, 2012.
- Sheikh, B.; Cort, R.P.; Kirkpatrick, W.R.; Jaques, R.S.; Asano, T. Monterey Wastewater Reclamation Study for Agriculture. Res. J. Water Pollut. Control Fed. 1990, 62, 216–226. [Google Scholar]
- Wu, X.; Conkle, J.L.; Ernst, F.; Gan, J. Treated wastewater irrigation: Uptake of pharmaceutical and personal care products by common vegetables under field conditions. Environ. Sci. Technol. 2014, 48, 11286–11293. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Dodgen, L.K.; Conkle, J.L.; Gan, J. Plant uptake of pharmaceutical and personal care products from recycled water and biosolids: A review. Sci. Total. Environ. 2015, 536, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Kinney, C.A.; Furlong, E.T.; Werner, S.L.; Cahill, J.D. Presence and distribution of wastewater-derived pharmaceuticals in soil irrigated with reclaimed water. Environ. Toxicol. Chem. 2006, 25, 317. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Aga, D.S. Potential, Ecological and Human Health Impacts of Antibiotics and Antibiotic-Resistant, Bacteria from Wastewater Treatment Plants. J. Toxicol. Environ. Heal. 2007, 10, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Center for Veterinary Medicine. CVM Updates—FDA Annual Summary Report on Antimicrobials Sold or Distributed in 2014 for Use in Food-Producing Animals. Available online: http://www.fda.gov/AnimalVeterinary/NewsEvents/CVMUpdates/ucm476256.htm (accessed on 8 February 2016).
- Kummerer, K. Drugs in the Environment: Emission of Drugs Diagnostic Aids and Disinfectants into Wastewater by Hospitals in Relation to Other Sources—A Review. Chemosphere 2001, 45, 957–969. [Google Scholar] [CrossRef]
- Pruden, A.; Larsson, D.G.J.; Amézquita, A.; Collignon, P.; Brandt, K.K.; Graham, D.W.; Lazorchak, J.M.; Suzuki, S.; Silley, P.; Snape, J.R.; et al. Management options for reducing the release of antibiotics and antibiotic resistance genes to the environment. Environ. Health Perspect. 2013, 121, 878–885. [Google Scholar] [CrossRef] [PubMed]
- USGS. Emerging Contaminants in the Environment. Available online: http://toxics.usgs.gov/regional/emc/ (accessed on 15 January 2016).
- Zhang, T.; Li, B. Occurrence Transformation, and Fate of Antibiotics in Municipal Wastewater Treatment Plants. Crit. Rev. Environ. Sci. Technol. 2011. Available online: http://www-tandfonline-com.proxy-um.researchport.umd.edu/doi/full/10.1080/10643380903392692 (accessed on 2 February 2016). [CrossRef]
- Negreanu, Y.; Pasternak, Z.; Jurkevitch, E.; Cytryn, E. Impact of treated wastewater irrigation on antibiotic resistance in agricultural soils. Environ. Sci. Technol. 2012, 46, 4800–4808. [Google Scholar] [CrossRef] [PubMed]
- Fahrenfeld, N.; Ma, Y.; O’Brien, M.; Pruden, A. Reclaimed water as a reservoir of antibiotic resistance genes: Distribution system and irrigation implications. Front. Microbiol. 2013, 4, 130. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.E.R.; Micallef, S.A.; Gibbs, S.G.; Davis, J.A.; He, X.; George, A.; Kleinfelter, L.M.; Schreiber, N.A.; Mukherjee, S.; Sapkota, A.; et al. Methicillin-resistant, Staphylococcus aureus (MRSA) detected at four U.S. wastewater treatment plants. Environ. Health Perspect. 2012, 120, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.E.R.; Micallef, S.A.; Gibbs, S.G.; George, A..; Claye, E..; Sapkota, A.; Joseph, S.W.; Sapkota, A.R. Detection of vancomycin-resistant enterococci (VRE) at four U.S. wastewater treatment plants that provide effluent for reuse. Sci. Total. Environ. 2014, 466–467, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Carey, S.A.; Goldstein, R.E.R.; Gibbs, S.G.; Claye, E.; He, X.; Sapkota, A.R. Occurrence of vancomycin-resistant and -susceptible, Enterococcus spp. in reclaimed water used for spray irrigation. Environ. Res. 2016, 147, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Ternes, T.A.; Bonerz, M.; Herrmann, N.; Teiser, B.; Andersen, H.R. Irrigation of treated wastewater in Braunschweig, Germany: An option to remove pharmaceuticals and musk fragrances. Chemosphere 2007, 66, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Wong, C.K.C.; Chu, L.M. Distribution of antibiotics in wastewater-irrigated soils and their accumulation in vegetable crops in the Pearl River Delta southern China. J. Agric. Food Chem. 2014, 62, 11062–11069. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-H.; Qiao, M.; Lv, Z.-E.; Guo, G.X.; Jia, Y.; Su, Y.H.; Zhu, Y.G. Impact of reclaimed water irrigation on antibiotic resistance in public parks Beijing China. Environ. Pollut. 2014, 184, 247–253. [Google Scholar] [CrossRef] [PubMed]
- U.S.National Library of Medicine. National Institutes of Health. Pharmaceutical Statistics. 2015. Available online: https://www.nlm.nih.gov/services/Subject_Guides/healthstatistics/pharmaceuticalstatistics/ (accessed on 19 April 2016).
- Sapkota, A.; Heidler, J.; Halden, R.U. Detection of triclocarban and two co-contaminating chlorocarbanilides in US aquatic environments using isotope dilution liquid chromatography tandem mass spectrometry. Environ. Res. 2007, 103, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Helsel, D.R. Statistics for Censored Environmental Data Using Minitab and R, 2nd ed.; Scott, M., Barnett, V., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 978-0-470-47988-9. [Google Scholar]
- Loganathan, B.; Phillips, M.; Mowery, H.; Jones-Lepp, T.L. Contamination profiles and mass loadings of macrolide antibiotics and illicit drugs from a small urban wastewater treatment plant. Chemosphere 2009, 75, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Get Smart: Know When Antibiotics Work. 2015. Available online: http://www.cdc.gov/getsmart/community/programs-measurement/measuring-antibiotic-prescribing.html (accessed on 21 April 2016).
- Hicks, L.A.; Bartoces, M.G.; Roberts, R.M.; Suda, K.J.; Hunkler, R.J.; Taylor, T.H., Jr.; Schrag, S.J. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin. Infect. Dis. 2015, 60, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Walters, E.; McClellan, K.; Halden, R.U. Occurrence and loss over three years of 72 pharmaceuticals and personal care products from biosolids-soil mixtures in outdoor mesocosms. Water Res. 2010, 44, 6011–6020. [Google Scholar] [CrossRef] [PubMed]
- Batt, A.L.; Kim, S.; Aga, D.S. Comparison of the occurrence of antibiotics in four full-scale wastewater treatment plants with varying designs and operations. Chemosphere 2007, 68, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Jelic, A.; Gros, M.; Petrovic, M.; Ginebreda, A.; Barcelo, D. Occurrence and Elimination of Pharmaceuticals During Conventional Wastewater Treatment. In Emerging and Priority Pollutants in Rivers: Bringing Science into River Management Plans; Guasch, H., Ginebreda, A., Geiszinger, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–23. ISBN 978-3-642-25721-6. [Google Scholar]
- Dorival-García, N.; Zafra-Gómez, A.; Navalón, A.; González-López, J.; Hontoria, E.; Vílchez, J.L. Removal and degradation characteristics of quinolone antibiotics in laboratory-scale activated sludge reactors under aerobic, nitrifying and anoxic conditions. J. Environ. Manag. 2013, 120, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Batt, A.L.; Kim, S.; Aga, D.S. Enhanced biodegradation of iopromide and trimethoprim in nitrifying activated sludge. Environ. Sci. Technol. 2006, 40, 7367–7373. [Google Scholar] [CrossRef] [PubMed]
- Popple, T.; Williams, J.B.; May, E.; Mills, G.A.; Oliver, R. Evaluation of a sequencing batch reactor sewage treatment rig for investigating the fate of radioactively labelled pharmaceuticals: Case study of propranolol. Water Res. 2016, 88, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Jones-Lepp, T.L.; Stevens, R. Pharmaceuticals and personal care products in biosolids/sewage sludge: The interface between analytical chemistry and regulation. Anal. Bioanal. Chem. 2007, 387, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Golet, E.M.; Strehler, A.; Alder, A.C.; Giger, W. Determination of Fluoroquinolone Antibacterial Agents in Sewage Sludge and Sludge-Treated Soil Using Accelerated Solvent Extraction Followed by Solid-Phase Extraction. Anal. Chem. 2002, 74, 5455–5462. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Eichhorn, P.; Pérez, S.; Wang, Y.; Barceló, D. Photodegradation of azithromycin in various aqueous systems under simulated and natural solar radiation: Kinetics and identification of photoproducts. Chemosphere 2011, 83, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K.; Al-Ahmad, A.; Mersch-Sundermann, V. Biodegradability of some antibiotics, elimination of the genotoxicity and affection of wastewater bacteria in a simple test. Chemosphere 2000, 40, 701–710. [Google Scholar] [CrossRef]
- Malchi, T.; Maor, Y.; Chefetz, B. Comments on “Human health risk assessment of pharmaceuticals and personal care products in plant tissue due to biosolids and manure amendments, and wastewater irrigation”. Environ. Int. 2015, 82, 110–112. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulkarni, P.; Olson, N.D.; Raspanti, G.A.; Rosenberg Goldstein, R.E.; Gibbs, S.G.; Sapkota, A.; Sapkota, A.R. Antibiotic Concentrations Decrease during Wastewater Treatment but Persist at Low Levels in Reclaimed Water. Int. J. Environ. Res. Public Health 2017, 14, 668. https://doi.org/10.3390/ijerph14060668
Kulkarni P, Olson ND, Raspanti GA, Rosenberg Goldstein RE, Gibbs SG, Sapkota A, Sapkota AR. Antibiotic Concentrations Decrease during Wastewater Treatment but Persist at Low Levels in Reclaimed Water. International Journal of Environmental Research and Public Health. 2017; 14(6):668. https://doi.org/10.3390/ijerph14060668
Chicago/Turabian StyleKulkarni, Prachi, Nathan D. Olson, Greg A. Raspanti, Rachel E. Rosenberg Goldstein, Shawn G. Gibbs, Amir Sapkota, and Amy R. Sapkota. 2017. "Antibiotic Concentrations Decrease during Wastewater Treatment but Persist at Low Levels in Reclaimed Water" International Journal of Environmental Research and Public Health 14, no. 6: 668. https://doi.org/10.3390/ijerph14060668