Evaluation of Trace Element and Metal Accumulation and Edibility Risk Associated with Consumption of Labeo umbratus from the Vaal Dam, South Africa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.1.1. 2011 Samples
2.1.2. 2016 Samples
2.2. Metal and Trace Element Analysis
2.3. Statistical Analysis
2.4. Health Risk Assessment
3. Results
3.1. Sediment and Fish Tissue Trace Element Concentrations
3.2. Health Risk Assessment
4. Discussion
4.1. Sediment and Fish Element Levels
4.2. Health Risk Assessment
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Paul-Pont, I.; Gonzalez, P.; Baudrimont, M.; Jude, F.; Raymond, N.; Bourrasseau, L.; Le Goïc, N.; Haynes, F.; Legeay, A.; Paillard, C.; et al. Interactive effects of metal contamination and pathogenic organisms on the marine bivalve Cerastoderma edule. Mar. Pollut. Bull. 2010, 60, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Boudou, A.; Ribeyre, F. Aquatic ecotoxicology: From the ecosystem to the cellular and molecular levels. Environ. Health Perspect. 1997, 105, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Wepener, V.; van Vuren, J.H.J.; Chatiza, F.P.; Mbizi, Z.; Slabbert, L.; Masola, B. Active biomonitoring in freshwater environments: Early warning signals from biomarkers in assessing biological effects of diffuse sources of pollutants. Phys. Chem. Earth 2005, 30, 751–761. [Google Scholar] [CrossRef]
- Ricciardi, F.; Binelli, A.; Provini, A. Use of two biomarkers (CYP450 and acetylcholinesterase) in zebra mussel for the biomonitoring of Lake Maggiore (northern Italy). Ecotoxicol. Environ. Saf. 2006, 63, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Viarengo, A.; Lowe, D.; Bolognesi, C.; Fabbri, E.; Koehler, A. The use of biomarkers in biomonitoring: A 2-tier approach assessing the level of pollutant-induced stress syndrome in sentinel organisms. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 146, 281–300. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zheng, B.; Liu, L. Biomonitoring and bioindicators used for river ecosystems: Definitions, approaches and trends. Procedia Environ. Sci. 2010, 2, 1510–1524. [Google Scholar] [CrossRef]
- Sobus, J.R.; Tan, Y.-M.; Pleil, J.D.; Sheldon, L.S. A biomonitoring framework to support exposure and risk assessments. Sci. Total Environ. 2011, 409, 4875–4884. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, J.; Fu, J.; Shi, J.; Jiang, G. Biomonitoring: An appealing tool for assessment of metal pollution in the aquatic ecosystem. Anal. Chim. Acta 2008, 606, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Kleynhans, C.J.; Louw, M.D. EcoClassification and EcoStatus Determination. In River EcoClassification: Manual for EcoStatus Determination, (version 2); Water & Forestry: Pretoria, South Africa, 2007. [Google Scholar]
- Gilbert, B.M.; Avenant-Oldewage, A. Arsenic, chromium, copper, iron, manganese, lead, selenium and zinc in the tissues of the largemouth yellowfish, Labeobarbus kimberleyensis (Gilchrist and Thompson, 1913), from the Vaal Dam, South Africa. Water SA 2014, 40, 739–748. [Google Scholar] [CrossRef]
- Addo-Bediako, A.; Marr, S.M.; Jooste, A.; Luus-powell, W.J. Human health risk assessment for silver catfish Schilbe intermedius Rüppell, 1832, from two impoundments in the Olifants River, Limpopo, South Africa. Water SA 2014, 40, 607–614. [Google Scholar] [CrossRef]
- Jooste, A.; Marr, S.M.; Addo-Bediako, A.; Luus-Powell, W.J. Metal bioaccumulation in the fish of the Olifants River, Limpopo province, South Africa, and the associated human health risk: A case study of rednose labeo Labeo rosae from two impoundments. Afr. J. Aquat. Sci. 2014, 39, 271–277. [Google Scholar] [CrossRef]
- Lynch, L.P.; Jirsa, F.; Avenant-Oldewage, A. Trace element accumulation and human risk assessment of Labeo capensis (Smith, 1841) from the Vaal Dam reservoir, South Africa. Water SA 2016, 42, 328–336. [Google Scholar] [CrossRef]
- Department of Water Affairs and Forestry Upper Vaal Water Management Area. Internal Strategic Perspective; Department of Water Affairs and Forestry Upper Vaal Water Management Area: Pretoria, South Africa, 2004.
- Tempelhoff, J.W.N. Civil society and sanitation hydropolitics: A case study of South Africa’s Vaal River Barrage. Phys. Chem. Earth 2009, 34, 164–175. [Google Scholar] [CrossRef]
- Grobler, D.C.; Toerien, D.F.; Rossouw, J.N. A review of sediment/water quality interaction with particular reference to the Vaal River system. Water SA 1987, 13, 15–22. [Google Scholar]
- Gouws, K.; Coetzee, P.P. Determination and partitioning of heavy metals in sediments of the Vaal Dam by sequential extraction. Water SA 1997, 23, 217–226. [Google Scholar]
- Gbem, T.T.; Balogun, J.K.; Lawal, F.A.; Annune, P.A. Trace metal accumulation in Clarias gariepinus (Teugels) exposed to sublethal levels of tannery effluent. Sci. Total Environ. 2001, 271, 1–9. [Google Scholar] [CrossRef]
- Brönmark, C.; Hansson, L.A. Environmental issues in lakes and ponds: Current state and perspectives. Environ. Conserv. 2002, 29, 290–307. [Google Scholar] [CrossRef]
- Retief, N.R.; Avenant-Oldewage, A.; du Preez, H. The use of cestode parasites from the largemouth yellowfish, Labeobarbus kimberleyensis (Gilchrist and Thompson, 1913) in the Vaal Dam, South Africa as indicators of heavy metal bioaccumulation. Phys. Chem. Earth 2006, 31, 840–847. [Google Scholar] [CrossRef]
- Crafford, D.; Avenant-Oldewage, A. Bioaccumulation of non-essential trace metals in tissues and organs of Clarias gariepinus (sharptooth catfish) from the Vaal River system—Strontium, aluminium, lead and nickel. Water SA 2010, 36, 621–640. [Google Scholar] [CrossRef]
- Crafford, D.; Avenant-Oldewage, A. Uptake of selected metals in tissues and organs of Clarias gariepinus (sharptooth catfish) from the Vaal River System—Chromium, copper, iron, manganese and zinc. Water SA 2011, 37, 181–200. [Google Scholar] [CrossRef]
- Pheiffer, W.; Pieters, R.; van Dyk, J.C.; Smit, N.J. Metal contamination of sediments and fish from the Vaal River, South Africa. Afr. J. Aquat. Sci. 2014, 39, 117–121. [Google Scholar] [CrossRef]
- Wepener, V.; van Dyk, C.; Bervoets, L.; O’Brien, G.; Covaci, A.; Cloete, Y. An assessment of the influence of multiple stressors on the Vaal River, South Africa. Phys. Chem. Earth 2011, 36, 949–962. [Google Scholar] [CrossRef]
- Skelton, P. A Complete Guide to the Freshwater Fishes of Southern Africa; Struiker: Cape Town, South Africa, 2001. [Google Scholar]
- Plessl, C.; Jandrisits, P.; Krachler, R.; Keppler, B.K.; Jirsa, F. Heavy metals in the mallard Anas platyrhynchos from eastern Austria. Sci. Total Environ. 2016, 580, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Squadrone, S.; Benedetto, A.; Brizio, P.; Prearo, M.; Abete, M.C. Mercury and selenium in European catfish (Silurus glanis) from Northern Italian Rivers: Can molar ratio be a predictive factor for mercury toxicity in a top predator? Chemosphere 2015, 119, 24–30. [Google Scholar] [CrossRef] [PubMed]
- US EPA (United States Environmental Protection Agency). Mid-Atlantic Risk Assessment. Available online: http://www.epa.gov/reg3hwmd/risk/human/rb-concentration_table/usersguide.htm (accessed on 15 June 2016).
- Heath, R.; Du Preez, H.; Genthe, B.; Avenant-Oldewage, A. Freshwater Fish and Human Health Reference Guide; Water Research Commission: Pretoria, South Africa, 2004. [Google Scholar]
- Gilbert, B.M.; Avenant-Oldewage, A. Effects of altered water quality and trace elements on the infection variables of Paradiplozoon ichthyoxanthon (Monogenea: Diplozoidae) from two sites in the Vaal River system, South Africa. Acta Parasitol. 2016, 61, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Musa, R.; Gerber, R.; Greenfield, R. A multivariate analysis of metal concentrations in two fish species of the Nyl River System, Limpopo Province, South Africa. Bull. Environ. Contam. Toxicol. 2017, 98, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Squadrone, S.; Prearo, M.; Brizio, P.; Gavinelli, S.; Pellegrino, M.; Scanzio, T.; Guarise, S.; Benedetto, A.; Abete, M.C. Heavy metals distribution in muscle, liver, kidney and gill of European catfish (Silurus glanis) from Italian Rivers. Chemosphere 2013, 90, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Otero-Muras, I.; Franco-Uría, A.; Alonso, A.A.; Balsa-Canto, E. Dynamic multi-compartmental modelling of metal bioaccumulation in fish: Identifiability implications. Environ. Model. Softw. 2010, 25, 344–353. [Google Scholar] [CrossRef]
- Ribecco, C.; Hardiman, G.; Šášik, R.; Vittori, S.; Carnevali, O. Teleost fish (Solea solea): A novel model for ecotoxicological assay of contaminated sediments. Aquat. Toxicol. 2012, 109, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Retief, N.R.; Avenant-Oldewage, A.; Du Preez, H.H. Seasonal study on Bothriocephalus as indicator of metal pollution in yellowfish, South Africa. Water SA 2009, 35, 315–322. [Google Scholar] [CrossRef]
- Coetzee, L.; Du Preez, H.H.; van Vuren, J.H.J. Metal concentrations in Clarias gariepinus and Labeo umbratus from the Olifants and Klein Olifants River, Mpumalanga, South Africa: Zinc, copper, manganese, lead, chromium, nickel, aluminium and iron. Water SA 2002, 28, 433–448. [Google Scholar] [CrossRef]
- Wagner, A.; Boman, J. Biomonitoring of trace elements in muscle and liver tissue of freshwater fish. Spectrochim. Acta 2003, 58, 2215–2226. [Google Scholar] [CrossRef]
- Vinodhini, R.; Narayanan, M. Bioaccumulation of heavy metals in organs of fresh water fish Cyprinus carpio (Common carp). Int. J. Environ. Sci. Technol. 2008, 5, 179–182. [Google Scholar] [CrossRef]
- Nussey, G.; Van Vuren, J.H.J.; Du Preez, H.H. Bioaccumulation of chromium, manganese, nickel and lead in the tissues of the moggel, Labeo umbratus (Cyprinidae), from Witbank Dam, Mpumalanga. Water SA 2000, 26, 269–284. [Google Scholar]
- Farombi, E.O.; Adelowo, O.A.; Ajimoko, R.R. Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in some selected fishes in Lagos, Nigeria. Int. J. Environ. Res. Public Health 2007, 4, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kiron, V.; Satoh, S. Trace minerals in fish nutrition. Aquaculture 1997, 151, 185–207. [Google Scholar] [CrossRef]
- Seymore, T.; Du Preez, H.H.; van Vuren, J.H.J. Manganese, lead and strontium bioaccumulation in the tissues of the yellowfish, Barbus marequensis from the lower Olifants River, Eastern Transvaal. Water SA 1995, 21, 159–172. [Google Scholar]
- Visnjic-Jeftic, Z.; Jaric, I.; Jovanovic, L.; Skoric, S.; Smederevac-Lalic, M.; Nikcevic, M.; Lenhardt, M. Heavy metal and trace element accumulation in muscle, liver and gills of the Pontic shad (Alosa immaculata Bennet 1835) from the Danube River (Serbia). Microchem. J. 2010, 95, 341–344. [Google Scholar] [CrossRef]
- Otachi, E.O.; Körner, W.; Avenant-Oldewage, A.; Fellner-Frank, C.; Jirsa, F. Trace elements in sediments, blue spotted tilapia Oreochromis leucostictus (Trewavas, 1933) and its parasite Contracaecum multipapillatum from Lake Naivasha, Kenya, including a comprehensive health risk analysis. Environ. Sci. Pollut. Res. 2014, 21, 7339–7349. [Google Scholar] [CrossRef] [PubMed]
- Fargar, A.M.; Stransbury, M.A.; Hogstrand, C.; Maccon-Nell, E.; Bergman, H. The physiological impairment of free-ranging brown trout exposed to metals in the Clark Fork River, Montana. Can. J. Fish. Aquat. Sci. 1995, 25, 2038–2050. [Google Scholar]
- Palaniappan, P.R.; Karthikeyan, S. Bioaccumulation and depuration of chromium in the selected organs and whole body tissues of freshwater fish Cirrhinus mrigala individually and in binary solutions with nickel. J. Environ. Sci. 2009, 21, 229–236. [Google Scholar] [CrossRef]
- Takatsu, A.; Kuroiwa, T.; Uchiumi, A. Arsenic accumulation in organs of the fresh water fish Tribolodon hakonensis. J. Trace Elem. Med. Biol. 1999, 13, 176–179. [Google Scholar] [CrossRef]
- Jezierska, B.; Witeska, M. The metal uptake and accumulation in fish living in polluted waters. Soil Water Pollut. Monit. Prot. Remediat. 2006, 3, 107–114. [Google Scholar]
- Martiniaková, M.; Omelka, R.; Stawarz, R.; Formicki, G. Accumulation of lead, cadmium, nickel, iron, copper, and zinc in bones of small mammals from polluted areas in Slovakia. Pol. J. Environ. Stud. 2012, 21, 153–158. [Google Scholar]
- Boening, D.W. Ecological effects, transport, and fate of mercury: A general review. Chemosphere 2000, 40, 1335–1351. [Google Scholar] [CrossRef]
- Ouédraogo, O.; Amyot, M. Mercury, arsenic and selenium concentrations in water and fish from sub-Saharan semi-arid freshwater reservoirs (Burkina Faso). Sci. Total Environ. 2013, 444, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Dušek, L.; Svobodová, Z.; Janoušková, D.; Vykusová, B.; Jarkovský, J.; Šmíd, R.; Pavliš, P. Bioaccumulation of mercury in muscle tissue of fish in the Elbe River (Czech Republic): Multispecies monitoring study 1991–1996. Ecotoxicol. Environ. Saf. 2005, 61, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Lebepe, J.; Marr, S.; Luus-Powell, W. Metal contamination and human health risk associated with the consumption of Labeo rosae from the Olifants River system, South Africa. Afr. J. Aquat. Sci. 2016, 41, 161–170. [Google Scholar] [CrossRef]
- Walters, C.R.; Somerset, V.S.; Leaner, J.J.; Nel, J.M. A review of mercury pollution in South Africa: Current status. J. Environ. Sci. Health 2011, 46, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- US EPA (United States Environmental Protection Agency). Mercury Update: Impact on Fish Advisories Mercury; EPA Fact Sheet EPA- 823-F-01-011 2001; USEPA: Washington, DC, USA, 2001; pp. 1–6.
- Chvojka, R.; Williams, R.J.; Fredrickson, S. Methyl mercury, total mercury, and selenium in Snapper from two areas of the New South Wales Coast, Australia. Mar. Pollut. Bull. 1990, 21, 570–573. [Google Scholar] [CrossRef]
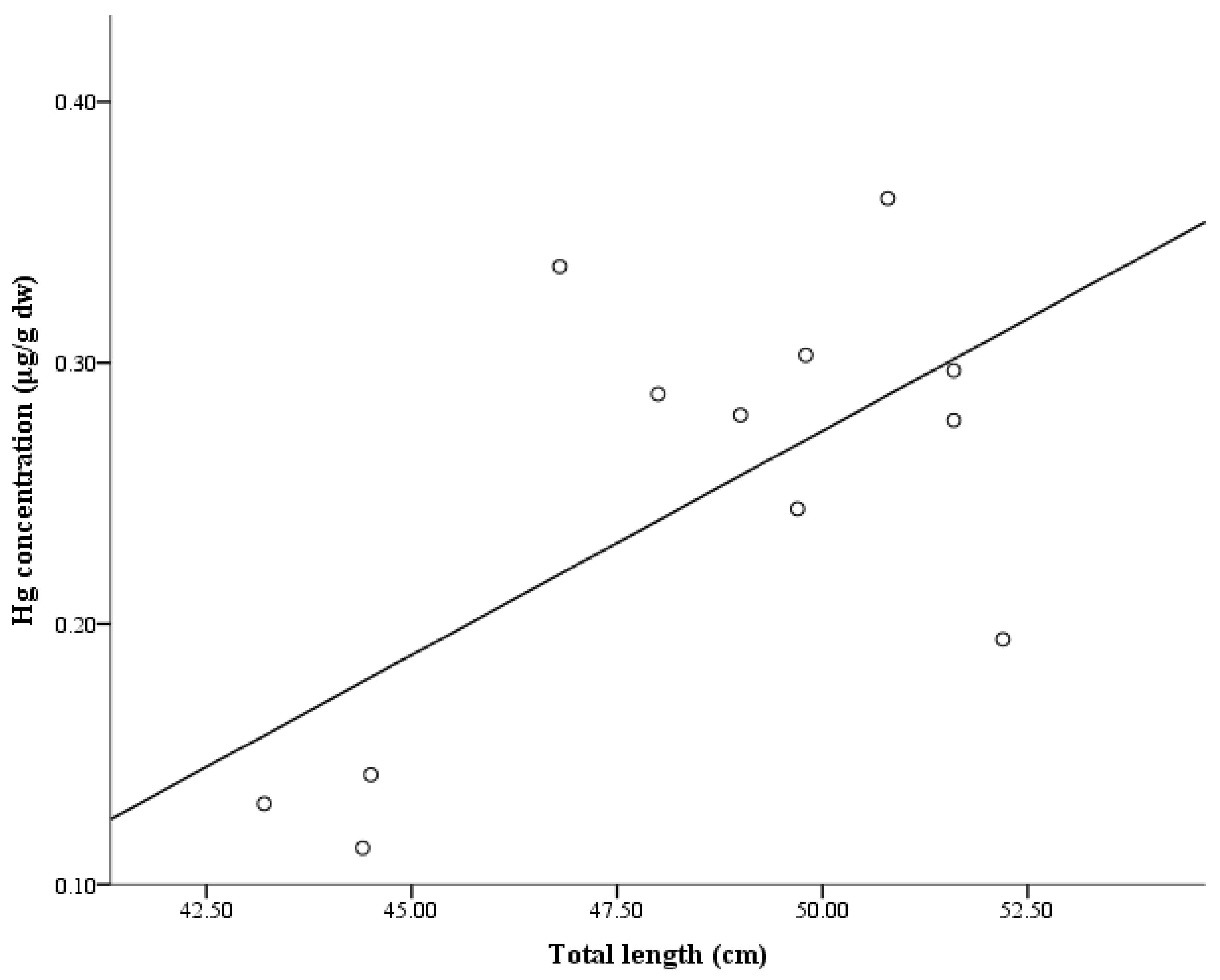
- Sackett, D.K.; Gregory Cope, W.; Rice, J.A.; Aday, D.D. The influence of fish length on tissue mercury dynamics: Implications for natural resource management and human health risk. Int. J. Environ. Res. Public Health 2013, 10, 638–659. [Google Scholar] [CrossRef] [PubMed]
- Cizdziel, J.V.; Hinners, T.A.; Pollard, J.E.; Heithmar, E.M.; Cross, C.L. Mercury concentrations in fish from Lake Mead, USA, related to fish size, condition, trophic level, location, and consumption risk. Arch. Environ. Contam. Toxicol. 2002, 43, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Simonin, H.A.; Loukmas, J.J.; Skinner, L.C.; Roy, K.M. Lake variability: Key factors controlling mercury concentrations in New York State fish. Environ. Pollut. 2008, 154, 107–115. [Google Scholar] [CrossRef] [PubMed]
| Average Weight (kg) | Average Time (Days) | Exposure Frequency (Days/Year) | Exposure Duration (Years) | Fish Consumption Weight (kg/Day) |
|---|---|---|---|---|
| BWa | AT | EFr | EDr | IRFa |
| 70 | 365 | 350 | 30 | 0.054 |
| Element | Sediment 2011 n = 3 (mg/kg) | Sediment 2016 n = 10 (mg/kg) | Liver 2011 n = 28 (mg/kg) | Liver 2016 n = 12 (mg/kg) | Musc le 2011 n = 28 (mg/kg) | Musc le 2016 n = 12 (mg/kg) | Spinal Cord W. Vertebrae 2011 n = 28 (mg/kg) | Gills with Arc hes 2011 n = 28 (mg/kg) | Kidney 2011 n = 28 (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|
| As | 2.59 (1.28) | <4.75 | 3.25 (1.11) | 6.33 (1.84) | 0.927 (0.703) | <4.6 | 3.09 (2.57) | 3.08 (0.979) | 13.2 (10.7) |
| Cd | 0.014 (0.006) | 0.010 (0.010) | <0.11 | 0.460 (0.17) | <0.11 | <0.006 | <0.11 | <0.11 | <0.11 |
| Cr | 133 (182) | 28.1 (14.4) | <0.01 | 0.130 (0.080) | 1.67 (5.17) | 0.049 (0.029) | 2.08 (3.46) | 2.79 (4.84) | 0.412 (1.34) |
| Cu | 11.9 (4.81) | 6.13 (2.63) | 844 (609) | 1302 (823) | 0.916 (0.592) | 1.71 (0.703) | 0.547 (0.281) | 1.43 (0.836) | 7.07 (5.06) |
| Hg | ND | 0.01 (0.003) | ND | 0.170 (0.050) | ND | 0.247 (0.083) | ND | ND | ND |
| Fe | 3586 (2070) | 4832 (2928) | 225 (232) | 284 (103) | 23.4 (72.3) | 19.2 (30.3) | 28.9 (44.4) | 93.1 (80.4) | 114 (138) |
| Mn | 62.4 (32.1) | 43.7 (28.1) | 3.73 (3.10) | 10.1 (7.60) | 0.921 (0.378) | <4.6 | 11.4 (7.45) | 14.2 (9.47) | 1.33 (1.22) |
| Ni | 12.1 (5.97) | 7.28 (4.59) | 0.395 (0.323) | 0.14 (0.09) | 0.360 (0.550) | <0.042 | 0.161 (0.146) | 0.553 (0.950) | 1.01 (1.11) |
| Se | 0.346 (0.205) | <4.75 | 19.3 (18.2) | 22.7 (6.60) | 0.354 (0.285) | <4.60 | 0.208 (0.644) | 0.563 (0.238) | 0.725 (0.582) |
| Sr | ND | 4.68 (1.51) | 6.75 (2.57) | <4.60 | 17.7 (6.27) | 12.5 (6.9) | 256 (188) | 293 (167) | 6.32 (9.41) |
| Zn | 21.0 (2.07) | 13.2 (9.0) | 257 (211) | 222 (56) | 32.1 (61.5) | 16.9 (1.32) | 30.4 (22.0) | 71.9 (49.1) | 45.6 (40.3) |
| Survey | Variable | As | Cd | Cr | Cu | Fe | Hg | Mn | Ni | Se | Sr | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RfDO (mg/kg/day) | 0.0003 | 0.001 | 0.003 | 0.04 | 0.7 | 0.0001 | 0.14 | 0.02 | 0.005 | 0.6 | 0.3 | |
| 2012 | Element concentration (mg/kg; ww) | 0.185 | <0.11 | 0.334 | 0.183 | 4.68 | - | 0.184 | 0.0720 | 0.0708 | 3.54 | 6.42 |
| THQ | 1.43 | - | 0.258 | 0.0106 | 0.0155 | - | 0.00305 | 0.00833 | 0.0328 | 0.0137 | 0.0495 | |
| IRFb (kg/day) | - | - | 0.0210 | 0.509 | 0.349 | - | 1.77 | 0.648 | 0.165 | 0.395 | 0.109 | |
| 2016 | Element concentration (mg/kg; ww) | <4.60 | <0.006 | 0.010 | 0.342 | 3.84 | 0.0494 | <4.60 | <0.042 | <4.60 | 2.50 | 3.38 |
| THQ | - | - | 0.00755 | 0.0198 | 0.0127 | 1.14 | - | - | - | 0.00964 | 0.0261 | |
| IRFb (kg/day) | - | - | 0.716 | 0.273 | 0.425 | - | - | - | - | 0.560 | 0.207 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilbert, B.M.; Hussain, E.; Jirsa, F.; Avenant-Oldewage, A. Evaluation of Trace Element and Metal Accumulation and Edibility Risk Associated with Consumption of Labeo umbratus from the Vaal Dam, South Africa. Int. J. Environ. Res. Public Health 2017, 14, 678. https://doi.org/10.3390/ijerph14070678
Gilbert BM, Hussain E, Jirsa F, Avenant-Oldewage A. Evaluation of Trace Element and Metal Accumulation and Edibility Risk Associated with Consumption of Labeo umbratus from the Vaal Dam, South Africa. International Journal of Environmental Research and Public Health. 2017; 14(7):678. https://doi.org/10.3390/ijerph14070678
Chicago/Turabian StyleGilbert, Beric M., Ebrahim Hussain, Franz Jirsa, and Annemariè Avenant-Oldewage. 2017. "Evaluation of Trace Element and Metal Accumulation and Edibility Risk Associated with Consumption of Labeo umbratus from the Vaal Dam, South Africa" International Journal of Environmental Research and Public Health 14, no. 7: 678. https://doi.org/10.3390/ijerph14070678






