Assessment of Vulnerability to Coccidioidomycosis in Arizona and California
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Descriptive Analysis
2.3. Coccidioidomycosis Vulnerability Indices
2.3.1. Methods for Creating Coccidioidomycosis Vulnerability Indices
2.3.2. Validating Coccidioidomycosis Vulnerability Indices
2.4. Climate Variability & Coccidioidomycosis Vulnerability
3. Results
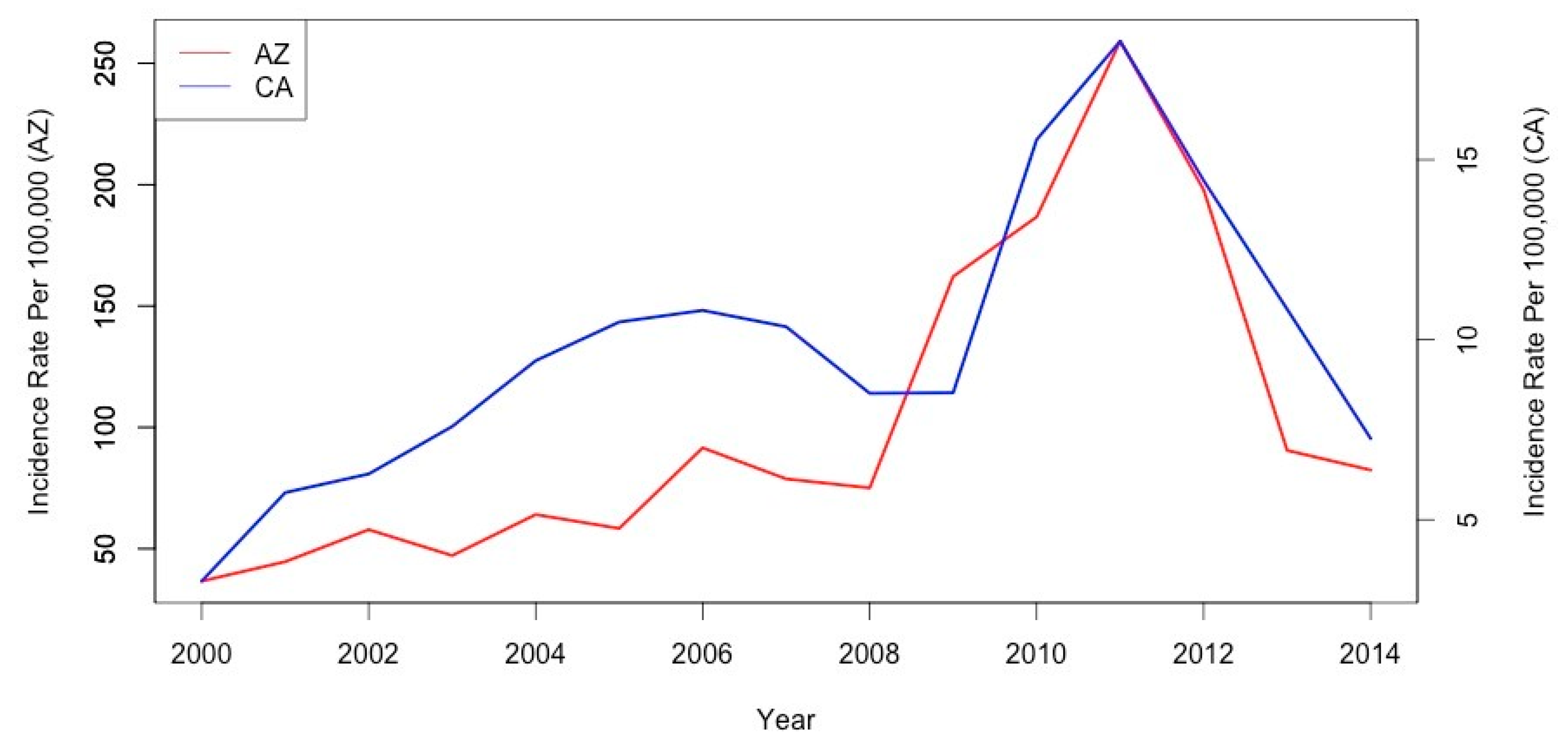
3.1. Descriptive Analysis
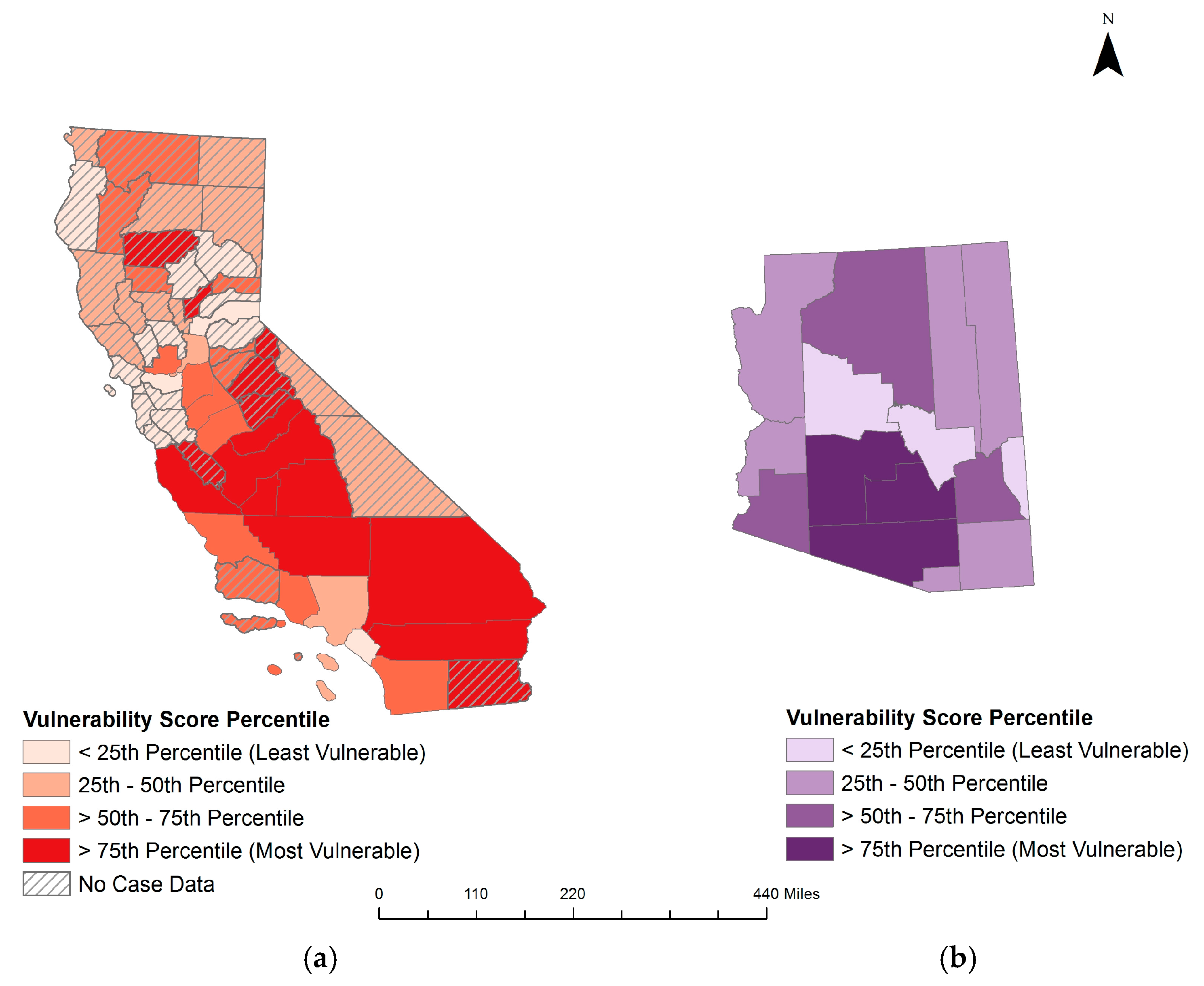
3.2. Coccidioidomycosis Vulnerability Indices
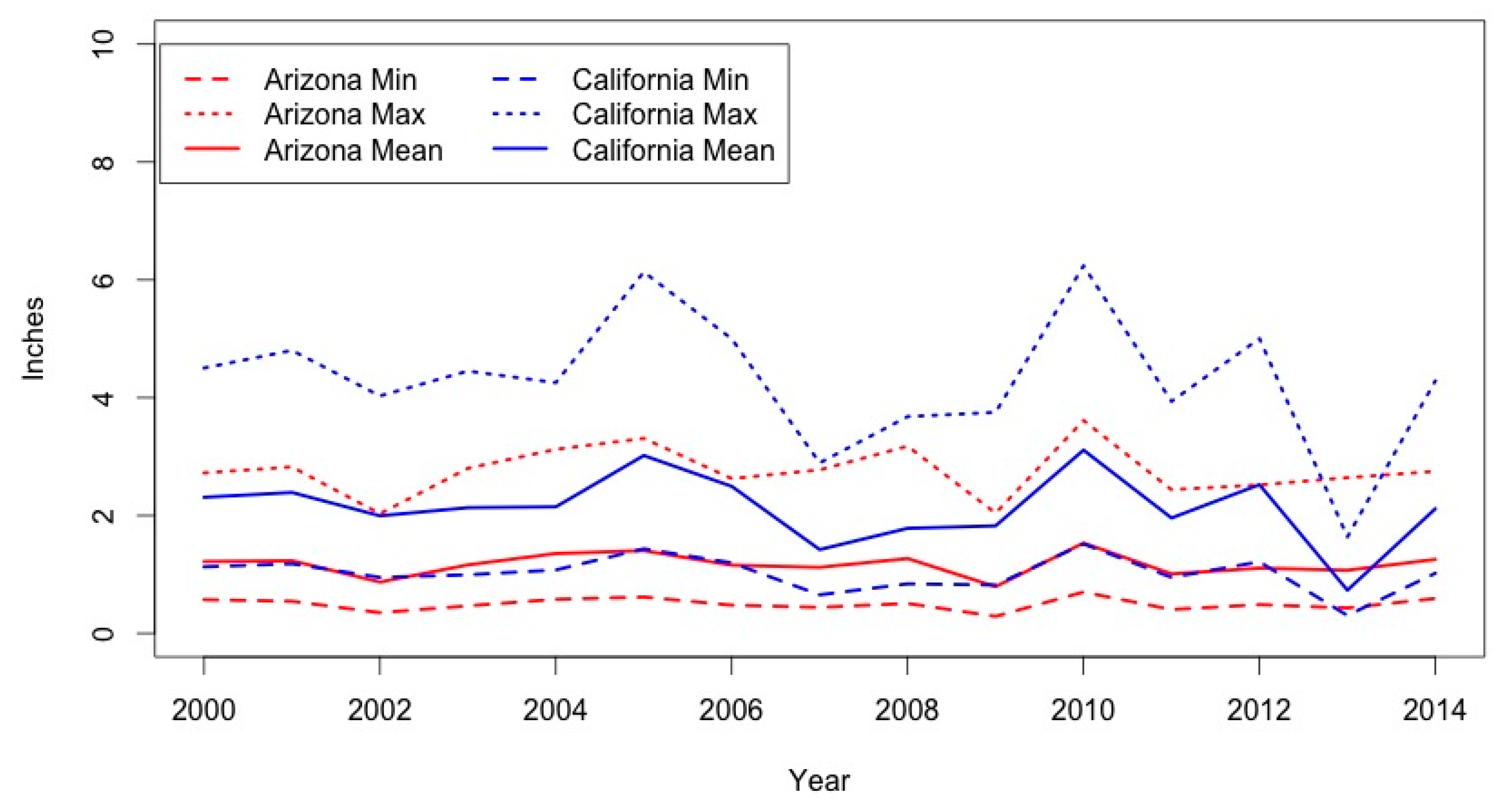
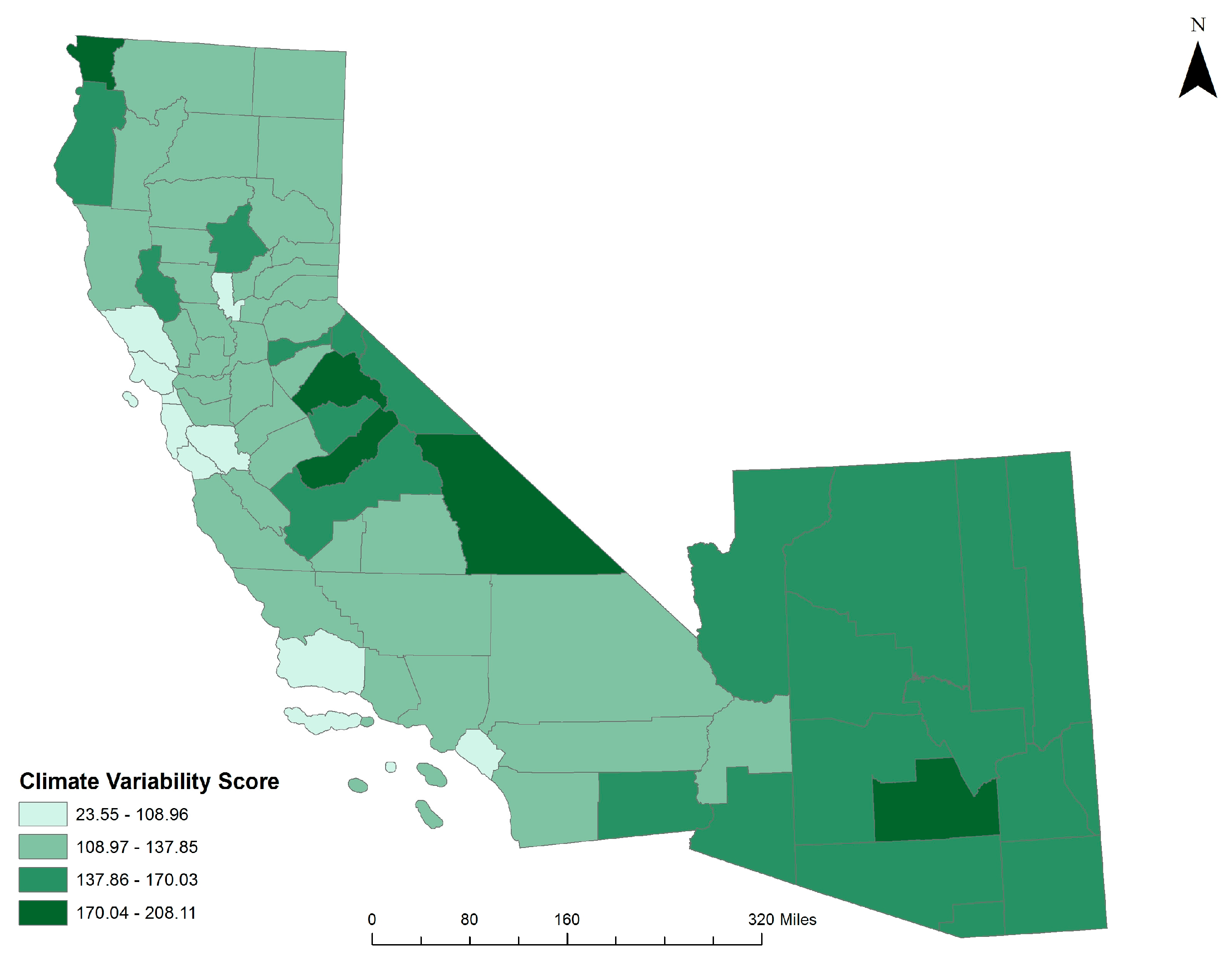
3.3. Climate Variability & Coccidioidomycosis Vulnerability
4. Discussion
Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Comrie, A.C. Climate factors influencing coccidioidomycosis seasonality and outbreaks. Environ. Health Perspect. 2005, 113, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Gregg, G. Assessment of Climate Change in the Southwest United States: A Report Prepared for the National Climate Assessment; Garfin, G., Jardine, A., Merideth, R., Black, M., LeRoy, S., Eds.; Island Press: Washington, DC, USA, 2013. [Google Scholar]
- Park, B.J.; Sigel, K.; Vaz, V.; Komatsu, K.; McRill, C.; Phelan, M.; Colman, T.; Comrie, A.C.; Warnock, D.W.; Galgiani, J.N.; et al. An epidemic of coccidioidomycosis in Arizona associated with climatic changes, 1998–2001. J. Infect. Dis. 2005, 191, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Tamerius, J.D.; Comrie, A.C. Coccidioidomycosis incidence in arizona predicted by seasonal precipitation. PLoS ONE 2011, 6, e21009. [Google Scholar] [CrossRef] [PubMed]
- Twarog, M.; Thompson, G.R., 3rd. Coccidioidomycosis: Recent updates. Semin. Respir. Crit. Care Med. 2015, 36, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Increase in reported coccidioidomycosis--United States, 1998–2011. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 217–221. [Google Scholar]
- Pappagianis, D. Epidemiology of coccidioidomycosis. Curr. Top. Med. Mycol. 1988, 2, 199–238. [Google Scholar] [PubMed]
- Sondermeyer, G.L.; Lee, L.A.; Gilliss, D.; McCarty, J.M.; Vugia, D.J. Epidemiology of pediatric coccidioidomycosis in California, 2000–2012. Pediatr. Infect. Dis. J. 2016, 35, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Guevara, R.E.; Motala, T.; Terashita, D. The changing epidemiology of coccidioidomycosis in Los Angeles (LA) County, California, 1973–2011. PLoS ONE 2015, 10, e0136753. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Gaab, E.M.; Sanchez, J.; Bui, P.Q.; Nobile, C.J.; Hoyer, K.K.; Peterson, M.W.; Ojcius, D.M. Valley fever: Danger lurking in a dust cloud. Microb. Infect. 2014, 16, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Kolivras, K.N.; Johnson, P.S.; Comrie, A.C.; Yool, S.R. Environmental variability and coccidioidomycosis (valley fever). Aerobiologia 2001, 17, 31–42. [Google Scholar] [CrossRef]
- Tabor, J.A.; O’Rourke, M.K. A risk factor study of coccidioidomycosis by controlling differential misclassifications of exposure and susceptibility using a landscape ecology approach. Sci. Total Environ. 2010, 408, 2199–2207. [Google Scholar] [CrossRef] [PubMed]
- Ampel, N.M. What’s behind the increasing rates of coccidioidomycosis in Arizona and California? Curr. Infect. Dis. Rep. 2010, 12, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.E.; Mayer, A.P.; Currier, J.; Files, J.A.; Wu, Q. Coccidioidomycosis in elderly persons. Clin. Infect. Dis. 2008, 47, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Children’s Environmental Health: Environmental Risks. Available online: http://www.who.int/ceh/risks/en/ (accessed on 6 April 2017).
- Laniado-Laborin, R. Expanding understanding of epidemiology of coccidioidomycosis in the Western Hemisphere. Ann. N. Y. Acad. Sci. 2007, 1111, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Barker, B.M.; Hoover, S.; Nix, D.E.; Ampel, N.M.; Frelinger, J.A.; Orbach, M.J.; Galgiani, J.N. Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clin. Microbiol. Rev. 2013, 26, 505–525. [Google Scholar] [CrossRef] [PubMed]
- Kolivras, K.N.; Comrie, A.C. Modeling valley fever (coccidioidomycosis) incidence on the basis of climate conditions. Int. J. Biometeorol. 2003, 47, 87–101. [Google Scholar] [PubMed]
- Leake, J.A.; Mosley, D.G.; England, B.; Graham, J.V.; Plikaytis, B.D.; Ampel, N.M.; Perkins, B.A.; Hajjeh, R.A. Risk factors for acute symptomatic coccidioidomycosis among elderly persons in Arizona, 1996–1997. J. Infect. Dis. 2000, 181, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Rosenstein, N.E.; Emery, K.W.; Werner, S.B.; Kao, A.; Johnson, R.; Rogers, D.; Vugia, D.; Reingold, A.; Talbot, R.; Plikaytis, B.D.; et al. Risk factors for severe pulmonary and disseminated coccidioidomycosis: Kern County, California, 1995–1996. Clin. Infect. Dis. 2001, 32, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Louie, L.; Ng, S.; Hajjeh, R.; Johnson, R.; Vugia, D.; Werner, S.B.; Talbot, R.; Klitz, W. Influence of host genetics on the severity of coccidioidomycosis. Emerg. Infect. Dis. 1999, 5, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Cutter, S.L.; Boruff, B.J.; Shirley, W.L. Social vulnerability to environmental hazards. Soc. Sci. Q. 2003, 84, 242–261. [Google Scholar] [CrossRef]
- Fisher, F.S.; Bultman, M.W.; Pappagianis, D. Operational Guidelines (Version 1.0) for Geological Fieldwork in Areas Endemic for Coccidioidomycosis (Valley Fever); U.S. Geological Survey: Reston, VA, USA, 2000. [Google Scholar]
- Edwards, P.Q.; Palmer, C.E. Prevalence of sensitivity to coccidioidin, with special reference to specific and nonspecific reactions to coccidioidin and to histoplasmin. Chest 1957, 31, 35–60. [Google Scholar] [CrossRef]
- Marsden-Haug, N.; Hill, H.; Litvintseva, A.P.; Engelthaler, D.M.; Driebe, E.M.; Roe, C.C.; Ralston, C.; Hurst, S.; Goldoft, M.; Gade, L.; et al. Notes from the field: Coccidioides immitis identified in soil outside of its known range—Washington, 2013. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 450. [Google Scholar] [PubMed]
- Talamantes, J.; Behseta, S.; Zender, C.S. Fluctuations in climate and incidence of coccidioidomycosis in Kern County, California: A review. Ann. N. Y. Acad. Sci. 2007, 1111, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Reed, R. Ecology and epizootiology of coccidioidomycosis. In Reprint of Reports of Papers Delivered at The 1959–1960 Conventions; The Intermountain Veterinary Medical Association: Salt Lake City, UT, USA, 1960. [Google Scholar]
- Komatsu, K.; Vaz, V.; McRill, C.; Colman, T.; Comrie, A.C.; Sigel, K.; Clark, T.; Phelan, M.; Hajjeh, R.; Park, B. Increase in coccidioidomycosis—Arizona, 1998–2001. MMWR Morb. Mortal. Wkly. Rep. 2003, 52, 109–112. [Google Scholar]
- Zender, C.S.; Talamantes, J. Climate controls on valley fever incidence in Kern County, California. Int. J. Biometeorol. 2006, 50, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Flynn, N.M.; Hoeprich, P.D.; Kawachi, M.M.; Lee, K.K.; Lawrence, R.M.; Goldstein, E.; Jordan, G.W.; Kundargi, R.S.; Wong, G.A. An unusual outbreak of windborne coccidioidomycosis. N. Engl. J. Med. 1979, 301, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Parker, G. An Exploratory Analysis of Associations between Drought and Coccidioidomycosis Incidence in Arizona and California. Master’s Thesis, Rollins School of Public Health of Emory University, Alanta, GA, USA, 2015. [Google Scholar]
- Comrie, A.C.; Glueck, M.F. Assessment of climate-coccidioidomycosis model: Model sensitivity for assessing climatologic effects on the risk of acquiring coccidioidomycosis. Ann. N. Y. Acad. Sci. 2007, 1111, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Coopersmith, E.J.; Bell, J.E.; Benedict, K.; Shriber, J.; McCotter, O.; Cosh, M.H. Relating coccidioidomycosis (valley fever) incidence to soil moisture conditions. GeoHealth 2017, 1, 51–63. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Summary for Policymakers; Working Group II Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Intergovernmental Panel on Climate Change: Genève, Switzerland, 2014. [Google Scholar]
- Balbus, J.; Crimmins, A.; Gamble, J.L.; Easterling, D.R.; Kunkel, K.E.; Saha, S.; Sarofim, M.C. Ch. 1: Introduction: Climate change and human health. In The Impacts of Climate Change on Human Health in the United States: A Scientific Assessment; U.S. Global Change Research Program: Washington, DC, USA, 2016; pp. 25–42. [Google Scholar]
- Bell, J.E.; Herring, S.C.; Jantarasami, L.; Adrianopoli, C.; Benedict, K.; Conlon, K.; Escobar, V.; Hess, J.; Luvall, J.; Garcia-Pando, C.P.; et al. Ch. 4: Impacts of Extreme Events on Human Health; U.S. Global Change Research Program: Washington, DC, USA, 2016; pp. 99–128. [Google Scholar]
- Litvintseva, A.P.; Marsden-Haug, N.; Hurst, S.; Hill, H.; Gade, L.; Driebe, E.M.; Ralston, C.; Roe, C.; Barker, B.M.; Goldoft, M.; et al. Valley fever: Finding new places for an old disease: Coccidioides immitis found in washington state soil associated with recent human infection. Clin. Infect. Dis. 2015, 60, e1–e3. [Google Scholar] [CrossRef] [PubMed]
- Tong, D.Q.; Wang, J.X.; Gill, T.E.; Lei, H.; Wang, B. Intensified dust storm activity and valley fever infection in the Southwestern United States. Geophys. Res. Lett. 2017, 44, 4304–4312. [Google Scholar] [CrossRef]
- IPCC. Summary for policymakers. In Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Parry, M.L., Canziani, O.F., Palutikof, J.P., van der Linden, P.J., Hanson, C.E., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 7–22. [Google Scholar]
- Wilhelmi, O.V.; Hayden, M.H. Connecting people and place: A new framework for reducing urban vulnerability to extremeheat. Environ. Res. Lett. 2010, 5. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on clImate Change; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Vincent, K. Creating an index of social vulnerability to climate change for Africa. In Working Paper 56; Tyndall Centre for Climate Change Research: Norwich, UK, 2004. [Google Scholar]
- Bradford, K.; Abrahams, L.; Hegglin, M.; Klima, K. A heat vulnerability index and adaptation solutions for Pittsburgh, Pennsylvania. Environ. Sci. Technol. 2015, 49, 11303–11311. [Google Scholar] [CrossRef] [PubMed]
- Cutter, S.L.; Barnes, L.; Berry, M.; Burton, C.; Evans, E.; Tate, E.; Webb, J. A place-based model for understanding community resilience to natural disasters. Glob. Environ. Chang. 2008, 18, 598–606. [Google Scholar] [CrossRef]
- Cutter, S.L.; Finch, C. Temporal and spatial changes in social vulnerability to natural hazards. Proc. Natl. Acad. Sci. USA 2008, 105, 2301–2306. [Google Scholar] [CrossRef] [PubMed]
- Dickin, S.K.; Schuster-Wallace, C.J.; Elliott, S. Developing a vulnerability mapping methodology: Applying the water-associated disease index to dengue in Malaysia. PLoS ONE 2013, 8, e63584. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, B.; Gregory, E.; Hallisey, E.; Heitgerd, J.; Lewis, B. A social vulnerability index for disaster management. J. Homel. Secur. Emerg. Med. 2011, 8. [Google Scholar] [CrossRef]
- Fussel, H.-M.; Ebi, K.L. Assessing vulnerability of human health. In Assessing Vulnerability to Global Environmental Change; Patt, A.G., Schroter, D., Klein, R.J.T., de la Vega-Leiner, A.C., Eds.; Earthscan: London, UK, 2009. [Google Scholar]
- Hahn, M.B.; Riederer, A.M.; Foster, S.O. The livelihood vulnerability index: A pragmatic approach to assessing risks from climate variability and change—A case study in Mozambique. Glob. Environ. Chang. 2009, 19, 74–88. [Google Scholar] [CrossRef]
- Johnson, D.P.; Stanforth, A.; Lulla, V.; Luber, G. Developing an applied extreme heat vulnerability index utilizing socioeconomic and environmental data. Appl. Geogr. 2012, 35, 23–31. [Google Scholar] [CrossRef]
- KC, B.; Shepherd, J.M.; Gaither, C.J. Climate change vulnerability assessment in Georgia. Appl. Geogr. 2015, 62, 62–74. [Google Scholar] [CrossRef]
- Luh, J.; Christenson, E.; Toregozhina, A.; Holcomb, D.; Witsil, T.; Hamrick, L.; Ojomo, E.; Bartram, J. Vulnerability assessment for loss of access to drinking water due to extreme weather events. Clim. Chang. 2015, 133, 1–15. [Google Scholar] [CrossRef]
- Malik, S.M.; Awan, H.; Khan, N. Mapping vulnerability to climate change and its repercussions on human health in Pakistan. Glob. Health 2012, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Reid, C.E.; O’Neill, M.S.; Gronlund, C.J.; Brines, S.J.; Brown, D.G.; Diez-Roux, A.V.; Schwartz, J. Mapping community determinants of heat vulnerability. Environ. Health Perspect. 2009, 117, 1730–1736. [Google Scholar] [CrossRef] [PubMed]
- Stanturf, J.A.; Goodrick, S.L.; Warren, M.L., Jr.; Charnley, S.; Stegall, C.M. Social vulnerability and ebola virus disease in rural Liberia. PLoS ONE 2015, 10, e0137208. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.E.; Ebi, K.L.; Vose, D.; Wint, W.; Alexander, N.; Mintiens, K.; Semenza, J.C. Vulnerabilities to the risks of changes in infectious disease transmission caused by climate change: A modelling study. Lancet 2014, 384. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, N.; Wu, W.; Wu, J.; Shi, P. Local spatial and temporal factors influencing population and societal vulnerability to natural disasters. Risk Anal. 2014, 34, 614–639. [Google Scholar] [CrossRef] [PubMed]
- United States Census Bureau. Population Estimates; United States Census Bureau: Suitland, ML, USA, 2011.
- United States Census Bureau. Tiger/Line Shapefiles; United States Census Bureau: Suitland, ML, USA, 2010.
- Environmental Systems Research Institute (ESRI). Arcgis Desktop; Environmental Systems Research Institute: Redlands, CA, USA, 2014. [Google Scholar]
- Homer, C.G.; Dewitz, J.A.; Yang, L.; Jin, S.; Danielson, P.; Xian, G.; Coulston, J.; Herold, N.D.; Wickham, J.D.; Megown, K. Completion of the 2011 National Land Cover Database for the conterminous United States-Representing a decade of land cover change information. Photogramm. Eng. Remote Sensing 2015, 81, 345–354. [Google Scholar]
- Centers for Disease Control and Prevention. Nchhstp Atlas; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2012.
- National Cancer Institute. State Cancer Profiles; National Cancer Institute: Rockville, ML, USA, 2015.
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A multiscalar drought index sensitive to global warming: The standardized precipitation evapotranspiration index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- American Hospital Association. Aha Annual Survey Database; American Hospital Association: Chicago, IL, USA, 2013. [Google Scholar]
- U.S. Department of Health and Human Services. Areal Health Resource File; U.S. Department of Health and Human Services: Washington, DC, USA, 2012.
- Centers for Disease Control and Prevention Mycotics Diseases Branch. Coccidioidomycosis Selected Counties—Arizona and California, 2000–2014; Centers for Disease Control and Prevention Mycotics Diseases Branch: Atlanta, GA, USA, 2015.
- National Oceanic and Atmoshperic Administration. National Centers for Environmental Information. Available online: https://www.ncdc.noaa.gov/ (accessed on 15 November 2016).
- Sabo, N.Č.; Popović, A.; Đorđević, D. Air pollution by pollen grains of anemophilous species: Influence of chemical and meteorological parameters. Water Air Soil Pollut. 2015, 226, 292. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Manangan, A.P.; Uejio, C.K.; Saha, S.; Schramm, P.J.; Marinucci, G.D.; Brown, C.L.; Hess, J.J.; Luber, G. Assessing Health Vulnerability to Climate Change: A Guide for Health Departments; National Center for Environmental Health: Atlanta, GA, USA, 2014. [Google Scholar]
- Bao, J.; Li, X.; Yu, C. The construction and validation of the heat vulnerability index, a review. Int. J. Environ. Res. Public Health 2015, 12, 7220. [Google Scholar] [CrossRef] [PubMed]
- Mayo Clinic. Valley Fever Risk Factors. Available online: http://www.mayoclinic.org/diseases-conditions/valley-fever/basics/risk-factors/con-20027390 (accessed on 6 April 2017).
- Fisher, F.S.; Bultman, M.W.; Johnson, S.M.; Pappagianis, D.; Zaborsky, E. Coccidioides niches and habitat parameters in the Southwestern United States: A matter of scale. Ann. N. Y. Acad. Sci. 2007, 1111, 47–72. [Google Scholar] [CrossRef] [PubMed]
- Baptista-Rosas, R.C.; Hinojosa, A.; Riquelme, M. Ecological niche modeling of Coccidioides spp. in western North American deserts. Ann. N. Y. Acad. Sci. 2007, 1111, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Lauer, A.; Baal, J.D.; Baal, J.C.; Verma, M.; Chen, J.M. Detection of Coccidioides immitis in Kern County, California, by multiplex PCR. Mycologia 2012, 104, 62–69. [Google Scholar] [CrossRef] [PubMed]
| Variable | Component | Source (Year) |
|---|---|---|
| % Land Suitable for Coccidioides spp. Growth | Exposure | Multi-Resolution Land Characteristics Consortium (2011) [61] |
| % Positive Skin Test | Exposure | Edwards & Palmer (1957) [24] |
| Population Density Per Square Mile | Exposure | U.S. Census (2010) [58] |
| % Population > 65 years | Sensitivity | U.S. Census (2010) [58] |
| % Population < 5 years | Sensitivity | U.S. Census (2010) [58] |
| % Population of African-American Race | Sensitivity | U.S. Census (2010) [58] |
| % Population of Filipino Race | Sensitivity | U.S. Census (2010) [58] |
| % Population Below Poverty Level | Sensitivity | U.S. Census (2010) [58] |
| % Population with No Higher Education | Sensitivity | U.S. Census (2010) [58] |
| % Population Living with HIV/AIDS | Sensitivity | CDC National Center for HIV/AIDS, Viral Hepatitis, STD, & TB Prevention (2012) [62] |
| Cancer Incidence Rate Per 100,000 (All Types) | Sensitivity | National Cancer Institute (2012) [63] |
| % Adults Who Smoke | Sensitivity | CDC Behavioral Risk Factor Surveillance System (2010) [64] |
| Number of Hospitals Per 100 Square Miles | Adaptive Capacity | American Hospital Association (2012) [65] |
| Number of Primary Care Physicians per 100,000 | Adaptive Capacity | HRSA Areal Health Resource File (2012) [66] |
| State | Incidence Rate | % ≥65 Years | % <5 Years | % African American | % Filipino | % Below Poverty Line | Cancer IR | % Adults Who Smoke | % PLWHA | % Adults with No Higher Education | % Suitable Land | % Positive Skin Tests | Population Density Per Sq. Mi. | PCPs Per 100k Population | Hospitals Per 100 Sq. Mi. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arizona Only | Mean Annual IR | 0.12 | −0.14 | 0.46 | 0.37 | −0.40 | 0.30 | 0.13 | 0.49 | −0.25 | 0.21 | 0.62 * | 0.36 | −0.06 | 0.56 * |
| 2010 IR | 0.16 | −0.12 | 0.37 | 0.32 | −0.23 | 0.16 | 0.22 | 0.56 * | −0.18 | 0.22 | 0.58 * | 0.38 | −0.04 | 0.58 * | |
| Mean Fall Monthly IR | 0.16 | −0.26 | 0.43 | 0.36 | −0.43 | 0.35 | 0.14 | 0.48 | −0.35 | 0.20 | 0.64 * | 0.38 | −0.01 | 0.57 * | |
| Mean Spring Monthly IR | 0.16 | −0.18 | 0.46 | 0.39 | −0.45 | 0.37 | 0.12 | 0.45 | −0.25 | 0.22 | 0.61 * | 0.35 | −0.01 | 0.56 * | |
| Mean Summer Monthly IR | 0.09 | −0.13 | 0.36 | 0.29 | −0.36 | 0.29 | 0.15 | 0.33 | −0.16 | 0.03 | 0.58 * | 0.33 | 0.07 | 0.46 | |
| Mean Winter Monthly IR | 0.16 | −0.18 | 0.45 | 0.39 | −0.43 | 0.34 | 0.11 | 0.39 | −0.17 | 0.28 | 0.45 | 0.22 | −0.04 | 0.44 | |
| California Only | Mean Annual IR | −0.47 * | 0.65 * | −0.20 | −0.46 * | 0.66 * | −0.51 * | 0.17 | −0.27 | 0.69 * | 0.07 | 0.66 * | −0.65 * | −0.65 * | −0.58 * |
| 2010 IR | −0.52 * | 0.67 * | −0.19 | −0.52 * | 0.70 * | −0.46 * | 0.24 | −0.32 | 0.76 * | 0.06 | 0.63 * | −0.70 * | −0.70 * | −0.62 * | |
| Mean Fall Monthly IR | −0.49 * | 0.66 * | −0.21 | −0.51 * | 0.69 * | −0.53 * | 0.22 | −0.30 | 0.72 * | 0.10 | 0.66 * | −0.71 * | −0.69 * | −0.63 * | |
| Mean Spring Monthly IR | −0.51 * | 0.68 * | −0.17 | −0.45 * | 0.69 * | −0.50 * | 0.18 | −0.29 | 0.69 * | 0.08 | 0.65 * | −0.65 * | −0.63 * | −0.59 * | |
| Mean Summer Monthly IR | −0.53 * | 0.67 * | −0.18 | −0.44 | 0.69 * | −0.49 * | 0.20 | −0.26 | 0.72 * | −0.01 | 0.64 * | −0.64 * | −0.64 * | −0.58 * | |
| Mean Winter Monthly IR | −0.44 | 0.64 * | −0.21 | −0.47 * | 0.63 * | −0.46 * | 0.15 | −0.29 | 0.70 * | 0.08 | 0.65 * | −0.66 * | −0.65 * | −0.59 * |
| State | Vulnerability Index Type | Mean Annual IR | 2010 IR | Mean Fall Monthly IR | Mean Spring Monthly IR | Mean Summer Monthly IR | Mean Winter Monthly IR |
|---|---|---|---|---|---|---|---|
| Both States | Quartile Index | 0.23 | 0.24 | 0.25 | 0.22 | 0.17 | 0.22 |
| Percentile Index | 0.26 | 0.27 | 0.28 | 0.24 | 0.21 | 0.25 | |
| Supervised Quartile Index | 0.26 | 0.30 | 0.29 | 0.23 | 0.25 | 0.23 | |
| Supervised Percentile Index | 0.34 * | 0.35 * | 0.36 * | 0.30 | 0.31 | 0.31 | |
| Arizona Only | Quartile Index | 0.22 | 0.23 | 0.14 | 0.18 | 0.02 | 0.17 |
| Percentile Index | 0.23 | 0.26 | 0.16 | 0.18 | 0.05 | 0.18 | |
| Supervised Quartile Index | 0.43 | 0.50 | 0.44 | 0.38 | 0.38 | 0.28 | |
| Supervised Percentile Index | 0.42 | 0.43 | 0.39 | 0.37 | 0.32 | 0.31 | |
| California Only | Quartile Index | 0.56 * | 0.60 * | 0.61 * | 0.56 * | 0.53 * | 0.56 * |
| Percentile Index | 0.62 * | 0.66 * | 0.66 * | 0.60 * | 0.60 * | 0.62 * | |
| Supervised Quartile Index | 0.63 * | 0.68 * | 0.66 * | 0.61 * | 0.62 * | 0.64 * | |
| Supervised Percentile Index | 0.72 * | 0.77 * | 0.75 * | 0.71 * | 0.72 * | 0.72 * |
| Climate Variability Score | Both States | Arizona Index | California Index |
|---|---|---|---|
| Overall Variability | 0.31 * | 0.34 | 0.42 * |
| Overall Precip. Variability | −0.23 * | 0.08 | −0.29 * |
| Overall Temp. Variability | 0.34 * | 0.15 | 0.45 * |
| Overall SPEI Variability | 0.30 * | 0.12 | 0.47 * |
| Fall Variability | 0.21 | 0.44 | 0.27 * |
| Fall Precip. Variability | −0.25 * | −0.15 | −0.31 * |
| Fall Temp. Variability | 0.24 * | 0.51 | 0.27 * |
| Fall SPEI Variability | 0.31 * | 0.14 | 0.47 * |
| Spring Variability | 0.15 | −0.44 | 0.29 * |
| Spring Precip. Variability | −0.25 * | −0.22 | −0.26 * |
| Spring Temp. Variability | 0.17 | −0.30 | 0.32 * |
| Spring SPEI Variability | 0.21 * | −0.07 | 0.37 * |
| Summer Variability | 0.34 * | 0.27 | 0.43 * |
| Summer Precip. Variability | −0.10 | 0.09 | −0.12 |
| Summer Temp. Variability | 0.39 * | 0.12 | 0.45 * |
| Summer SPEI Variability | 0.26 * | 0.21 | 0.40 * |
| Winter Variability | 0.15 | 0.41 | 0.10 |
| Winter Precip. Variability | −0.16 | 0.17 | −0.29 * |
| Winter Temp. Variability | 0.21 | 0.29 | 0.19 |
| Winter SPEI Variability | 0.15 | 0.26 | 0.19 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shriber, J.; Conlon, K.C.; Benedict, K.; McCotter, O.Z.; Bell, J.E. Assessment of Vulnerability to Coccidioidomycosis in Arizona and California. Int. J. Environ. Res. Public Health 2017, 14, 680. https://doi.org/10.3390/ijerph14070680
Shriber J, Conlon KC, Benedict K, McCotter OZ, Bell JE. Assessment of Vulnerability to Coccidioidomycosis in Arizona and California. International Journal of Environmental Research and Public Health. 2017; 14(7):680. https://doi.org/10.3390/ijerph14070680
Chicago/Turabian StyleShriber, Jennifer, Kathryn C. Conlon, Kaitlin Benedict, Orion Z. McCotter, and Jesse E. Bell. 2017. "Assessment of Vulnerability to Coccidioidomycosis in Arizona and California" International Journal of Environmental Research and Public Health 14, no. 7: 680. https://doi.org/10.3390/ijerph14070680





