Residential Proximity to Roadways and Ischemic Placental Disease in a Cape Cod Family Health Study
Abstract
:1. Introduction
2. Materials and Methods
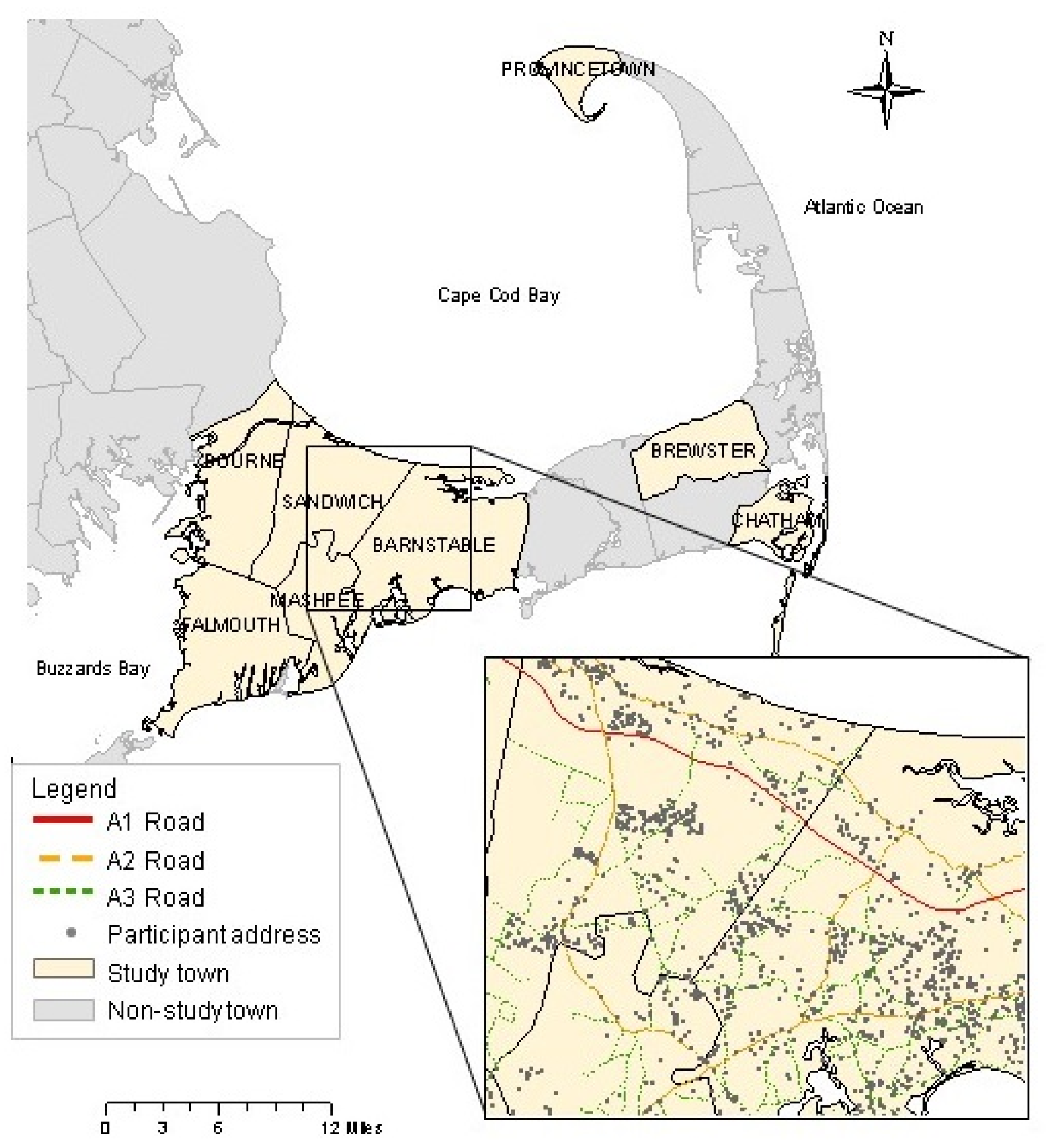
2.1. Study Population
2.2. Exclusions
2.3. Assessment of Ischemic Placental Disease
2.4. Assessment of Exposure to Traffic-Related Air Pollution
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hettfleisch, K.; Bernardes, L.S.; Carvalho, M.A.; Pastro, L.D.; Vieira, S.E.; Saldiva, S.R.; Saldiva, P.; Francisco, R.P. Short-term exposure to urban air pollution and influences on placental vascularization indexes. Environ. Health Perspect. 2017, 125. [Google Scholar] [CrossRef] [PubMed]
- Ananth, C.V.; Vintzileos, A.M. Ischemic placental disease: Epidemiology and risk factors. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 159, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Ananth, C.V.; Peltier, M.R.; Chavez, M.R.; Kirby, R.S.; Getahun, D.; Vintzileos, A.M. Recurrence of ischemic placental disease. Obstet. Gynecol. 2007, 110, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.; Pijnenborg, R.; Vercruysse, L.; Romero, R. The “great obstetrical syndromes” are associated with disorders of deep placentation. Am J Obstet. Gynecol. 2011, 204, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Ananth, C.V.; Savitz, D.A.; Bowes, W.A., Jr. Hypertensive disorders of pregnancy and stillbirth in North Carolina, 1988 to 1991. Acta Obstet. Gynecol. Scand. 1995, 74, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Basso, O.; Rasmussen, S.; Weinberg, C.R.; Wilcox, A.J.; Irgens, L.M.; Skjaerven, R. Trends in fetal and infant survival following preeclampsia. JAMA 2006, 296, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Ounsted, M.; Moar, V.; Scott, W.A. Perinatal morbidity and mortality in small-for-dates babies: The relative importance of some maternal factors. Early Hum. Dev. 1981, 5, 367–375. [Google Scholar] [CrossRef]
- Parker, S.E.; Werler, M.M. Epidemiology of ischemic placental disease: A focus on preterm gestations. Semin. Perinatol. 2014, 38, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A.; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [PubMed]
- De Melo, J.O.; Soto, S.F.; Katayama, I.A.; Wenceslau, C.F.; Pires, A.G.; Veras, M.M.; Furukawa, L.N.; de Castro, I.; Saldiva, P.H.; Heimann, J.C. Inhalation of fine particulate matter during pregnancy increased IL-4 cytokine levels in the fetal portion of the placenta. Toxicol. Lett. 2015, 232, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Misra, D.P.; Dvonch, J.T.; Krishnakumar, A. Exposures to airborne particulate matter and adverse perinatal outcomes: A biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ. Health Perspect. 2006, 114, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Slama, R.; Darrow, L.; Parker, J.; Woodruff, T.J.; Strickland, M.; Nieuwenhuijsen, M.; Glinianaia, S.; Hoggatt, K.J.; Kannan, S.; Hurley, F.; et al. Meeting report: Atmospheric pollution and human reproduction. Environ. Health Perspect. 2008, 116, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Veras, M.M.; Damaceno-Rodrigues, N.R.; Caldini, E.G.; Maciel Ribeiro, A.A.; Mayhew, T.M.; Saldiva, P.H.; Dolhnikoff, M. Particulate urban air pollution affects the functional morphology of mouse placenta. Biol. Reprod. 2008, 79, 578–584. [Google Scholar] [CrossRef] [PubMed]
- HEI Panel on the Health Effects of Traffic-Related Air Pollution. Traffic-Related Air Pollution: A Critical Review of the Literature on Emissions, Exposure, and Health Effects; Health Effects Institute: Boston, MA, USA, 2010. [Google Scholar]
- Wu, M.; Ries, J.J.; Proietti, E.; Vogt, D.; Hahn, S.; Hoesli, I. Development of late-onset preeclampsia in association with road densities as a proxy for traffic-related air pollution. Fetal Diagn. Ther. 2016, 39, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wilhelm, M.; Chung, J.; Ritz, B. Comparing exposure assessment methods for traffic-related air pollution in an adverse pregnancy outcome study. Environ. Res. 2011, 111, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.L.; Edwards, S.E.; Chang, H.H.; Auten, R.L. Proximity to roadways and pregnancy outcomes. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Yorifuji, T.; Naruse, H.; Kashima, S.; Murakoshi, T.; Tsuda, T.; Doi, H.; Kawachi, I. Residential proximity to major roads and placenta/birth weight ratio. Sci. Total Environ. 2012, 414, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, S.L.; Eliot, M.N.; Whitsel, E.A.; Huang, Y.T.; Kelsey, K.T.; Marsit, C.J.; Wellenius, G.A. Maternal residential proximity to major roadways, birth weight, and placental DNA methylation. Environ. Int. 2016, 92, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, A.F.; Rifas-Shiman, S.L.; Koutrakis, P.; Schwartz, J.D.; Kloog, I.; Melly, S.; Coull, B.A.; Zanobetti, A.; Gillman, M.W.; Gold, D.R.; et al. Prenatal exposure to traffic pollution: Associations with reduced fetal growth and rapid infant weight gain. Epidemiology 2015, 26, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Genereux, M.; Auger, N.; Goneau, M.; Daniel, M. Neighbourhood socioeconomic status, maternal education and adverse birth outcomes among mothers living near highways. J. Epidemiol. Community Health 2008, 62, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Brauer, M.; Lencar, C.; Tamburic, L.; Koehoorn, M.; Demers, P.; Karr, C. A cohort study of traffic-related air pollution impacts on birth outcomes. Environ. Health Perspect. 2008, 116, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Zeka, A.; Melly, S.J.; Schwartz, J. The effects of socioeconomic status and indices of physical environment on reduced birth weight and preterm births in Eastern Massachusetts. Environ. Health 2008, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Kashima, S.; Naruse, H.; Yorifuji, T.; Ohki, S.; Murakoshi, T.; Takao, S.; Tsuda, T.; Doi, H. Residential proximity to heavy traffic and birth weight in Shizuoka, Japan. Environ. Res. 2011, 111, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Yorifuji, T.; Naruse, H.; Kashima, S.; Murakoshi, T.; Doi, H. Residential proximity to major roads and obstetrical complications. Sci. Total Environ. 2015, 508, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Malmqvist, E.; Jakobsson, K.; Tinnerberg, H.; Rignell-Hydbom, A.; Rylander, L. Gestational diabetes and preeclampsia in association with air pollution at levels below current air quality guidelines. Environ. Health Perspect. 2013, 121, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Van den Hooven, E.H.; Jaddoe, V.W.; de Kluizenaar, Y.; Hofman, A.; Mackenbach, J.P.; Steegers, E.A.; Miedema, H.M.; Pierik, F.H. Residential traffic exposure and pregnancy-related outcomes: A prospective birth cohort study. Environ. Health 2009, 8. [Google Scholar] [CrossRef] [PubMed]
- Sram, R.J.; Binkova, B.; Dejmek, J.; Bobak, M. Ambient air pollution and pregnancy outcomes: A review of the literature. Environ. Health Perspect. 2005, 113, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Jerrett, M.; Arain, A.; Kanaroglou, P.; Beckerman, B.; Potoglou, D.; Sahsuvaroglu, T.; Morrison, J.; Giovis, C. A review and evaluation of intraurban air pollution exposure models. J. Expo. Anal. Environ. Epidemiol. 2005, 15, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Aschengrau, A.; Weinberg, J.; Rogers, S.; Gallagher, L.; Winter, M.; Vieira, V.; Webster, T.; Ozonoff, D. Prenatal exposure to tetrachloroethylene-contaminated drinking water and the risk of adverse birth outcomes. Environ. Health Perspect. 2008, 116, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Aschengrau, A.; Weinberg, J.M.; Gallagher, L.G.; Winter, M.R.; Vieira, V.M.; Webster, T.F.; Ozonoff, D.M. Exposure to tetrachloroethylene-contaminated drinking water and the risk of pregnancy loss. Water Qual. Expo. Health 2009, 1, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Aschengrau, A.; Weinberg, J.M.; Janulewicz, P.A.; Gallagher, L.G.; Winter, M.R.; Vieira, V.M.; Webster, T.F.; Ozonoff, D.M. Prenatal exposure to tetrachloroethylene-contaminated drinking water and the risk of congenital anomalies: A retrospective cohort study. Environ. Health 2009, 8. [Google Scholar] [CrossRef] [PubMed]
- Carwile, J.L.; Mahalingaiah, S.; Winter, M.R.; Aschengrau, A. Prenatal drinking-water exposure to tetrachloroethylene and ischemic placental disease: A retrospective cohort study. Environ. Health 2014, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.L.; Creasy, R.K.; Cunningham, G.C.; Hawes, W.E.; Norris, F.D.; Tashiro, M. Fetal growth and perinatal viability in california. Obstet. Gynecol. 1982, 59, 624–632. [Google Scholar] [PubMed]
- Dadvand, P.; Ostro, B.; Figueras, F.; Foraster, M.; Basagana, X.; Valentin, A.; Martinez, D.; Beelen, R.; Cirach, M.; Hoek, G.; et al. Residential proximity to major roads and term low birth weight: The roles of air pollution, heat, noise, and road-adjacent trees. Epidemiology 2014, 25, 518–525. [Google Scholar] [CrossRef] [PubMed]
- MassGIS. Massgis Data-1990 U.S. Census-Tiger Linework. Available online: http://www.mass.gov/anf/research-and-tech/it-serv-and-support/application-serv/office-of-geographic-information-massgis/datalayers/cen1990tiger.html (accessed on 29 December 2016).
- MassGIS. Massgis Data-Level 3 Assessors’ Parcel Mapping. Available online: http://www.mass.gov/anf/research-and-tech/it-serv-and-support/application-serv/office-of-geographic-information-massgis/datalayers/l3parcels.html (accessed on 1 April 2014).
- Karner, A.A.; Eisinger, D.S.; Niemeier, D.A. Near-roadway air quality: Synthesizing the findings from real-world data. Environ. Sci. Technol. 2010, 44, 5334–5344. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hinds, W.C.; Kim, S.; Sioutas, C. Concentration and size distribution of ultrafine particles near a major highway. J. Air Waste Manag. Assoc. 2002, 52, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Zeger, S.L.; Liang, K.Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986, 42, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.Y.; Zeger, S.L. Longitudinal data analysis using generalized linear models. Biometrika 1986, 73, 13–22. [Google Scholar] [CrossRef]
- Rasmussen, S.; Irgens, L.M.; Dalaker, K. A history of placental dysfunction and risk of placental abruption. Paediatr. Perinat. Epidemiol. 1999, 13, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Dahabreh, I.J.; Kent, D.M. Index event bias as an explanation for the paradoxes of recurrence risk research. JAMA 2011, 305, 822–823. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.A.; Loomis, D.; Conceicao, G.M.; Braga, A.L.; Arcas, R.M.; Kishi, H.S.; Singer, J.M.; Bohm, G.M.; Saldiva, P.H. Association between air pollution and intrauterine mortality in Sao Paulo, Brazil. Environ. Health Perspect. 1998, 106, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Landgren, O. Environmental pollution and delivery outcome in Southern Sweden: A study with central registries. Acta Paediatr. 1996, 85, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Bobak, M.; Leon, D.A. Pregnancy outcomes and outdoor air pollution: An ecological study in districts of the Czech Republic 1986-8. Occup. Environ. Med. 1999, 56, 539–543. [Google Scholar] [CrossRef] [PubMed]
- DeFranco, E.; Hall, E.; Hossain, M.; Chen, A.; Haynes, E.N.; Jones, D.; Ren, S.; Lu, L.; Muglia, L. Air pollution and stillbirth risk: Exposure to airborne particulate matter during pregnancy is associated with fetal death. PLoS ONE 2015, 10, e0120594. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Sarovar, V.; Malig, B.; Basu, R. Association of stillbirth with ambient air pollution in a California cohort study. Am. J. Epidemiol. 2015, 181, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Faiz, A.S.; Rhoads, G.G.; Demissie, K.; Kruse, L.; Lin, Y.; Rich, D.Q. Ambient air pollution and the risk of stillbirth. Am. J. Epidemiol. 2012, 176, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.F.; Lee, Y.L.; Jaakkola, J.J. Air pollution and stillbirth: A population-based case-control study in Taiwan. Environ. Health Perspect. 2011, 119, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Pearce, M.S.; Glinianaia, S.V.; Rankin, J.; Rushton, S.; Charlton, M.; Parker, L.; Pless-Mulloli, T. No association between ambient particulate matter exposure during pregnancy and stillbirth risk in the north of England, 1962–1992. Environ. Res. 2010, 110, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Gardosi, J.; Kady, S.M.; McGeown, P.; Francis, A.; Tonks, A. Classification of stillbirth by relevant condition at death (recode): Population based cohort study. BMJ 2005, 331, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- Van den Hooven, E.H.; Pierik, F.H.; de Kluizenaar, Y.; Hofman, A.; van Ratingen, S.W.; Zandveld, P.Y.; Russcher, H.; Lindemans, J.; Miedema, H.M.; Steegers, E.A.; et al. Air pollution exposure and markers of placental growth and function: The Generation R study. Environ. Health Perspect. 2012, 120, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.G.; Williams, J.W. Williams Obstetrics; McGraw-Hill Medical: New York, NY, USA, 2010. [Google Scholar]
- Stieb, D.M.; Chen, L.; Eshoul, M.; Judek, S. Ambient air pollution, birth weight and preterm birth: A systematic review and meta-analysis. Environ. Res. 2012, 117, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Rice, F.; Lewis, A.; Harold, G.; van den Bree, M.; Boivin, J.; Hay, D.F.; Owen, M.J.; Thapar, A. Agreement between maternal report and antenatal records for a range of pre and peri-natal factors: The influence of maternal and child characteristics. Early Hum. Dev. 2007, 83, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Yawn, B.P.; Suman, V.J.; Jacobsen, S.J. Maternal recall of distant pregnancy events. J. Clin. Epidemiol. 1998, 51, 399–405. [Google Scholar] [CrossRef]
- Stuart, J.J.; Bairey Merz, C.N.; Berga, S.L.; Miller, V.M.; Ouyang, P.; Shufelt, C.L.; Steiner, M.; Wenger, N.K.; Rich-Edwards, J.W. Maternal recall of hypertensive disorders in pregnancy: A systematic review. J. Womens Health (Larchmt) 2013, 22, 37–47. [Google Scholar]
- Dietz, P.; Bombard, J.; Mulready-Ward, C.; Gauthier, J.; Sackoff, J.; Brozicevic, P.; Gambatese, M.; Nyland-Funke, M.; England, L.; Harrison, L.; et al. Validation of self-reported maternal and infant health indicators in the pregnancy risk assessment monitoring system. Matern. Child Health J. 2014, 18, 2489–2498. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J.; Greenland, S.; Lash, T.L. Modern Epidemiology, 3rd ed.; Lippincott, Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Ritz, B.; Wilhelm, M. Ambient air pollution and adverse birth outcomes: Methodologic issues in an emerging field. Basic Clin. Pharmacol. Toxicol. 2008, 102, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Schafer, J.L. Multiple imputation: A primer. Stat. Methods Med. Res. 1999, 8, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Brauer, M.; Hoek, G.; van Vliet, P.; Meliefste, K.; Fischer, P.; Gehring, U.; Heinrich, J.; Cyrys, J.; Bellander, T.; Lewne, M.; et al. Estimating long-term average particulate air pollution concentrations: Application of traffic indicators and geographic information systems. Epidemiology 2003, 14, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.B.; Beckerman, B.; Jerrett, M.; Brauer, M. Application of land use regression to estimate long-term concentrations of traffic-related nitrogen oxides and fine particulate matter. Environ. Sci. Technol. 2007, 41, 2422–2428. [Google Scholar] [CrossRef] [PubMed]
- Hajat, A.; Hsia, C.; O’Neill, M.S. Socioeconomic disparities and air pollution exposure: A global review. Curr. Environ. Health Rep. 2015, 2, 440–450. [Google Scholar] [PubMed]
- Green, R.S.; Malig, B.; Windham, G.C.; Fenster, L.; Ostro, B.; Swan, S. Residential exposure to traffic and spontaneous abortion. Environ. Health Perspect. 2009, 117, 1939–1944. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Ischemic Placental Disease | |
|---|---|---|
| Yes | No | |
| Number, n (%) | 270 (8.2) | 3039 (91.8) |
| Year of pregnancy, n (%) | ||
| Before 1974 | 61 (22.6) | 570 (18.8) |
| 1975–1980 | 118 (43.7) | 1243 (40.9) |
| After 1980 | 91 (33.7) | 1226 (40.3) |
| Maternal age (years), mean (SD) | 26.6 (4.5) | 27.7 (4.6) |
| Paternal age (years), mean (SD) | 30.1 (6.1) | 30.7 (5.8) |
| White, n (%) | 256 (94.8) | 2956 (97.3) |
| Maternal education, n (%) | ||
| Less than high school | 2 (0.7) | 39 (1.3) |
| High school graduate | 55 (20.4) | 569 (18.7) |
| Some college | 105 (38.9) | 1047 (34.5) |
| Four-year college graduate or more | 108 (40.0) | 1384 (45.5) |
| Paternal occupation, n (%) | ||
| White collar | 114 (42.2) | 1521 (50.1) |
| Blue collar | 106 (39.3) | 1020 (33.6) |
| Other | 50 (18.5) | 498 (16.4) |
| Number of prior pregnancies and prior IPD, n (%) | ||
| Nulligravid | 118 (43.7) | 905 (29.8) |
| Previous pregnancy, no prior IPD | 122 (45.2) | 1962 (64.6) |
| Previous pregnancy, prior IPD | 30 (11.1) | 172 (5.7) |
| Gestational weight gain <20 lb, n (%) | 70 (25.9) | 415 (13.7) |
| Cigarette smoking during first trimester, n (%) | 119 (44.1) | 744 (24.5) |
| Alcohol consumption during first trimester, n (%) | 110 (40.7) | 1108 (36.5) |
| Live <50 m from A1–A3 roadway, n (%) | 35 (13.0) | 443 (14.6) |
| Live <50 m from A1–A2 roadway, n (%) | 11 (4.1) | 111 (3.7) |
| Live <50 m from A1 roadway, n (%) | 0 (0.0) | 0 (0.0) |
| Any A1–A3 roads within 500 m buffer, n (%) | 237 (87.8) | 2650 (87.2) |
| Any A1–A2 roads within 500 m buffer, n (%) | 149 (55.2) | 1532 (50.4) |
| Any A1 roads within 500 m buffer, n (%) | 12 (4.4) | 150 (4.9) |
| Any A1–A3 roads within 200 m buffer, n (%) | 150 (55.6) | 1718 (56.5) |
| Any A1–A2 roads within 200 m buffer, n (%) | 117 (43.3) | 1086 (35.7) |
| Any A1 roads within 200 m buffer, n (%) | 5 (1.9) | 39 (1.3) |
| Any PCE exposure in year prior to pregnancy, n (%) | 126 (46.7) | 1536 (50.5) |
| Shortest Euclidean Distance Between Residence and the Closest Major Roadway (m) | Length of Major Roadways within 500 m Buffer Around Residence (m) | Length of Major Roadways within 200 m Buffer Around Residence (m) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A1–A3 Roads | A1–A2 Roads | A1 Roads | A1–A3 Roads | A1–A2 Roads | A1 Roads | A1–A3 Roads | A1–A2 Roads | A1 Roads | |
| Minimum | 12 | 13 | 67 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5th percentile | 30 | 68 | 565 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10th percentile | 38 | 136 | 911 | 0 | 0 | 0 | 0 | 0 | 0 |
| 25th percentile | 85 | 339 | 2075 | 592 | 0 | 0 | 0 | 0 | 0 |
| Median | 188 | 728 | 3694 | 1008 | 16 | 0 | 120 | 0 | 0 |
| 75th percentile | 362 | 1541 | 5276 | 1571 | 658 | 0 | 373 | 94 | 0 |
| 90th percentile | 570 | 2136 | 7310 | 2000 | 1771 | 0 | 411 | 335 | 0 |
| 95th percentile | 743 | 2524 | 8372 | 2307 | 2686 | 0 | 555 | 527 | 0 |
| Maximum | 1923 | 5306 | 37,192 | 3989 | 11,836 | 1463 | 1087 | 2721 | 423 |
| Shortest Euclidean Distance of Residence from Closest A1–A3 Roadway (m) | ||||
|---|---|---|---|---|
| <50 | 50–99 | 100–199 | ≥200 | |
| Number, n (%) | 478 (14.5) | 443 (13.4) | 877 (26.5) | 1511 (45.7) |
| Year of pregnancy, n (%) | ||||
| Before 1974 | 96 (20.1) | 108 (24.4) | 151 (17.2) | 276 (18.3) |
| 1975–1980 | 216 (45.2) | 177 (40.0) | 357 (40.7) | 611 (40.4) |
| After 1980 | 166 (34.7) | 158 (35.7) | 369 (42.1) | 624 (41.3) |
| Maternal age (years), mean (SD) | 27.3 (4.8) | 27.6 (4.5) | 26.9 (4.4) | 28.1 (4.7) |
| Paternal age (years), mean (SD) | 30.2 (6.0) | 30.8 (6.0) | 29.9 (6.0) | 31.2 (5.6) |
| White, n (%) | 457 (95.6) | 430 (97.1) | 850 (96.9) | 1475 (97.6) |
| Maternal education, n (%) | ||||
| Less than high school | 10 (2.1) | 2 (0.5) | 17 (1.9) | 12 (0.8) |
| High school graduate | 72 (15.1) | 81 (18.3) | 210 (24.0) | 261 (17.3) |
| Some college | 181 (37.9) | 152 (34.3) | 301 (34.3) | 518 (34.3) |
| Four-year college graduate or more | 215 (45.0) | 208 (47.0) | 349 (39.8) | 720 (47.7) |
| Paternal occupation, n (%) | ||||
| White collar | 230 (48.1) | 218 (49.2) | 395 (45.0) | 792 (52.4) |
| Blue collar | 171 (35.8) | 165 (37.3) | 269 (30.7) | 521 (34.5) |
| Other | 77 (16.1) | 60 (13.5) | 213 (24.3) | 198 (13.1) |
| Number of prior pregnancies and prior IPD, n (%) | ||||
| Nulligravid | 158 (33.1) | 130 (29.4) | 262 (29.9) | 473 (31.3) |
| Previous pregnancy, no prior IPD | 291 (60.9) | 287 (64.8) | 559 (63.7) | 947 (62.7) |
| Previous pregnancy, prior IPD | 29 (6.1) | 26 (5.9) | 56 (6.4) | 91 (6.0) |
| Gestational weight gain <20 lb, n (%) | 66 (13.8) | 57 (12.9) | 122 (13.9) | 240 (15.9) |
| Cigarette smoking during first trimester, n (%) | 124 (25.9) | 116 (26.2) | 254 (29.0) | 369 (24.4) |
| Alcohol consumption during first trimester, n (%) | 161 (33.7) | 179 (40.4) | 324 (36.9) | 554 (36.7) |
| Any PCE exposure in year prior to pregnancy, n (%) | 238 (49.8) | 221 (49.9) | 399 (45.5) | 804 (53.2) |
| Exposure | Events/N | Unadjusted RR (95% CI) | Adjusted a RR (95% CI) |
|---|---|---|---|
| Distance from closest A1–A3 road (m) | |||
| ≥200 | 127/1511 | Reference | Reference |
| 100–199 | 68/877 | 0.91 (0.66, 1.27) | 0.87 (0.63, 1.21) |
| 50–99 | 40/443 | 1.11 (0.77, 1.60) | 1.08 (0.75, 1.55) |
| <50 | 35/478 | 0.77 (0.49, 1.23) | 0.74 (0.47, 1.17) |
| Length of A1–A3 roads in 500 m buffer (m) | |||
| 0 | 33/422 | Reference | Reference |
| 1–1075 | 124/1443 | 1.14 (0.74, 1.76) | 1.12 (0.73, 1.71) |
| ≥1076 | 113/1444 | 1.11 (0.67, 1.85) | 1.08 (0.65, 1.77) |
| Length of A1–A3 roads in 200 m buffer (m) | |||
| 0 | 120/1441 | Reference | Reference |
| 1–351 | 80/933 | 1.01 (0.73, 1.40) | 0.95 (0.69, 1.31) |
| ≥352 | 70/935 | 0.88 (0.62, 1.24) | 0.85 (0.60, 1.19) |
| Exposure | Preeclampsia | Placental Abruption | SGA | Stillbirth | Vaginal Bleeding | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Events/N | Adjusted a RR (95% CI) | Events/N | Adjusted a RR (95% CI) | Events/N | Adjusted a RR (95% CI) | Events/N | Adjusted a RR (95% CI) | Events/N | Adjusted a RR (95% CI) | |
| Distance from closest A1–A3 road (m) | ||||||||||
| ≥200 | 17/1511 | Reference | 13/1511 | Reference | 108/1505 | Reference | 6/1511 | Reference | 112/1511 | Reference |
| 100–199 | 9/877 | 0.89 (0.37, 2.17) | 10/877 | 1.34 (0.54, 3.30) | 51/870 | 0.81 (0.55, 1.19) | 7/877 | 2.02 (0.65, 6.30) | 61/877 | 0.96 (0.68, 1.35) |
| <100 | 5/921 | 0.46 (0.16, 1.29) | 13/921 | 1.75 (0.82, 3.76) | 61/915 | 0.91 (0.63, 1.31) | 6/921 | 1.71 (0.56, 5.23) | 42/921 | 0.67 (0.47, 0.95) |
| Length of A1–A3 roads in 500 m buffer (m) | ||||||||||
| 0 | 5/422 | Reference | 3/422 | Reference | 25/420 | Reference | 2/422 | Reference | 28/422 | Reference |
| 1–1075 | 14/1443 | 0.81 (0.29, 2.24) | 16/1443 | 1.57 (0.46, 5.33) | 97/1436 | 1.17 (0.70, 1.95) | 7/1443 | 0.97 (0.21, 4.48) | 97/1443 | 1.08 (0.68, 1.73) |
| ≥1076 | 12/1444 | 0.69 (0.23, 2.06) | 17/1444 | 1.67 (0.49, 5.72) | 91/1434 | 1.14 (0.63, 2.05) | 10/1444 | 1.43 (0.33, 6.28) | 90/1444 | 1.02 (0.63, 1.65) |
| Length of A1–A3 roads in 200 m buffer (m) | ||||||||||
| 0 | 16/1441 | Reference | 12/1441 | Reference | 96/1435 | Reference | 6/1441 | Reference | 107/1441 | Reference |
| 1–351 | 9/933 | 0.86 (0.35, 2.09) | 13/933 | 1.76 (0.75, 4.15) | 60/924 | 0.86 (0.59, 1.26) | 9/933 | 2.38 (0.81, 7.00) | 62/933 | 0.89 (0.63, 1.26) |
| ≥352 | 6/935 | 0.56 (0.21, 1.47) | 11/935 | 1.49 (0.65, 3.38) | 57/931 | 0.86 (0.58, 1.28) | 4/935 | 1.08 (0.31, 3.75) | 46/935 | 0.71 (0.50, 1.01) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wesselink, A.K.; Carwile, J.L.; Fabian, M.P.; Winter, M.R.; Butler, L.J.; Mahalingaiah, S.; Aschengrau, A. Residential Proximity to Roadways and Ischemic Placental Disease in a Cape Cod Family Health Study. Int. J. Environ. Res. Public Health 2017, 14, 682. https://doi.org/10.3390/ijerph14070682
Wesselink AK, Carwile JL, Fabian MP, Winter MR, Butler LJ, Mahalingaiah S, Aschengrau A. Residential Proximity to Roadways and Ischemic Placental Disease in a Cape Cod Family Health Study. International Journal of Environmental Research and Public Health. 2017; 14(7):682. https://doi.org/10.3390/ijerph14070682
Chicago/Turabian StyleWesselink, Amelia K., Jenny L. Carwile, María Patricia Fabian, Michael R. Winter, Lindsey J. Butler, Shruthi Mahalingaiah, and Ann Aschengrau. 2017. "Residential Proximity to Roadways and Ischemic Placental Disease in a Cape Cod Family Health Study" International Journal of Environmental Research and Public Health 14, no. 7: 682. https://doi.org/10.3390/ijerph14070682





